The levels of oxidative stress markers in maternal and umbilical cord blood of pregnant women with diabetes mellitus in terms of blood flow redistribution in the fetal venous system
Zalozniaia I.V., Kopteeva E.V., Milyutina Yu.P., Korenevsky A.V., Arutjunyan A.V., Shelaeva E.V., Kapustin R.V., Kogan I.Yu.
Oxidative stress is a significant contributing factor in the development of congenital anomalies, morbidity, and mortality in newborns during pregnancies complicated by diabetes mellitus. Alterations in the antioxidant system that occur during this process, along with transformations of the fetal venous circulation, represent crucial adaptive mechanisms of the developing organism to adverse conditions in the intrauterine environment.
Objective: To examine the levels of oxidative stress markers in pregnant women with pregestational diabetes mellitus (type 1 or 2 diabetes mellitus) in relation to the redistribution of blood flow in the fetal venous system, compared to a control group.
Materials and methods: A prospective study was conducted in the D.O. Ott Research Institute for OG&R from February 2022 to September 2023. The study included 70 women divided into the following groups: Group I included pregnant women with pregestational diabetes mellitus and a ductus venosus shunt fraction ≤16.5% (n=22); Group II included women with pregestational diabetes mellitus and a ductus venosus shunt fraction >16.5% (n=24); and Group III, the control group (n=24). The levels of 3-nitrotyrosine, malondialdehyde, catalase activity, and total antiradical activity were determined in maternal and cord blood serum at 37–41 weeks of pregnancy. Ultrasound examination was also performed to assess venous hemodynamics in the vessels of the umbilical portal venous system of the fetus.
Results: The threshold level for the redistribution of highly oxygenated blood in the ductus venosus to the fetal brain and heart was 16.5%. Analysis of oxidative stress markers in patients with pregestational diabetes mellitus based on the shunt fraction in the ductus venosus showed a significant increase in the levels of 3-nitrotyrosine and malondialdehyde in the group with a reduced shunt fraction in the ductus venosus (≤16.5%) compared to the control group and the group of women with a normal shunt fraction in the ductus venosus (>16.5%). The levels of catalase and antiradical activity were significantly increased in women in the group with a normal shunt fraction in the ductus venosus and remained unchanged compared to the control group in the group with a reduced fraction.
Conclusion: The study results indicate that oxidative stress is induced in patients with pregestational diabetes mellitus, accompanied by a decrease in the shunt fraction in the ductus venosus. This associated change reflects a violation of the compensatory capabilities of the fetus, aimed primarily at preserving the structure and function of the newborn brain. This may underlie the pathogenesis of irreversible changes in the formation and development of the fetal nervous system during pregnancy complicated by diabetes mellitus.
Authors' contributions: All authors made a significant contribution to the conceptualization, execution and preparation of the study, read and approved the final version prior to submission. Zalozniaia I.V. – material collection and processing, drafting of the manuscript, Kopteeva E.V. – material collection and processing, statistical analysis, conception and design of the study, Milyutina Yu.P., Shelaeva E.V. – material collection and processing, Korenevsky A.V., Arutjunyan A.V. – editing of the manuscript, Kapustin R.V., Kogan I.Yu. – conception and design of the study.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The study was conducted in accordance with the research theme for the 2022–2024 “Optimization of methods for prediction, prevention and treatment of “great obstetric syndromes”, as well as delivery strategies in pregnant women from high-risk groups, in order to improve obstetric and perinatal outcomes” (state registration number 1021062812133-0 3.2.2).
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the D.O. Ott Research Institute for OG&R (Ref. No: 115 of 03.02.2022).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Zalozniaia I.V., Kopteeva E.V., Milyutina Yu.P., Korenevsky A.V., Arutjunyan A.V., Shelaeva E.V.,
Kapustin R.V., Kogan I.Yu. The levels of oxidative stress markers in maternal and umbilical cord blood
of pregnant women with diabetes mellitus in terms of blood flow redistribution in the fetal venous system.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (10): 41-51 (in Russian)
https://dx.doi.org/10.18565/aig.2024.178
Keywords
Diabetes mellitus (DM) is a significant public health issue, and its prevalence is increasing annually worldwide. Of particular concern is the rapid rise in the number of women experiencing pregnancy against the backdrop of various carbohydrate metabolism disorders [1]. DM during pregnancy is associated with a higher incidence of congenital anomalies, obstetric complications, and perinatal complications, the most serious of which is antenatal fetal death. Pregestational diabetes mellitus (PGDM), which includes type 1 and type 2 diabetes, carries a significantly higher risk of perinatal complications than DM diagnosed for the first time during pregnancy [1].
It is widely accepted that the mechanisms underlying DM have multiple pathological effects mediated by chronic hypoxia, hyperglycemia, hyperinsulinemia, iron deficiency, alterations in epigenetic regulation, and transcriptional activity of key cellular response modulators. These factors lead to the overproduction of free radicals, such as reactive oxygen species (ROS) and nitrogen, resulting in oxidative stress (OS) that affects both the mother and fetus during pregnancy [2].
Chronic fetal hyperglycemia and hyperinsulinemia resulting from maternal DM accelerate metabolism and increase oxygen consumption in tissues, contributing to a hypoxic state [1]. The influence of OS, defined as a disruption in the balance between free radical synthesis and the antioxidant redox system, is considered a key factor in the development of pregnancy complications associated with DM [3]. Excessive ROS stimulation can pose a risk for miscarriage, fetal malformations, impaired fetal growth (either fetal growth restriction or macrosomia), premature birth, and preeclampsia [4].
In contrast to the pro-oxidant system, the neutralization of free radicals and the toxic products of their metabolism occurs through the combined actions of both enzymatic and non-enzymatic antioxidant systems. Following prolonged exposure to chronic hyperglycemia, a decrease in antioxidant activity is often observed after an initial increase [5]. This decrease triggers a series of chain reactions that promote free-radical synthesis, leading to irreversible damage to cells and macromolecules. This, in turn, initiates lipid peroxidation, depletes thiol proteins, causes hydroxylation and nitration of nucleic acids, results in DNA chain rupture, and exacerbates OS through the formation of additional ROS pools [6]. A significant damaging effect in maternal DM is observed only when the buffer capacity of the protective system is depleted, whereby antioxidant consumption exceeds synthesis, leading to an imbalance between the production of activated oxygen metabolites and their utilization [7].
The markers of free radical oxidation and antioxidant protection are widely used to assess the severity of nitrosative and oxidative damage to macromolecules. Malondialdehyde (MDA), the end product of lipid peroxidation, has been shown in numerous studies to consistently increase in type 1 and type 2 DM [8]. 3-Nitrotyrosine, a marker of nitrosative stress formed during the nitration of proteins at tyrosine residues, contributes to the pathogenesis of DM [9] and can directly damage endothelial cells [9].
The key enzymes involved in antioxidant protection include glutathione peroxidase and catalase. However, with increased ROS formation in the body, catalase is the key enzyme that regulates hydrogen peroxide levels in cells [10]. Total antiradical activity (ARA) serves as an integral indicator of the antiradical capacity of all low-molecular antioxidants in the cell, such as vitamins C and E, reduced glutathione, uric acid, bilirubin, and coenzyme Q [11]. The increased induction of OS observed in various pathological conditions during pregnancy, including maternal hyperglycemia, is a critical factor underlying negative fetal programming [12]. These changes are often attributed to a disruption in endogenous antioxidant protection and decreased adaptive capacity of the fetus in response to adverse environmental conditions [13].
The mechanisms of compensatory adaptation in the fetus under hypoxic conditions also involve redistribution of blood flow in the umbilical-portal venous system, which varies among fetuses with normal growth, macrosomia, and growth restriction [14]. The ductus venosus is a crucial fetal shunt that directs oxygen-rich blood from the umbilical vein to the heart and brain, while bypassing the fetal liver [15]. The shunt fraction in the ductus venosus (SFDV) is an important indicator of fetal hemodynamics and reflects the percentage of highly oxygenated blood entering through this pathway. Previous studies indicate that the fetal adaptive response to hypoxia in placental insufficiency – termed the "brain-sparing effect" – is characterized by a significant increase in SFDV [16]. However, in cases of maternal DM, hemodynamic changes in the fetal umbilical-portal venous system prioritize blood flow to the fetal liver, resulting in a decrease in SFDV throughout gestation [16, 17]. It has also been shown that a marked reduction in oxygenated blood flow through the ductus venosus in maternal DM is associated with fetal lactacidemia [17].
Thus, it can be inferred that in maternal DM, a pathological decrease in fetal SFDV may indicate a reduction in compensatory capabilities in response to chronic hypoxia associated with oxidative and nitrosative stress [18].
This study aimed to examine the levels of oxidative stress markers in pregnant women with pregestational diabetes mellitus (type 1 or 2) concerning the redistribution of blood flow in the fetal venous system compared to a control group.
Materials and methods
Study design
This prospective single-center study was conducted in the D.O. Ott Research Institute for OG&R from February 2022 to November 2023; the study was approved by the local Research Ethics Committee (Ref. No: 115 of 03.02.2022).
The inclusion criteria were singleton pregnancies and signed informed consent to participate in the study. The inclusion criterion for the comparison groups was PGDM, including type 1 or type 2 DM. For the control group, patients without carbohydrate metabolism disorders or severe somatic comorbidities were selected. As a result, 86 patients with PGDM and 58 without carbohydrate metabolism disorders (control group) were included in the study.
In the first phase of the study, to determine SFDV, patients included in the study underwent ultrasound examination at 30+0–41+3 weeks of pregnancy. A detailed description of this phase of the study and calculation of the SFDV can be found in our previous studies [19, 20].
Based on our hypothesis regarding the association of a decrease in SFDV in fetuses from patients in the PGDM group with adverse pregnancy outcomes [19, 20], a logistic regression model was constructed. The presence of at least one and a possible combination of several adverse pregnancy outcomes was considered a combined adverse outcome, which included preterm delivery, intrauterine fetal hypoxia, emergency cesarean section, fetal macrosomia, diabetic fetopathy, hospitalization in the neonatal intensive care unit, or more than 5 days. Patients whose preterm delivery could have been caused by other causes were excluded from the analysis, such as severe preeclampsia and placental abruption. A model of the dependence of adverse outcomes (dependent variable) on the ductus venosus shunt fraction (which is a predictor in this study) was constructed using logistic regression. The Z-test was used to test the null hypothesis using a logistic regression. The characteristics of the logistic regression model are presented in Tables 1 and 2, respectively.
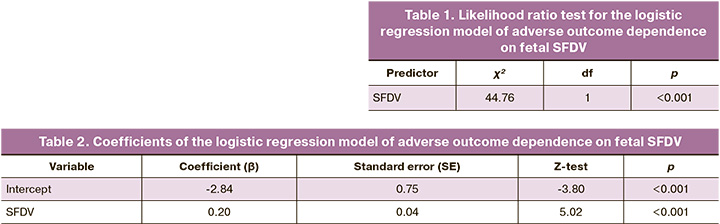
A model of adverse outcome dependence on the SFDV was constructed using logistic regression. ROC analysis was performed to determine the threshold value for SFDV reduction in patients with PGDM (n=86). As a result, different SFDV threshold values were analyzed with the same area under the curve (AUC=0.822). Based on a maximum Youden's index of 0.537, an SFDV threshold of 16.5% was selected. When reached, the sensitivity and specificity for predicting combined adverse outcome were 70.59 and 83.11%, respectively.
Depending on the SFDV, the patients with PGDM were divided into groups (Figure).
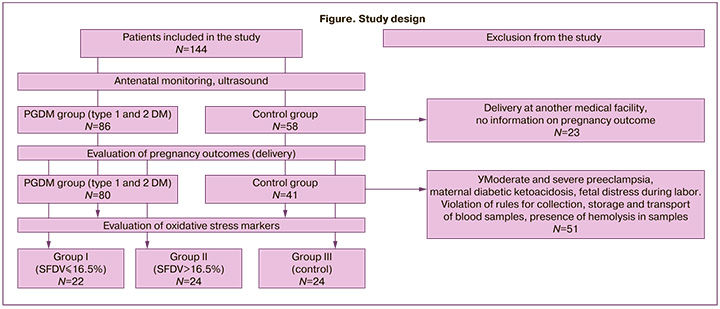
Study methods
Maternal and umbilical cord blood samples were collected during the second phase of this study. Peripheral venous blood was collected for the study once at 37+0-41+3 weeks of gestation. Cord blood was collected after the cord was excised during delivery. The exclusion criteria for this phase of the study were moderate and severe preeclampsia, maternal diabetic ketoacidosis, and fetal distress during labor, as confirmed by biochemical analysis of cord blood and acid-base balance parameters. Blood samples were excluded from further analysis if the rules for collection, storage, and transport of blood samples were violated and if blood samples showed hemolysis.
As a result, 70 pregnant women participated in the study of biochemical markers of OS: group I included patients with PGDM and SFDV £16.5% (n=22); group II included patients with PGDM and SFDV >16.5% (n=24); group III consisted of patients without carbohydrate metabolism disorders (n=24).
Analysis of OS markers included MDA, 3-nitrotyrosine, catalase activity, and ARA. The contents of the studied parameters were evaluated in maternal blood (sampling was carried out from 37+0 to 41+3 weeks of pregnancy) and umbilical cord blood (sampling was carried out from the umbilical vein before the beginning of placental separation). Blood samples were excluded from further analysis if the rules for collection, storage, and transport of blood samples were violated and if blood samples showed hemolysis.
Serum levels of 3-nitrotyrosine in maternal and cord blood were determined by solid-phase enzyme immunoassay using the commercial test system HBT Nitrotyrosine ELISA (Hycult Biotech, Netherlands). Serum MDA levels, as well as the activity of catalase and ARA in maternal and cord blood, were determined by colorimetric methods using reagents of domestic (AO Vekton, Russia) and foreign (Sigma-Algrich, USA) manufacture.
The level of lipid peroxidation in serum was measured by the intensity of the color of complex of active forms of thiobarbituric acid (TBA) formed during the interaction of the final product of lipid peroxidation, MDA, with 2-TBA at high temperature in an acidic medium. The amount of TBA-active products in the butanol fraction was measured at wavelengths of 535 and 580 nm [21].
Catalase activity was determined using a modified Goth method (Góth, 1991) with a solution of ammonium molybdate. It is based on the determination of the amount of a stable colored complex formed as a result of the interaction of a peroxide solution with an ammonium molybdate solution (Vekton, Russia). The absorption maximum of the resulting colored complex occurred at λ=374 nm [22].
Serum ARA was assessed using a calibration curve, and the results were compared with the degree of reduction of the DPPH free radical by the “classical” bioantioxidant ascorbic acid and expressed in μmol g-eq. AK / l [23].
Statistical analysis
Statistical analysis was performed using Jamovi v. 1.6 2021 (Jamovi Project, Australia). Logistic regression and receiver operating characteristic (ROC) analyses were used to determine the SFDV threshold. The distribution of continuous variables was tested for normality using the Kolmogorov–Smirnov test. Continuous parameters with a normal distribution were described using arithmetic means (M) and standard deviations (SD) with 95% confidence intervals [95% CI]. For non-normal distributions, continuous variables were described using the median (Me) and interquartile range (IQR). Categorical variables are described using counts and percentages. Comparison of two groups for a continuous variable with normal distribution was performed using the Student's t-test for non-normal distribution using the Mann–Whitney U test. Comparison of three or more groups for continuous variables with normal distribution was performed using one-way analysis of variance, and post hoc comparisons were performed using Tukey's test (when variances were equal). Comparisons of three or more groups for continuous variables with non-normal distribution were performed using the Kruskal–Wallis test, and post hoc comparisons were performed using the Dunn test with Holm's correction. The Pearson χ2 test was used to compare proportions in the analysis of the multi-way contingency tables.
Results
Clinical characteristics of the study groups
The patients in the study groups were comparable in age, body mass index (BMI), and parity (p> 0.05). Among the patients with PGDM, there was a higher prevalence of obesity and chronic arterial hypertension than in the control group (Table 3).
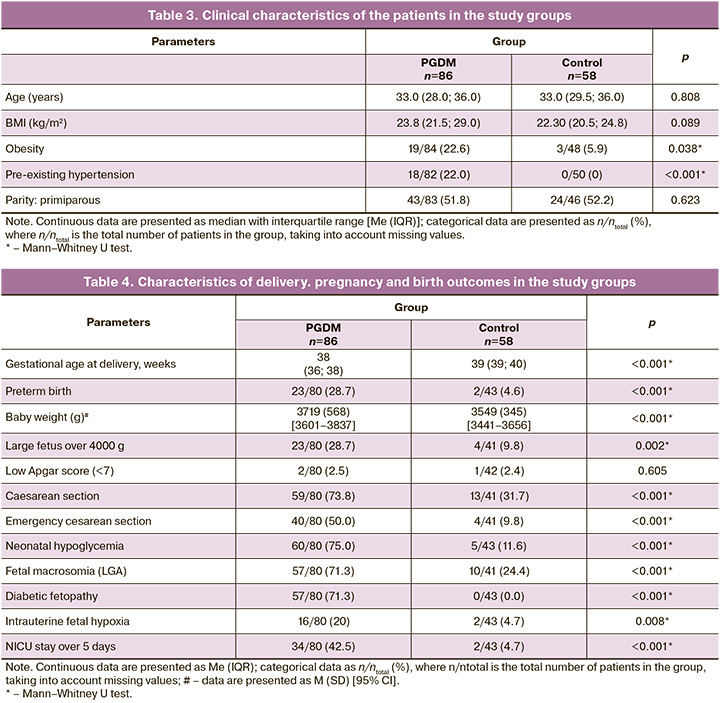
The incidence of neonatal hypoglycemia was significantly higher in patients with PGDM (75.0%) than in the control group (11.6%) (p<0.001). The percentage of large for gestational age (LGA) infants was significantly higher in the PGDM group (71.3%, p<0.001). In addition, infants in the PGDM group were characterized by a high prevalence of diabetic fetopathy (71.3%) and hypertrophic cardiomyopathy (26.3%) (p<0.001) (Table 4). Intrauterine fetal hypoxia was more common in patients with PGDM (20.0%), whereas the frequency of this complication in the control group was 4.7%. In PGDM patients, in 42.5% of cases, newborns required a long stay in the neonatal intensive care unit (NICU) (more than 5 days), while in the control group, it was 4.7% (p<0.001) (Table 4).
Evaluation of oxidative stress markers
Patients from group I (PGDM, SFDV £16.5%) showed a significant increase in maternal (7.93 (2.87) μmol/l) and cord (11.71 (2.35) μmol/l) MDA levels compared to those in groups II and III (p<0.05), between which no significant differences were found (Table 5). The level of 3-nitrotyrosine in maternal (32.54 nmol/l) and umbilical cord (1.45 nmol/l) blood was also increased in group I compared to groups II and III (p<0.05). No differences were found between group II – PGDM (SFDV>16.5%) and the control group (Table 5).
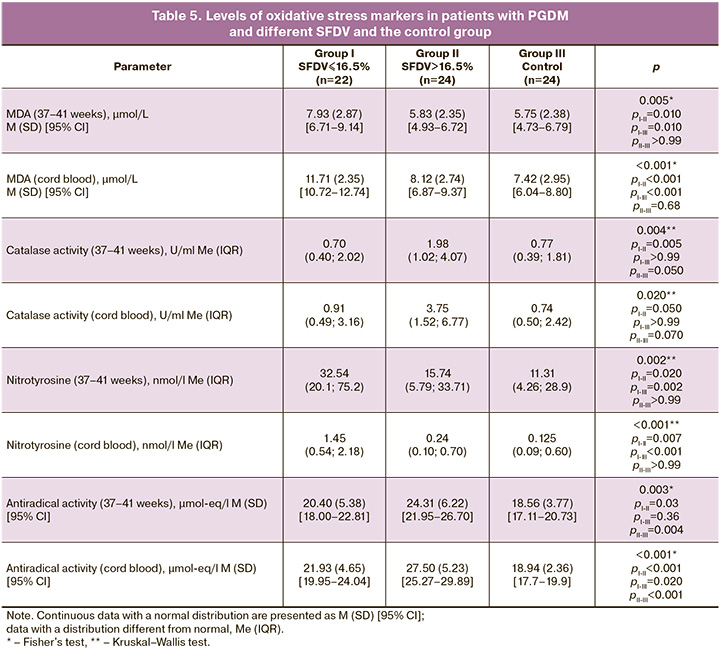
Analysis of catalase activity in maternal blood showed a significant increase in group II (1.98 U/ml) compared to groups I (0.70 U/ml, pI-II=0.005) and III (0.77 U/ml, pII-III=0.05). No differences were found between groups I and III (p>0.99). This trend was preserved when analyzing catalase activity in cord blood, which was increased in group II (3.75 U/ml) compared to groups I (0.91 U/ml, pI-II=0.05) and III (0.74 U/ml, pII-III=0.07). Among patients with PGDM and SFDV>16.5% (group II), an increase in ARA in maternal blood was observed (24.31 (6.22) μmol-eq/l) compared to that in group I (pI-II=0.03) and control group III (pII-III=0.004). No differences were found between groups I and III (p=0.360). In group II, an increase in ARA in umbilical cord blood was also observed (27.50 (5.23) μmol-eq/l) compared to that in group I (pI-II<0.001) and control group III (pI-III=0.020) (Table 4). As a result of the analysis of OS markers in the study groups, it was established that in patients with PGDM and SFDV >16.5%, MDA and 3-nitrotyrosine indicators were comparable with the control group data, whereas a significant increase in these biomarkers was noted only in patients with reduced SFDV (≤16.5%). In patients with PGDM and normal SFDV, an increase in catalase activity was noted compared with the control group and patients with PGDM and reduced SFDV. Total ARA increased in patients with PGDM, both in maternal and cord blood, mainly because of patients with normal SFDV.
Discussion
The study evaluated oxidative stress markers and antioxidant protection parameters in the maternal and cord blood of women with PGDM and a control group. Patients with a history of type 1 and type 2 diabetes mellitus were combined into one group because of the significant influence of chronic hyperglycemia on the development of maternal and fetal free radical processes during pregnancy. The data obtained demonstrate that the nature of OS activation in patients with PGDM differs depending on SFDV. The analysis revealed that in patients with PGDM and normal SFDV (>16.5%), the indices of MDA and 3-nitrotyrosine were comparable to those in the control group. However, a significant increase in these biomarkers was observed only in patients with a reduced SFDV (≤16.5%).
These results are consistent with those of previous studies. Lipid peroxidation is activated in pregnant women with DM, as indicated by an increase in serum MDA levels. Additionally, patients with PGDM exhibit a significantly higher level of free MDA than those with gestational diabetes, suggesting greater activation of OS [8]. MDA is one of the most sensitive indicators of neonatal hypoxia, and its levels are positively correlated with maternal blood glucose concentration and fetal erythropoietin production [24]. The degree of OS activation is also influenced by DM compensation, duration of hyperglycemia, and need for insulin therapy, which are directly related to the increased formation of OS markers and development of perinatal complications [25]. An increase in MDA levels and a decrease in superoxide dismutase (SOD) activity among mothers with diabetic pregnancies led to a significant increase in the incidence of macrosomia in newborns compared to that in the control group [26]. In our study, elevated 3-nitrotyrosine levels in the PGDM group with reduced SFDV indicated the activation of nitrosative stress. Nitration of protein-bound tyrosine is considered a post-translational modification with significant pathophysiological consequences that can adversely affect the antioxidant system either directly or indirectly. For instance, the nitration of Mn-SOD inactivates the enzyme, leading to a self-amplifying chain reaction of OS [27]. Excessive nitrosative stress during pregnancy complicated by diabetes, along with the resultant endothelial dysfunction, plays a crucial role in perinatal complications and adverse outcomes in offspring [28].
In our study, an increase in the activity of markers for both the enzymatic (catalase) and non-enzymatic (ARA) components of the antioxidant system was noted only in patients with preserved SFDV (>16.5%) in the PGDM group. Other studies have reported conflicting results regarding catalase activity in patients with DM. Some researchers found no difference in catalase activity between women with DM and healthy pregnant women [29]. In contrast, others noted an increase in plasma catalase levels among those with gestational diabetes [30], while another study reported a decrease [31]. Some studies have indicated elevated catalase levels in women with type 1 DM, whereas in those with gestational DM, the levels did not differ from the control [32]. Notably, a study on patients with type 2 DM showed decreased SOD activity while maintaining catalase activity [31], potentially indicating a direct relationship between catalase activity and the severity of carbohydrate metabolism disorders as well as an increased role of catalase in decomposing peroxides during OS.
ARA levels reflect the overall antiradical capacity of the non-enzymatic components of the antioxidant system (vitamins C, E, and uric acid). While previous studies, including ours, have predominantly demonstrated decreased total antioxidant capacity in pregnant women with different types of DM [25], changes in ARA levels have varied across studies and are directly influenced by the severity of carbohydrate metabolism disorders, correction methods, and the duration of DM. For example, some studies have noted decreased levels of vitamin A, SOD, glutathione, and total antioxidant capacity in gestational DM patients, along with increased vitamin E content, which acts as a primary “radical trap” in cells, and elevated MDA levels compared to the control group [5]. Conversely, pregnant women with diabetes showed decreased vitamin E levels and increased vitamin C concentrations, with no change in vitamin A levels compared to the control group [26].
Thus, in women with PGDM and normal SFDV, there is a trend towards increased antioxidant activity, with OS marker levels comparable to those in the control group, reflecting the presence of compensatory adaptation mechanisms. In contrast, women with PGDM and reduced SFDV exhibited a shift towards increased OS, as evidenced by elevated prooxidant levels and unchanged antioxidant activity, indicating diminished compensatory capabilities in this group.
The components of the adaptive-compensatory mechanisms of the fetus in maternal DM are diverse: beyond the activation of antioxidant systems, these include anaerobic glycolysis activation, fetal hemoglobin structure with high oxygen affinity, the release of blood from the fetal liver into the bloodstream, increased heart rate, heightened workload of the left heart sections under stress, and enhanced splenic blood flow to boost fetal erythropoietin production and other hematopoiesis factors [33]. Among these components, the centralization of fetal blood flow is crucial, as it effectively protects the fetal brain from hypoxic damage. However, in maternal DM, the ability to centralize the blood circulation appears to be compromised. Possible causes of this hemodynamic redistribution include increased blood flow to the fetal liver [34], expanded cross-sectional areas of fetal liver vessels, and reduced vascular resistance in response to catecholamine release in maternal DM [35]. The interrelationships and cross-influences of various compensatory mechanisms remain unclear. Recent studies have focused on the activation of nuclear factor erythroid-related 2 (Nrf2), the primary modulator of antioxidant activity in enzymatic systems [36]. Nrf2 is a transcription factor that enters the nucleus in response to OS and stimulates the expression of numerous cytoprotective genes, including those encoding antioxidant enzymes and related proteins, such as SOD, catalase, heme oxygenase-1, glutathione S-transferase, and thioredoxin. Dysregulation of Nrf2 has been implicated in diabetes etiology, with increased Nrf2 expression leading to overexpression of catalase and SOD, providing protective effects against complications [36].
Some studies have shown OS activation in the placentas of women with PGDM along with decreased expression of Nrf2 and its downstream antioxidant enzymes. Increased antioxidant enzyme production coincided with elevated Nrf2 expression in the placentas of gestational DM rats.
It seems that, in compensated PGDM, as in gestational DM, compensatory mechanisms are fully operational, initially reflected by increased Nrf2 levels and accompanying antioxidants to counteract OS. Conversely, decompensated DM is associated with a breakdown of these compensatory mechanisms, evident in decreased Nrf2 expression and antioxidant activity, leading to increased OS due to increased free radical production [36].
Thus, at the transcriptional and centralized regulatory levels of antioxidant synthesis in cells, the adaptive compensatory mechanisms of the fetus in response to chronic hypoxia and hyperglycemia during pregnancy complicated by DM are evident. Of particular interest is the study of Nrf2’s biological roles that extend beyond its canonical cytoprotective functions, including its impact on angiogenesis. Recent studies have indicated that mice with hereditary Nrf2 deficiency develop a congenital intrahepatic shunt connecting the portal vein with the inferior vena cava, exhibiting characteristics of a patent venous duct with a high prevalence (~66%) [37].
The mechanisms underlying the formation of a portocaval shunt, similar to that of the venous duct, in mice with Nrf2 deficiency, are unclear. This portocaval shunt is a remnant of the embryonic structure that allows the fetus to maintain brain function during pregnancy when exposed to various unfavorable factors; it typically does not function in the postnatal period. This shunt was observed in young mice with Nrf deficiency and may have performed functions similar to those in the embryo. Since increased expression of Nrf2 is primarily observed in neurons, where this factor plays a vital role [1], preservation of the liver shunt in adult animals, facilitating the access of oxygenated blood to the brain, may serve as a last reserve of adaptation aimed at preserving brain function [38].
In light of the results obtained in this study and based on the reviewed literature, it can be argued that the adaptive and compensatory capabilities of the fetus with diabetes mellitus (DM) demonstrate extreme diversity and pleiotropy of action. Nrf2 may be a key link in the system of adaptive mechanisms, providing a connection between the compensatory reaction of antioxidant defense proteins, angiogenic factors, and changes in fetal venous hemodynamics in pathologies associated with the activation of oxidative stress (OS). Investigation of Nrf2 in this context is promising for further scientific research. In this complex interconnected system of responses to OS, the initial compensatory increase in antioxidant defense products likely represents the first step, which subsequently transitions into a decrease in their activity associated with prolonged pathological effects and overstrain of antioxidant systems.
Conclusion
The study found that a decrease in fetal SFDV (≤16.5%) in pregnant women with pregestational diabetes mellitus was associated with an increase in OS and a lack of increase in the activity of antioxidant systems, indicating a decrease in the adaptive capacity of this patient group. Conversely, when SFDV was preserved (>16.5%), there was a notable shift towards increased antioxidant activity, and the content of prooxidants was comparable to their levels in the control group. This reflects the presence of active compensatory adaptation mechanisms and the mitigation of the effects of hyperglycemia in this group of patients.
References
- Reece E.A. Diabetes-induced birth defects: what do we know? What can we do? Curr. Diab. Rep. 2012; 12(1): 24-32. https://dx.doi.org/10.1007/s11892-011-0251-6.
- Vento M. Oxidative stress in the perinatal period. Free Radic. Biol. Med. 2019; 142: 1-2. https://dx.doi.org/10.1016/j.freeradbiomed.2019.07.028.
- Jozwik M., Wolczynski S., Jozwik M., Szamatowicz M. Oxidative stress markers in preovulatory follicular fluid in humans. Mol. Hum. Reprod. 1999; 5(5): 409-13. https://dx.doi.org/10.1093/molehr/5.5.409.
- Скрипниченко Ю.П., Пятаева С.В, Володина М.А., Цвиркун Д.В., Баранов И.И., Высоких М.Ю., Кузьмич И.Н. Особенности течения окислительно-восстановительных реакций в крови у женщин с физиологически протекающей и осложненной беременностью. Акушерство и гинекология. 2017; 8: 60-6. [Skripnichenko Yu.P., Pyataeva S.V., Volodina M.A., Tsvirkun D.V., Baranov I.I., Vysokikh M.Yu., Kuz’mich I.N. Specific features of redox reactions in the blood of women with physiological or complicated pregnancy. Obstetrics and Gynecology. 2017; (8): 60-6. (in Russian)]. https://dx.doi.org/10.18565/aig.2017.8.60-6.
- Ma H., Qiao Z., Li N., Zhao Y., Zhang S. The relationship between changes in vitamin A, vitamin E, and oxidative stress levels, and pregnancy outcomes in patients with gestational diabetes mellitus. Ann. Palliat. Med. 2021; 10(6): 6630-6. https://dx.doi.org/10.21037/apm-21-1036.
- Guérin P., El Mouatassim S., Ménézo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum. Reprod. Update. 2001; 7(2): 175-89. https://dx.doi.org/10.1093/humupd/7.2.175.
- Dennery P.A. Role of redox in fetal development and neonatal diseases. Antioxid. Redox Signal. 2004; 6(1): 147-53. https://dx.doi.org/10.1089/152308604771978453.
- Peuchant E., Brun J.L., Rigalleau V., Dubourg L., Thomas M.J., Daniel J.Y. et al. Oxidative and antioxidative status in pregnant women with either gestational or type 1 diabetes. Clin. Biochem. 2004; 37(4): 293-8. https://dx.doi.org/10.1016/j.clinbiochem.2003.12.005.
- Pacher P., Obrosova I.G., Mabley J.G., Szabó C. Role of nitrosative stress and peroxynitrite in the pathogenesis of diabetic complications. Emerging new therapeutical strategies. Curr. Med. Chem. 2005; 12(3): 267-75. https://dx.doi.org/10.2174/0929867053363207.
- Djordjevic A., Spasic S., Jovanovic-Galovic A., Djordjevic R., Grubor-Lajsic G. Oxidative stress in diabetic pregnancy: SOD, CAT and GSH-Px activity and lipid peroxidation products. J. Matern. Fetal Neonatal Med. 2004; 16(6): 367-72. https://dx.doi.org/10.1080/14767050400018270.
- Aouache R., Biquard L., Vaiman D., Miralles F. Oxidative stress in preeclampsia and placental diseases. Int. J. Mol. Sci. 2018; 19(5): 1496. https://dx.doi.org/10.3390/ijms19051496.
- Márquez-Valadez B., Valle-Bautista R., García-López G., Díaz N.F., Molina-Hernández A. Maternal diabetes and fetal programming toward neurological diseases: beyond neural tube defects. Front. Endocrinol. (Lausanne). 2018; 9: 664. https://dx.doi.org/10.3389/fendo.2018.00664.
- Ornoy A., Reece E.A., Pavlinkova G., Kappen C., Miller R.K. Effect of maternal diabetes on the embryo, fetus, and children: congenital anomalies, genetic and epigenetic changes and developmental outcomes. Birth Defects Res. C Embryo Today. 2015; 105(1): 53-72. https://dx.doi.org/10.1002/bdrc.21090.
- Kiserud T., Kessler J., Ebbing C., Rasmussen S. Ductus venosus shunting in growth-restricted fetuses and the effect of umbilical circulatory compromise. Ultrasound Obstet. Gynecol. 2006; 28(2): 143-9. https://dx.doi.org/10.1002/uog.2784.
- Ярыгина Т.А., Гасанова Р.М., Марзоева О.В., Сыпченко Е.В., Гус А.И. Все о венозном протоке - в помощь практикующим специалистам. Акушерство и гинекология. 2023; 9: 22-32. [Yarygina T.A., Gasanova R.M., Marzoeva O.V., Sypchenko E.V., Gus A.I. All that practitioners should know about ductus venosus. Obstetrics and Gynecology. 2023; (9): 22-32. (in Russian)]. https://dx.doi.org/10.18565/aig.2023.127.
- Godfrey K.M., Haugen G., Kiserud T., Inskip H.M., Cooper C., Harvey N.C. et al.; Southampton Women's Survey Study Group; Hanson MA. Fetal liver blood flow distribution: role in human developmental strategy to prioritize fat deposition versus brain development. PLoS One. 2012; 7(8): e41759. https://dx.doi.org/10.1371/journal.pone.0041759.
- Lund A., Ebbing C., Rasmussen S., Kiserud T., Kessler J. Maternal diabetes alters the development of ductus venosus shunting in the fetus. Acta Obstet. Gynecol. Scand. 2018; 97(8): 1032-40. https://dx.doi.org/10.1111/aogs.13363.
- Behrman H.R., Kodaman P.H., Preston S.L., Gao S. Oxidative stress and the ovary. J. Soc. Gynecol. Investig. 2001; 8(1 Suppl. Proceedings): S40-S42. https://dx.doi.org/10.1016/s1071-5576(00)00106-4.
- Коптеева Е.В., Шелаева Е.В., Алексеенкова Е.Н., Капустин Р.В., Коган И.Ю. Особенности гемодинамики в умбиликально-портальной венозной системе плода при беременности, осложненной сахарным диабетом. Журнал акушерства и женских болезней. 2024; 73(2): 27-41. [Kopteeva E.V., Shelaeva E.V., Alekseenkova E.N., Kapustin R.V., Kogan I.Yu. Blood flow redistribution in the fetal umbilical-portal venous system in pregnancy complicated by diabetes mellitus. Journal of Obstetrics and Women’s Diseases. 2024; 73(2): 27-41. (in Russian)]. https://dx.doi.org/10.17816/JOWD625384.
- Шелаева Е.В., Коптеева Е.В., Алексеенкова Е.Н., Капустин Р.В., Коган И.Ю. Роль умбилико-портальной венозной гемодинамики в патогенезе макросомии плода при беременности, осложненной сахарным диабетом. Журнал акушерства и женских болезней. 2024; 73(3): 89-104. [Shelaeva E.V., Kopteeva E.V., Alekseenkova E.N., Kapustin R.V., Kogan I.Yu. The role of umbilical-portal venous hemodynamics in fetal macrosomia pathogenesis in pregnancy complicated by diabetes mellitus. Journal of Obstetrics and Women’s Diseases. 2024; 73(3): 89-104. (in Russian)]. https://dx.doi.org/10.17816/JOWD629597.
- Гаврилов В.Б., Гаврилова А.Р., Мажуль Л.М. Анализ методов определения продуктов перекисного окисления липидов в сыворотке крови по тесту с тиобарбитуровой кислотой. Вопросы медицинской химии. 1987; 33(1): 118-22. [Gavrilov V.B., Gavrilova A.R., Mazhul' L.M. Methods of determining lipid peroxidation products in the serum using a thiobarbituric acid test. Voprosy Meditsinskoi Khimii. 1987; 33(1): 118-22. (in Russian)].
- Góth L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta. 1991; 196(2-3): 143-51. https://dx.doi.org/10.1016/0009-8981(91)90067-m.
- Евсюкова И.И., Арутюнян А.В., Ковалевская О.В., Прокопенко В.М., Опарина Т.И., Додхоев Д.С. Интенсивность свободнорадикального окисления и состояние антиоксидантной системы у новорожденных детей, развивавшихся в условиях хронической плацентарной недостаточности. Журнал акушерства и женских болезней. 2007; 56(3): 50-5. [Evsyukova I.I., Arutyunyan A.V., Kovalevskaya O.V., Prokopenko V.M., Oparina T.I., Dodkhoev D.S. The intensity of free radical oxidation and antioxidant system state in the newborn after chronic placental deficiency. Journal of Obstetrics and Women’s Diseases. 2007; 56(3): 50-5. (in Russian)].
- Mentese A., Güven S., Demir S., Sümer A., Yaman S.Ö., Alver A. et al. Circulating parameters of oxidative stress and hypoxia in normal pregnancy and HELLP syndrome. Adv. Clin. Exp. Med. 2018; 27(11): 1567-72. https://dx.doi.org/ 10.17219/acem/74653.
- Kapustin R., Chepanov S., Kopteeva E., Arzhanova O. Maternal serum nitrotyrosine, 8-isoprostane and total antioxidant capacity levels in pre-gestational or gestational diabetes mellitus. Gynecol. Endocrinol. 2020; 36(Supp1. 1): S36-S42. https://dx.doi.org/10.1080/09513590.2020.1816727.
- Grissa O., Atègbo J.M., Yessoufou A., Tabka Z., Miled A., Jerbi M. et al. Antioxidant status and circulating lipids are altered in human gestational diabetes and macrosomia. Transl. Res. 2007; 150(3): 164-71. https://dx.doi.org/10.1016/j.trsl.2007.03.007.
- Quijano C., Hernandez-Saavedra D., Castro L., McCord J.M., Freeman B.A., Radi R. Reaction of peroxynitrite with Mn-superoxide dismutase. Role of the metal center in decomposition kinetics and nitration. J. Biol. Chem. 2001; 276(15): 11631-8. https://dx.doi.org/10.1074/jbc.M009429200.
- Horváth E.M., Magenheim R., Kugler E., Vácz G., Szigethy A., Lévárdi F. et al. Nitrative stress and poly(ADP-ribose) polymerase activation in healthy and gestational diabetic pregnancies. Diabetologia. 2009; 52(9): 1935-43. https://dx.doi.org/10.1007/s00125-009-1435-3.
- Biri A., Onan A., Devrim E., Babacan F., Kavutcu M., Durak I. Oxidant status in maternal and cord plasma and placental tissue in gestational diabetes. Placenta. 2006; 27(2-3): 327-32. https://dx.doi.org/10.1016/j.placenta.2005.01.002.
- Lappas M., Permezel M., Ho P.W., Moseley J.M., Wlodek M.E., Rice G.E. Effect of nuclear factor-kappa B inhibitors and peroxisome proliferator-activated receptor-gamma ligands on PTHrP release from human fetal membranes. Placenta. 2004; 25(8-9): 699-704. https://dx.doi.org/10.1016/j.placenta.2004.02.003.
- López-Tinoco C., Roca M., García-Valero A., Murri M., Tinahones F.J., Segundo C. et al. Oxidative stress and antioxidant status in patients with late-onset gestational diabetes mellitus. Acta Diabetol. 2013; 50(2): 201-8. https://dx.doi.org/10.1007/s00592-011-0264-2.
- Orhan H., Onderoglu L., Yücel A., Sahin G. Circulating biomarkers of oxidative stress in complicated pregnancies. Arch. Gynecol. Obstet. 2003; 267(4): 189-95. https://dx.doi.org/10.1007/s00404-002-0319-2.
- Desoye G., Carter A.M. Fetoplacental oxygen homeostasis in pregnancies with maternal diabetes mellitus and obesity. Nat. Rev. Endocrinol. 2022; 18(10): 593-607. https://dx.doi.org/10.1038/s41574-022-00717-z.
- Tchirikov M., Schröder H.J., Hecher K. Ductus venosus shunting in the fetal venous circulation: regulatory mechanisms, diagnostic methods and medical importance. Ultrasound Obstet. Gynecol. 2006; 27(4): 452-61. https://dx.doi.org/10.1002/uog.2747.
- Tchirikov M., Kertschanska S., Schröder H.J. Differential effects of catecholamines on vascular rings from ductus venosus and intrahepatic veins of fetal sheep. J. Physiol. 2003; 548(Pt. 2): 519-26. https://dx.doi.org/10.1113/jphysiol.2002.034470.
- Manoharan B., Bobby Z., Dorairajan G., Jacob S.E., Gladwin V., Vinayagam V. et al. Increased placental expressions of nuclear factor erythroid 2-related factor 2 and antioxidant enzymes in gestational diabetes: protective mechanisms against the placental oxidative stress? Eur. J. Obstet. Gynecol. Reprod. Biol. 2019; 238: 78-85. https://dx.doi.org/10.1016/j.ejogrb.2019.05.016.
- Skoko J.J., Wakabayashi N., Noda K., Kimura S., Tobita K., Shigemura N. et al. Loss of Nrf2 in mice evokes a congenital intrahepatic shunt that alters hepatic oxygen and protein expression gradients and toxicity. Toxicol. Sci. 2014; 141(1): 112-9. https://dx.doi.org/10.1093/toxsci/kfu109.
- Jin Y., Wang G., Han S.S., He M.Y., Cheng X., Ma Z.L. et al. Effects of oxidative stress on hyperglycaemia-induced brain malformations in a diabetes mouse model. Exp. Cell Res. 2016; 347(1): 201-11. https://dx.doi.org/10.1016/j.yexcr.2016.08.002.
Received 22.07.2024
Accepted 24.09.2024
About the Authors
Irina V. Zalozniaia, PhD (Bio), D.O. Ott Research Institute for OG&R, 3 Mendeleevskaya Line, Saint Petersburg, 199034, Russia, irinabiolog2012@yandex.ru,eLibrary SPIN: 2488-3790, https://orcid.org/0000-0002-0576-9690
Ekaterina V. Kopteeva, MD, D.O. Ott Research Institute for OG&R, 3 Mendeleevskaya Line, Saint Petersburg, 199034, Russia, ekaterina_kopteeva@bk.ru,
eLibrary SPIN: 9421-6407, https://orcid.org/0000-0002-9328-8909
Yulia P. Milyutina, PhD (Bio), D.O. Ott Research Institute for OG&R, 3 Mendeleevskaya Line, Saint Petersburg, 199034, Russia, milyutina1010@mail.ru,
eLibrary SPIN: 6449-5635, https://orcid.org/0000-0003-1951-8312
Andrey V. Korenevsky, Dr. Bio. Sci., D.O. Ott Research Institute for OG&R, 3 Mendeleevskaya Line, Saint Petersburg, 199034, Russia, a.korenevsky@yandex.ru,
eLibrary SPIN: 7942-6016, https://orcid.org/0000-0002-0365-8532
Alexander V. Arutjunyan, Dr. Bio. Sci., Professor, D.O. Ott Research Institute for OG&R, 3 Mendeleevskaya Line, Saint Petersburg, 199034, Russia, alexarutiunjan@gmail.com, eLibrary 9938-5277, https://orcid.org/0000-0002-0608-9427
Elizaveta V. Shelaeva, PhD, D.O. Ott Research Institute for OG&R, 3 Mendeleevskaya Line, Saint Petersburg, 199034, Russia, eshelaeva@yandex.ru, eLibrary SPIN: 7440-0555, https://orcid.org/0000-0002-9608-467X
Roman V. Kapustin, Dr. Med. Sci., D.O. Ott Research Institute for OG&R, 3 Mendeleevskaya Line, Saint Petersburg, 199034, Russia, kapustin.roman@gmail.com,
eLibrary SPIN: 7300-6260, https://orcid.org/0000-0002-2783-3032
Igor Yu. Kogan, Dr. Med. Sci., Professor, Corresponding Member of the Russian Academy of Sciences, D.O. Ott Research Institute for OG&R, 3 Mendeleevskaya Line,
Saint Petersburg, 199034, Russia, ikogan@mail.ru, eLibrary SPIN: 6572-6450, https://orcid.org/0000-0002-7351-6900
Corresponding author: Irina V. Zalozniaia, irinabiolog2012@yandex.ru



