Newborn and fetal cardiovascular system in twin-to-twin transfusion syndrome
Relevance. Twin-to-twin transfusion syndrome (TTTS) is associated with fetal cardiovascular disorders carrying the risk of antenatal death and congenital heart disease.Kostyukov K.V., Sakalo V.A., Gladkova K.A., Bokeriya E.L.
Aim. To investigate the effect of TTTS on the fetal cardiovascular system.
Materials and methods. The study included 145 pregnant women with monochorionic diamniotic twin pregnancies complicated by TTTS. The participants were divided into two groups based on the Quintero staging: stage I–II (group I, n=100) and stage III–IV (group II, n=45). Myocardial hypertrophy, cardiomegaly, the state of the heart valves, and venous hemodynamics were examined by expert echocardiography.
Results. In the whole study cohort, 54.2% of recipient twins had cardiomyopathy. Women with Quintero stage III and IV TTTS were more likely (100%) to have twins with myocardial hypertrophy than those with stage I and II TTTS (73.8%), p=0.001. Recipient twins with cardiomyopathy had a high antenatal mortality rate (19.3%), p=0.01. Cardiomyopathy persisted in 25.4% of newborns from pregnancies affected by TTTS.
Conclusion. Cardiomyopathy of the recipient twin is a TTTS complication, which increases in frequency as the disease progresses. The presence of cardiomyopathy increases the risk of antenatal death of the recipient. Surgical correction of TTTS aimed to reverse hemodynamic imbalance may improve fetal cardiac function, which illustrates the adaptability of the developing heart. However, a quarter of surviving recipient twins has cardiomyopathy and remains at risk for pulmonary stenosis.
Keywords
Monochorionic multiple pregnancies carry a high risk of perinatal mortality and morbidity. Even not complicated by twin-to-twin transfusion syndrome (TTTS), 4–11% of monochorionic multiple pregnancies are associated with fetal cardiovascular abnormalities [1].
In TTTS, the blood that would normally go to the donor twin is diverted to the recipient twin, causing a reduction in blood volume in the donor twin and excess of blood in the recipient twin. It results from unbalanced unidirectional blood flow through placental arteriovenous anastomoses. To compensate for hypovolemia, the donor twin releases endothelin II and natriureticpeptide, thusactivatingtherenin-angiotensin-aldosterone system (RAAS). As a potent vasoconstrictor, angiotensin II increases peripheral vascular resistance and causes the secretion of aldosterone from the adrenal cortex. Aldosterone increases tubular reabsorption of sodium and fluid to regulate hypovolemia, which results in an increase in blood volume and reabsorption in the renal tubules. Chronic hyper-perfusion of the recipient twin and activation of the RAAS of the donor twin aggravates renal hypoperfusion, leading to persistent activation of the RAAS and maintaining a vicious circle. An increase in circulating blood volume and peripheral vascular resistance leads to myocardial remodeling in the recipient twin, accompanied by a wide range of cardiovascular changes [2, 3]. The progression of cardiomyopathy may ultimately lead to heart failure, which is the leading cause of the recipient's death.
The fetal cardiovascular system was assessed by fetal echocardiography (ECHO-KG) that can be used for the detailed diagnosis and evaluation of morphologic and functional changes. ECHO-KG provides a four-chamber view of the heart, left and right ventricular outflow tracts, projections of three vessels and trachea, and also allows evaluation of venous hemodynamics [4, 5]. In recent years, studies investigating Doppler fetal venous hemodynamics have been increasingly focused on blood flow in the venous duct. With an overload of the right heart, abnormal blood flow in the venous duct is visualized as zero and reversed ductus venous flow in the phase of atrial contraction.
The Myocardial Performance Index, also known as the Tei index, is a measure of global systolic and diastolic myocardial function. Several studies have shown that the Tei index correlates with a degree of myocardial dysfunction, even in the early stages of TTTS [6].
Estimates of the heart size, cardio-thoracic index, ventricular muscle, interventricular septal thickness, atrioventricular valve regurgitation, Tei index, E/A ratio, and ventricular outflow tract obstruction can be used to assess the progression of TTTS.
ECHO-KG in TTTS with pronounced polyhydramnios of one twin and oligohydramnios of the co-twin may be technically challenging and sets high demands for specialists and equipment.
 The present study aimed to investigate the effect of TTTS on the fetal cardiovascular system.
The present study aimed to investigate the effect of TTTS on the fetal cardiovascular system.
Materials and methods
This prospective study included 145 patients with monochorionic diamniotic twins complicated by TTTS who were managed, underwent fetoscopic coagulation of placental vascular anastomoses (FLCA), and delivered at the V.I. Kulakov NMRC for OG&P of Ministry of Health of Russia from 2014 to 2019.
Inclusion criteria were monochorionic diamniotic twin pregnancy complicated by TTTS and managed with FLCA. Exclusion criteria were malformations of one or both twins.
The participants were divided into two groups based on the Quintero staging: stage I–II (group I, n=100) and stage III–IV (group II, n = 45). All patients underwent FLCA. The groups did not differ significantly concerning gestational age at surgery (20.5 ± 1.7 weeks). Newborns from pregnancies affected by TTTS, who were prenatally diagnosed with cardiomyopathy, were also divided into two groups including newborns with stage I–II TTTS (group I, n=37) and stage III–IV TTTS (group II, n=30).
The groups underwent expert fetal echocardiography using the Voluson E8 ultrasound system (GE Medical Systems, Zipf, Austria) at 16–26 weeks’ gestation, as well as in the early neonatal period (first seven days of life) and before the child was discharged from the hospital (on average on day 21).
 ECHO-KG examination included the study of the following parameters:
ECHO-KG examination included the study of the following parameters:
• estimation of ventricular wall thickness.
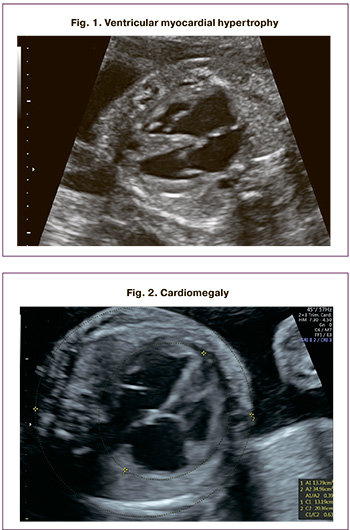
The diagnostic criterion for ventricular hypertrophy was the excess of the ventricular wall thickness or interventricular septum of two standard deviations from the expected mean value for gestational age (Fig. 1).
• heart size (degree of cardiomegaly);
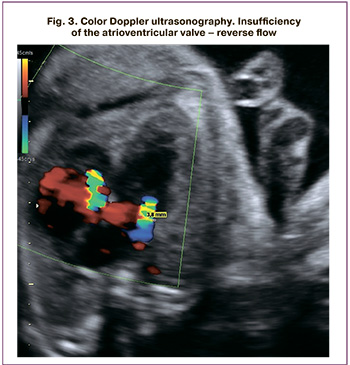
Cardiothoracic ratio (CTI)>1/3but<50% was considered moderate cardiomegaly, and CTI >50% was regarded as pronounced cardiomegaly with a (Fig. 2).
• the state of the valve apparatus;
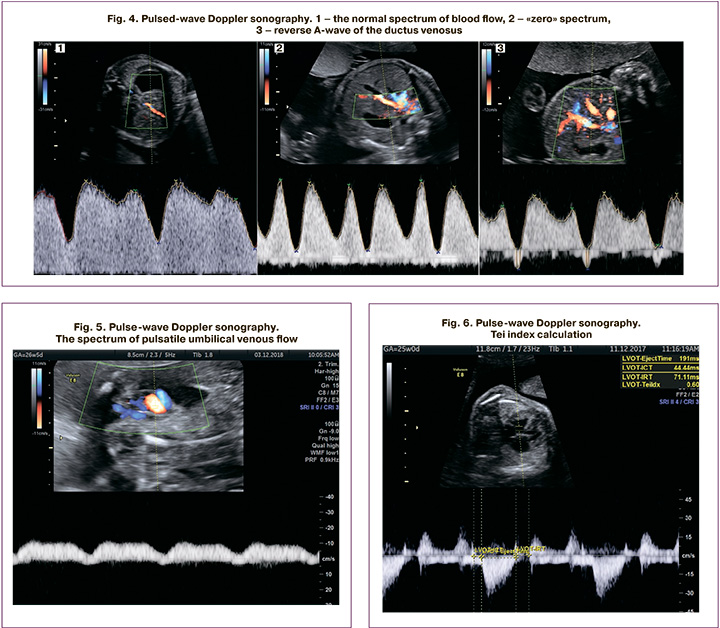
Valvular insufficiency was considered moderate and pronounced when the regurgitant jet width was<25% and>25% of the atrial area, respectively (Fig. 3)
• venous hemodynamics;
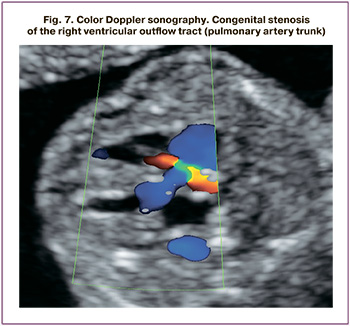 Fetal ductus venosus flow was assessed in a sagittal or transverse plane through the fetal abdomen using color Doppler mapping. Right heart overload results in decreased ductus venosus blood flow (an increase in the pulsation index of more than 95 percentile), and subsequently in a zero and reverse curve type. Ductus venosus flow pattern was classified as normal (positive A-wave) or abnormal (absent or negative A-wave) (Fig.4). With the progression of cardiomyopathy, the pulsating spectrum of blood flow in the umbilical vein is determined (Fig. 5).
Fetal ductus venosus flow was assessed in a sagittal or transverse plane through the fetal abdomen using color Doppler mapping. Right heart overload results in decreased ductus venosus blood flow (an increase in the pulsation index of more than 95 percentile), and subsequently in a zero and reverse curve type. Ductus venosus flow pattern was classified as normal (positive A-wave) or abnormal (absent or negative A-wave) (Fig.4). With the progression of cardiomyopathy, the pulsating spectrum of blood flow in the umbilical vein is determined (Fig. 5).
• myocardial function;
Myocardial function was estimated using the myocardial performance index (Tei Index) (Fig. 6). An increase in the Tei index of more than 0.35 ± 0.05 was considered a sign of cardiomyopathy.
• identification of congenital heart defects arising from TTTS (Fig. 7).
The diagnostic criteria for fetal and newborn cardiomyopathy were cardiovascular abnormalities, including myocardial hypertrophy, cardiomegaly, atrioventricular valve insufficiency, impaired venous hemodynamics (absent or reverse A-wave of the venous duct, pulsation of the umbilical vein), myocardial performance (Tei index>0.33), and right ventricular outflow tract obstruction.
Statistical analysis
Statistical analysis and plotting were performed using Excel spreadsheets (Microsoft, USA) and the GraphPadPrism 8 software (GraphPadSoftware, USA). The distribution of continuous variables was tested for normality using the generalized D'Agostino–Pearson test. Quantitative variables showing normal distribution were expressed as means (standard deviation) and compared by t-test. Data with non-normal distribution were reported as medians (interquartile range) and compared by the Mann–Whitney test. Qualitative variables were summarized as counts and percentages and compared by Fisher's exact test and χ2. Differences were considered statistically significant at p <0.05. The Research Ethics Committee of the V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology, and Perinatology approved this study.
Results
In the whole study cohort, 54.2% (83 of 145) of recipient twins had cardiomyopathy. Recipient twins with stage III and IV TTTS were significantly more likely (87.9% and 100%) to have cardiomyopathy than those with stage I and II TTTS (36% and 44%), p <0.001.
Antenatal mortality of recipient twins with cardiomyopathy was significantly higher than among twins without this complication (19.3% and 4.8%, respectively), p=0.01, odds ratio 4.7 (95% CI 1.3–15.7). The antenatal mortality rate among recipients with cardiomyopathy did not depend on the TTTS stages accounting for 11.9% (5 out of 42) in stages I and II and 26.8% (11 out of 41) in stages III–IV, p=0.11.
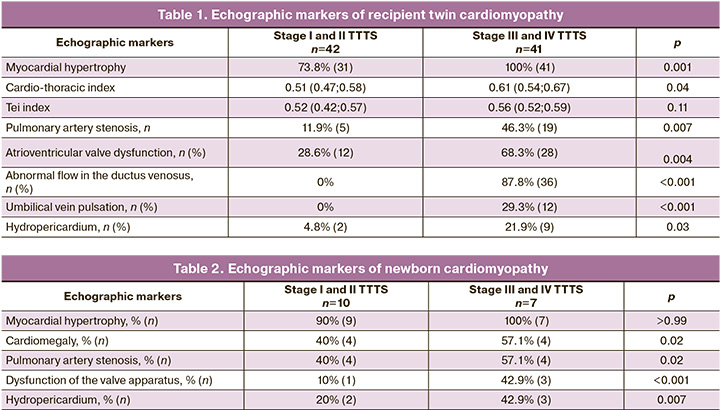
The most commonly diagnosed echographic signs of recipient cardiomyopathy were myocardial hypertrophy and cardiomegaly. Myocardial hypertrophy was more common in stage III and IV TTTS (100%) than in stages I and II (73.8%), p=0.001. Also, twins with stages III–IV TTTS had more pronounced cardiomegaly (the median of the cardio-thoracic index of 0.61 compared with 0.51 in those with stages I–II, p=0.04).
The left-ventricular myocardial performance index (Tay-index) was similar in twins with different stages of TTTS. At the same time, medians of the Tei index were greater in twins with cardiomyopathy (0.53) than in those without cardiomyopathy (0.31), p <0.001.
Pulmonary artery stenosis and atrioventricular valve dysfunction were more common in stage III and IV TTTS (46.3% and 68.3%) compared with stage I and II (11.9% and 28.6%), p=0.007 and 0.004.
Stage III and IV TTTS were characterized by abnormal blood flow in the ductus venosus and pulsation of the umbilical vein, which was not observed in stages I and II.
The detection rate of hydropericardium was higher in twins with stage III and IV TTTS (21.9%) compared with that in stage I and II (4.8%), p=0.03.
Table 1 summarizes the detection rate of echographic markers of recipient cardiomyopathy at different stages of TTTS.
Among newborns from pregnancies affected by TTTS and antenatally diagnosed cardiomyopathy, only 25.4% (17 out of 67) maintained the signs of cardiomyopathy. The overall incidence of postnatal cardiomyopathy in TTTS stages I–II and III–IV did not differ statistically (27% and 23.3%, respectively), p=0.78. At the same time, detection rates of cardiomegaly, pulmonary artery stenosis, atrioventricular valve dysfunction, hydropericardium were significantly different in twins with stages I–II and III–IV TTTS. Echocardiography detection rates of neonatal cardiomyopathy are presented in Table 2.
Discussion
According to echocardiographic findings, cardiovascular abnormalities occur in 70% of recipient twins [1]. Among them, the most common are abnormal ductus venosus flow, myocardial hypertrophy, and atrioventricular valve regurgitation [3]. These changes are due to hemodynamic changes that arise from unbalanced inter-twin blood transfusion through placental vascular anastomoses.
In this study, 54.8% of recipient twins had cardiomyopathy. The incidence of cardiovascular disorders increased with the progression of TTTS. It was relatively low in stage I (36%) and highest in stage IV (100%), p <0.001. According to several authors, the presence of cardiomyopathy in a recipient twin increases the risk of intrauterine death during laser coagulation of placental vascular anastomoses [7, 8]. The antenatal mortality rate of recipient twins with cardiomyopathy was almost five times higher (19.3%) than among twins without this complication (4.8%), p=0.01.
Fetoscopic laser coagulation of placental vascular anastomoses is an etiotropic treatment for TTTS that affects fetal hemodynamics. Successful operation leads to the improvement of hemodynamic parameters within several days [9, 10]. In our study, successful intrauterine correction of TTTS resulted in a 4-fold reduction in the incidence of recipient cardiomyopathy.
Only 25.4% of newborns had signs of cardiomyopathy. However, altered hemodynamics in the recipient fetus may lead to congenital heart disease such as pulmonary artery stenosis, which occurs in 7.8% of cases. In comparison, its prevalence in the population is only 0.03% [11]. In this study cohort, pulmonary artery stenosis was detected in 11.9% (8) of newborns.
Conclusion
Recipient twin cardiomyopathy is a common complication of TTTS, and its incidence increases along with the disease progression. Recipient twin cardiomyopathy is associated with an increased risk of antenatal death. Surgical correction of TTTS aimed to reverse hemodynamic imbalance may improve fetal cardiac function, which illustrates the adaptability of the developing heart. However, in surviving recipient twins, the risk of pulmonary artery stenosis and cardiomyopathy may persist.
References
- Martins Y., Silva S., Matias A., Blickstein I. Cardiac morbidity in twin-twin transfusion syndrome? J. Perinat. Med. 2012; 40(2): 107-14. https://dx. doi.org/ 10.1515/jpm-2011-0208.
- Van Mieghem T., Lewi L., Gucciardo L., Dekoninck P., Van Schoubroeck D., Devlieger R. et al. The fetal heart in twin-to-twin transfusion syndrome. Int. J. Pediatr. 2010; 2010: 379792. https://dx. doi.org/10.1155/2010/379792.
- Wohlmuth C., Boudreaux D., Moise K.J.Jr., Johnson A., Papanna R., Bebbington M. et al. Cardiac pathophysiology in twin–twin transfusion syndrome: new insights into its evolution. Ultrasound Obstet. Gynecol. 2018; 51(3): 341-8. https://dx. doi.org/10.1002/uog.17480.
- International Society of Ultrasound in Obstetrics and Gynecology, Carvalho J.S., Allan L.D., Chaoui R., Copel J.A., DeVore G.R., Hecher K. et al. ISUOG Practice Guidelines (updated): sonographic screening examination of the fetal heart. Ultrasound Obstet. Gynecol. 2013; 41(3): 348-59. https://dx.doi.org/ 10.1002/uog.12403.
- Leszczyńska K., Preis K., Respondek-Liberska M., Słodki M., Wood D., Weiner S. et al. Recommendations for fetal echocardiography in twin pregnancy in 2016. Prenat. Cardiol. 2016; 6(1): 6-15. https://dx.doi.org/10.1515/pcard-2016-0001.
- Gapp-Born E., Sananes N., Weingertner A.S., Guerra F., Kohler M., Fritz G. et al. Predictive value of cardiovascular parameters in twin-to-twin transfusion syndrome. Ultrasound Obstet. Gynecol. 2014; 44(4): 427-33. https://dx.doi.org/10.1002/uog.13351.
- Habli M., Michelfelder E., Cnota J., Wall D., Polzin W., Lewis D. et al. Prevalence and progression of recipient-twin cardiomyopathy in early-stage twin-twin transfusion syndrome. Ultrasound Obstet. Gynecol. 2012; 39(1): 63-8. https://dx.doi.org/10.1002/uog.10117.
- Eixarch E., Valsky D., Deprest J., Baschat A.A., Lewi L., Ortiz J.U. et al. Preoperative prediction of the individualized risk of early fetal death after laser therapy in twin-to-twin transfusion syndrome. Prenat. Diagn. 2013; 33(11): 1033-8. https://dx.doi.org/10.1002/pd.4191.
- Gheorghe C.P., Boring N., Mann L., Donepudi R., Lopez S.M., Chauhan S.P. et al. Neonatal outcomes and maternal characteristics in monochorionic diamniotic twin pregnancies: uncomplicated versus twin-to-twin transfusion syndrome survivors after fetoscopic laser surgery. Fetal Diagn. Ther. 2020; 47(2): 165-70. https://dx.doi.org/10.1159/000500858.
- Сакало В.А., Костюков К.В., Гладкова К.А., Гасанова Р.М., Тетруашвили Н.К., Бокерия Е.Л. Патология сердечно-сосудистой системы плодов при фето-фетальном трансфузионном синдроме. Детские болезни сердца и сосудов. 2018; 15(3): 137-43. [Sakalo V.A., Kostyukov K.V., Gladkova K.A., Gasanova R.M., Tetruashvili N.K., Bockeria E.L. Fetal cardiovascular hemodynamics in twin-to-twin transfusion syndrome. Children’s Heart and Vascular Diseases. 2018; 15(3): 137-43. (in Russian)].
- Peyvandi S., Rychik J., McCann M., Soffer D., Tian Z., Szwast A. Pulmonary artery blood flow patterns in fetuses with pulmonary outflow tract obstruction. Ultrasound Obstet. Gynecol. 2014; 43(3): 297-302. https://dx.doi.org/ 10.1002/uog.12472.
Received 10.06.2020
Accepted 21.08.2020
About the Authors
Kirill V. Kostyukov, M.D., Ph.D., Senior Researcher at the Department of Fetal Medicine, Institute of Obstetrics; Physician at the Unit of Functional and Ultrasound Diagnostics, Department of Diagnostic Imaging, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia. Tel.: +7(926)214-97-84. E-mail: kostyukov_k@yahoo.com.117997, Russia, Moscow, Ac. Oparina str., 4.
Victoria A. Sakalo, Physician at the 1st Obstetric Department of Pathology of Pregnancy, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Tel.: +7(929)588-72-08. E-mail: v_sakalo@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Kristina A. Gladkova, Ph.D., Senior Researcher at the Department of Fetal Medicine, Institute of Obstetrics; Head of the 1st Obstetric Department
of Pathology of Pregnancy, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia. Tel.: +7(916)321-10-07. E-mail: k_gladkova@oparina4.ru.
117997, Russia, Moscow, Ac. Oparina str., 4.
Ekaterina L. Bokeria, Dr.Med.Sci., Head of the 2nd Department of Pathology of Newborn and Preterm Babies, V.I. Kulakov NMRC for OG&P
of Minzdrav of Russia. E-mail: e_bokeriya@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
For citation: Kostyukov K.V., Sakalo V.A., Gladkova K.A., Bokeriya E.L. Newborn and fetal cardiovascular system in twin-to-twin transfusion syndrome
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2020; 9: 82-87 (in Russian)
https://dx.doi.org/10.18565/aig.2020.9.82-87



