Somatic mutations in MED12 gene exon 2 in women with a single uterine fibroid or multiple ones
Objective. To search for genetic markers for uterine fibroids in order to optimize diagnosis and management tactics in patients and to predict the risk of recurrence.Sogoyan N.S., Kuznetsova M.V., Asaturova A.V., Adamyan L.V., Trofimov D.Yu.
Subjects and methods. Tissue samples from 167 fibroids and blood aliquots from 65 patients were collected. DNA was extracted and MED12 gene exon 2 amplified. Polymerase chain reaction was carried out to determine sequences using the Sanger method.
Results. Women with compromised and uncompromised histories showed different somatic mutation ratios in MED12 gene exon 2 and differences in the distribution of mutations when comparing women with a single uterine fibroid or multiple ones.
Conclusion. The findings could reveal a possible interaction of a mutation in the gene under study with a compromised history. Somatic mutation saturation in the subgroups of patients with multiple myomatous nodules indicates that multiple uterine fibroids mainly arise from MED12 gene mutations, which may be less typical for single fibroids.
Keywords
Uterine fibroids are benign monoclonal, well-demarcated, capsulated tumors that originate from the smooth muscle cells of the uterus. It is one of the most common benign tumors of the female reproductive system. It occurs in 40-50% of women of reproductive age, more often in the late reproductive and premenopausal periods [1, 2]. However, in the last ten years, this disease has been diagnosed more frequently in young women under 30 years of age [3].
Uterine fibroids can be represented as single or multiple nodes, herewith multiple uterine fibroids being diagnosed more often. Symptoms of the disease, as well as predictions, can be very different, depending on the size, location, growth rate and development of tumors. Most often, pain, bleeding and impaired function of neighboring organs are detected in patients with uterine myoma due to the compression by the expanding tumor. Modern methods of treatment include hormonal therapy and surgical methods performed by laparoscopic, laparotomic and vaginal myomectomy. Unfortunately, uterine myoma is the most common cause of hysterectomy worldwide, which is especially undesirable in women of reproductive age [4]. However, none of the treatment methods of uterine fibroids are ideal and they can result in complications and relapses. The development of fibroids can lead to infertility, complicate the course of pregnancy, and also cause problems during pregnancy and childbirth [5]. All that makes uterine myoma one of the most important threats to the reproductive health of women and determines the relevance of studying the pathogenesis of uterine fibroids, optimizing diagnosis, managing patients and predicting the risk of recurrence of this disease.
Despite numerous studies of factors affecting the development and growth of uterine leiomyoma, the pathogenesis of this disease remains insufficiently studied. The genetic predisposition to this disease presents particular interest among the factors causing the development of uterine fibroids, which is confirmed by the discovery of “family forms” of fibroids in 5-10% of patients [3], but the specific mechanisms of this predisposition were not described until recently [6, 7].
The most significant discovery of recent years in the study of the genetic mechanisms of uterine fibroids has been the description of somatic mutations in the MED12 gene, which encodes one of the subunits of the mediator complex of RNA polymerase II. Numerous studies published over the last 6 years have shown that with a frequency of 50-85%, somatic mutations in myomas occur exactly in exon 2 of the MED12 gene [8-12]. In this case, single-nucleotide substitutions are most often found in 43 and 44 codons.
In 2015, a group of authors published a study [13], according to which integration into the genome of mice of the mutant allele of the MED12 gene (the most common mutation, 131G > A) led to the development of myomas from those cells where only the mutant allele worked. The generation of MED12 in some cell membranes meant that in this case there was no operability so that there was no expression of it, and no changes in the myometrial tissues were found in such mice.
Based on these results, the authors concluded that the complete loss of the product function of the MED12 gene did not cause the development of myomas. In the case of mice, in some myometrial cells where only the mutant version of the MED12 gene was expressed, pathological changes in the myometrium histologically similar to leiomyomas were detected.
In our previous study, we found that somatic mutations in exon 2 of the gene MED12 were present in 50% of the cases. Although the data on frequencies we received turned out to be less than the data for other populations, there is no doubt that in the Russian population the frequency of somatic mutations in the MED12 gene is quite high (about 50% according to our data and about 70% in the previously published work of the Russian authors) [14].
We also detected a single nucleotide substitution at position 107 (T > G) in two myomatous nodes of the same patient. This replacement was unique; it was not described in the literature and mentioned in databases [15]. In a previously published paper on the Russian population [14], a somatic mutation 107 T > C was found in the same position.
Given the presence of “family cases” for the development of fibroids, it is extremely important to study somatic mutations in the MED 12 gene in women with burdened heredity for fibroids. A separate study also begs the question of how heterogeneous can be such mutations in different myomatous nodes, developing in patients with multiple fibroids.
The purpose of the study was to identify the genetic marker of uterine myoma development in order to optimize the diagnosis, management of patients with uterine myoma and to predict the risk of recurrence by assessing and comparing the frequency and nature of somatic mutations in exon 2 of the MED12 gene in women with single and multiple myomas. The patients were divided into 2 groups: group 1 included the women with a history of myoma, group 2 included the women without its history. The study also aimed at determining the variability of the above-mentioned exon in different nodes in patients with multiple nodes.
Materials and Methods
Tissue samples of 167 fibroids obtained from 65 patients (1 to 5 myomatous nodes from each) and an aliquot of the blood of all patients were collected in the Department of Surgical Gynecology at National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov, Moscow in 2016.
All patients underwent a complete clinical and anamnestic examination: anamnesis, general and gynecological examination, ultrasound examination of the pelvic organs, clinical and laboratory examination. Endoscopic surgery was carried out according to a standardized method with the use of endovideosurgical equipment manufactured by Karl Storz (Germany). Collection of tissue samples of myomas was carried out directly during myomectomy or hysterectomy depending on the size and number of nodes. Tissue fragments were placed in saline and sent to the Biobank of National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov; they were frozen at -70ºC for subsequent storage in the collection. The samples of each node were also subjected to histological examination in order to confirm the presence of exclusively myomatous node tissue in the sample and the absence of capsule or myometrial tissue in it, in which, according to the literature data [16], somatic mutations in the MED12 gene do not occur.
DNA extraction was performed by Qiagen kit (USA). Amplification of DNA was carried out on the device DT Prime (LLC “DNA-Technology”, Russia). The PCR reaction was conducted with primers for a portion of MED12 gene exon 2 (total length of the chunk 320 n). Sequence fragments were identified by sequencing the method of Sanger on ABI PRISM 3130 instrument (Applied BioSystems, USA).
In the case of detection of mutation in exon 2 of the gene MED12, DNA extraction was carried out from blood of the patient followed by analysis for mutations in exon 2 of the MED12 gene. The absence of mutations in genomic DNA served as the basis for confirming the fact that mutations discovered in the DNA of fibroids are somatic.
Information about patients
The study involved 65 patients divided into 2 groups: women with burdened history of myoma, whose fibroids were diagnosed in close maternal relatives (mother, grandmother, sister, aunt). Group 1 included 35 patients, who were obtained 95 samples of myomatous nodes: 9 of them were diagnosed with single fibroids, therefore, they were taken 9 samples of nodes; multiple fibroids were revealed in 26 patients who were collected 86 samples of myomas.
Group 2 included the women without a history of uterine fibroids (information about their case histories was obtained during the conversation with the patients). There were 30 patients in it, 72 samples of nodes were collected: 13 patients were diagnosed with a single fibroid, they were taken 13 samples of fibroids; 17 patients had multiple fibroids, 59 samples of myomatous nodes were collected.
The average age in the first group was 38.7 years, in the second group it was 39.5 years.
Results
In group 1, a single node was found in 9 patients, 2 nodes in 7 patients, 3 nodes in 3 patients, 4 nodes in 5 patients, 5 nodes and more in 11 patients. In group 2, a similar pattern was observed: a single node was revealed in 13 patients, 2 nodes in 4 patients, 3 nodes in 4 patients, 4 nodes in 3 patients, 5 nodes and more in 6 patients. Figure 1 shows a comparison of both samples, which demonstrates the fact that the difference in the number of samples of myomatous nodes in both groups is insignificant and does not affect the reliability of the results.

Analysis of the obtained sequences showed that different variants of somatic mutations in exon 2 of the MED12 gene were found in different amounts not only in groups 1 and 2, but also in subgroups with single and multiple nodes. The proportion of patients with mutations in group 1 was 68% (24 patients out of 35), the proportion of myomatous nodes with mutations was 61% (58 nodes out of 95). In group 2, the proportion of patients with mutations was 63% (19 patients out of 30), while the proportion of myomatous nodes with mutations was 48% (35 nodes out of 72 samples). The data are given in Table 1.

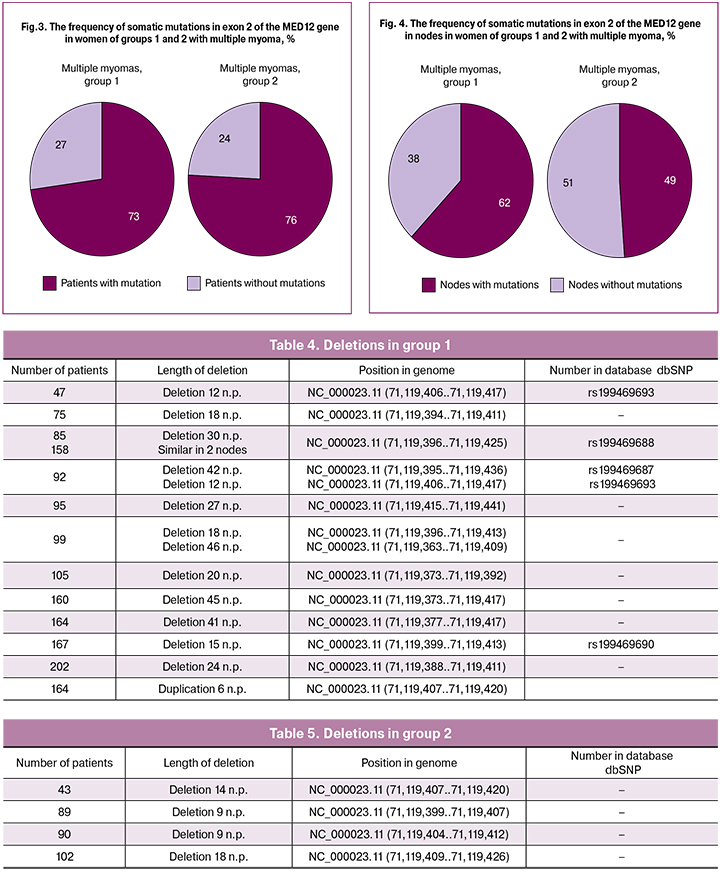
As a result of the analysis of mutations found in subgroups of women with single and multiple myomas, we were able to identify a possible relationship between the number of nodes in women with mutations in exon 2 of the MED12 gene. Thus, we found that 55% of patients with a burdened history of myoma and diagnosed single myoma (5 patients out of 9) had mutations in the MED12 gene. Multiple uterine fibroids were detected in 26 women from group 1, the proportion of patients with mutations in the MED12 gene was 73% (19 patients out of 26), the proportion of myomatous nodes in this subgroup with somatic mutations in the studied gene was 62% (53 nodes out of 86). Only 9 patients out of 26 (35%) had the same mutations.
In group 2, a single node was diagnosed in 13 patients, 6 of them had different variants of somatic mutations in exon 2 of the MED12 gene, which was 46%. Multiple myoma was revealed in 13 patients, there were also mutations in this gene; their proportion was 76% (13 patients out of 17), while the proportion of myomatous nodes with mutations was 49% (29 nodes out of 50 samples). Same mutations were observed only in 41 % (7 out of 17). For comparison of the two groups, the data are shown in Figures 2, 3 and 4 and in Table 2.
When we compared these indicators with the results obtained in the previous study, where the proportion of mutations in a random sample was 50% [15], we could conclude that mutations in the MED12 gene were likely to have a relationship with anamnesis. The high frequency of mutations in group 2 was most likely due to the lack of awareness of patients about their family history.
This pattern is also observed when comparing patients and nodes with mutations in cases of single and multiple myomas, where the frequency of mutations is higher in patients with a burdened history of myoma.
Single nucleotide substitutions
In exon 2 of the MED12 gene there were 8 variants of single nucleotide substitutions in 4 positions (Table 3).
Thus, one variant of single-nucleotide replacement (A/C) was found in position 128 in one patient from group 2; 3 variants of mutations occurring in both groups were described for position 130. Mutations 131 (G/A, G/C, G/T) were detected more often than others, with the replacement of G/A detected in more patients. For position 107, one variant of mutation was described, which was also found in both groups of patients.
All patients with detected mutations had their blood samples tested. All mutations were found to be somatic, as no changes in blood DNA in exon 2 of the MED12 gene were found.
Summary:
- A greater variety and number of mutations occurred in patients from group 1, which indicated a possible relationship of these somatic mutations with a burdened patients’ history.
- In women with multiple myomas the frequency of somatic mutations in exon 2 of the MED12 gene was higher than in women with single myoma, indicating the predominant development of multiple myomatosis as a result of somatic mutation in the MED12 gene. This fact can be used as a prognostic factor for the possible development of multiple fibroids in a woman with one node during the examination.
- In patients with multiple myomas there was also a simultaneous carriage of several variants of mutations in different nodes and a combination of different single nucleotide substitutions and deletions in one patient. Probably it was the result of non-specific mechanism that caused somatic mutations.
Deletions
The analysis of the obtained sample revealed another variant of somatic mutation that is deletions. They are less common than single nucleotide substitutions in the studied sequences, but the localization of these deletions in most cases are the two codons of exon 2 (43 and 44), in which substitutions are most frequently found.
In group 1, deletions were found in 12 patients (34%). The deletions are presented in Table 4. In 3 cases different deletions were found in 2 different nodes in the case of multiple myomas in the same patient; also, in multiple myomas deletions were combined with single nucleotide substitutions.
In group 2, as it was expected, deletions were found in 4 patients (13%). As in group 1, deletions in multiple myomas were combined with single nucleotide substitutions (Table 5).
The proportion of deletions in the studied samples was higher in women with a burdened anamnesis and was 34% (12 patients out of 35) versus 13% in group 2 (4 patients out of 30).
Summary:
- In women with a burdened anamnesis of myoma there was a greater number and variety of deletions, compared with women without a history of myoma.
- Deletions were localized within the same areas where single nucleotide substitutions were usually detected.
- In cases of multiple myomas a simultaneous combination of deletions of different lengths and a combination of deletions and single nucleotide substitutions in different nodes in one patient were detected.
- Only some deletions from the detected ones are registered in dbSNP, while all the others described in the article, as well as the insertion, are new.
In one case in a patient with diagnosed multiple uterine myoma in one of the nodes there was insert length of 6 n.p. This mutation was unique, as it was not previously described in the literature and was absent in the databases. Patient 164 was from group of women with a history of uterine myoma. Her mother and twin sister were also diagnosed myoma. The patient was taken 4 samples of myomatous nodes, two of them were found mononucleotide substitutions: 1 node - G/T131, 2 node - G/A 131, 3 node - insert 6 n.p., 4 node - deletion 41 n.p.
Discussion
Comparing the above-mentioned results with the data obtained in our previous study, where the proportion of somatic mutations in exon 2 of the MED12 gene in a random sample was 50% [15], we believe that it is possible to hypothesize the role of the hereditary factor in the development of these mutations, and, as a consequence, the development of uterine fibroids, because the hereditary component in this work has determined a greater frequency of mutations in women with uterine myomas.
Although the mutations in exon 2 of the MED12 gene are somatic, and the mechanism that causes these mutations is directed at a specific target – exon 2, but it is obvious that the hypothetical mechanism that causes this kind of mutation is not specific, that is, it causes a very diverse change in exon 2 of the MED12 gene, sometimes affecting the intron located in front of it. An illustration of the non-specificity of this mechanism is the fact that different mutations can be detected in one patient with multiple uterine myoma in different nodes. The most striking clinical example of this assumption is the case described above. In a patient with “a family form” of uterine fibroids, 4 samples of myomatous nodes were taken, and in each of them various mutations were found: 1 node – G/T 131, 2 node – G/A 131, 3 node – insert 6 n. p., 4 node – deletion 41 n. p. The insert we found was a unique somatic mutation in exon 2 of the MED12 gene. Data on it in the literature and databases are not available.
The most common single nucleotide replacement in exon 2 of the MED12 gene was G/A replacement at position 131, which has been described in numerous studies in recent years [7]. According to our data, this mutation was found in 43% of women in group 1 and 33% of patients in group 2, which, indeed, confirmed its high prevalence.
We found a single nucleotide replacement 107 T/G, which was first described in our previous study [15]; in this study we revealed a larger number of women: 3 patients with “family form” uterine fibroids and 2 patients without a history of uterine myoma. This fact allows us to conclude that single-nucleotide substitution at position 107 is probably typical for the Russian population. Earlier at this position another replacement T/S was found [18]. This fact proves that this position, along with codons 43 and 44, is also a “hot spot” for somatic mutations in exon 2 of the MED12 gene.
Recent publications in the field of genetic factors of uterine fibroids development are mostly devoted to somatic mutations in exon 2 of the MED12 gene. According to the data obtained in the work of Osinovskaya N. S. and co-authors, mutations in women with multiple myomas were found in 61% of samples of myomatous nodes, while in patients with single myoma this figure was 32.5% [18]. The authors of this study concluded that multiple myomas develop mainly as a result of mutations in the MED12 gene, which is probably not typical of single myomas. The frequencies of somatic mutations in the MED12 gene in women with multiple and single myomas confirm this hypothesis, and also indicate the relationship of the hereditary component that increases the likelihood of somatic mutations in the MED12 gene that cause the development of myomas.
Further studies of somatic mutations in the MED12 gene may radically change the approach to the management and treatment of patients with mutations in the studied gene. We have assumptions that the pathogenesis of tumors with mutations is somewhat different from the pathogenesis of tumors in which mutations are not detected. Perhaps the study of these differences will help to find the relationship between the type of mutation and the prognosis of the disease, the risk of recurrence after surgical removal of fibroids, that is, the “aggressiveness” of the disease, as well as to identify a new genetic marker of the development of uterine fibroids to optimize the diagnosis, tactics of managing patients with uterine myoma and predict the risk of recurrence.
Conclusion
The study of somatic mutations in exon of the MED12 gene confirmed the fact that this gene is one of the key genes associated with the pathogenesis of uterine fibroids.
Our data confirmed the prevalence of somatic mutations in the MED12 gene in women with uterine myoma, and, most importantly, confirmed a direct relationship between the mutation of the studied gene with burdened anamnesis. According to our results, genetic component determines the saturation of the sample by mutations of the MED12 gene.
We have discovered a somatic mutation, namely insertion length 6 n. p., which is localized in exon 2 of the MED12 gene; previously published sources have not mentioned about it. Perhaps it is specific to the Russian population.
The saturation of subgroups of patients with multiple myomatous nodes with somatic mutations in exon 2 of the MED12 gene indicates the predominant development of multiple uterine fibroids as a result of somatic mutations in the MED12 gene, which, according to our data, is less characteristic of single nodes.
Thus, the modern approach to the development of methods for early assessment of risk for the development of uterine leiomyomas is the establishment of a “molecular portrait” of each tumor in order to classify it to a particular type. Careful collection of clinical data and comparison of dynamic development of “molecular portrait” of the tumor might have value in the early diagnosis of difficult cases of multiple myomas, especially when they are burdened by this disease in family history.
The main task for further research is the study of molecular mechanisms of somatic mutations (primarily the hereditary factors responsible for the increased frequency of occurrence of such mutations) and finding relationships between the type of mutation and the prognosis of the disease and the risk of recurrence after surgical removal of the fibroids, and “aggressiveness” of the disease, which is very important for practical public health.
References
1. Адамян Л.В. Миома матки: диагностика, лечение и реабилитация. М.: ФГБУ Научный центр акушерства, гинекологии и перинатологии им. В.И. Кулакова Минздрава России; 2015. 72с. [Adamyan L.V. Uterine fibroids: diagnosis, treatment and rehabilitation. M.: Scientific Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov, Ministry of Health of Russia. 2015; 72 p. (in Russian)]
2. Сидорова И.С., Унанян А.Л., Коган Е.А., Гуриев Т.Д. Миома матки у больных молодого возраста: клинико-патогенетические особенности. Акушерство, гинекология и репродукция. 2010; 1: 16-20. [Sidorova I.S., Unanyan A.L., Kogan E.A., Guriev and etc. Uterine fibroids in young patients: clinical and pathogenetic features, obstetrics, gynecology and reproduction. 2010; (1): 16-20. (in Russian)]
3. Адамян Л.В., Спицын В.А., Андреева Е.Н. Генетические аспекты гинекологических заболеваний. Руководство для врачей. М.: ГЭОТАР-Медиа; 2008. 215с. [Adamyan L.V. Spitsyn V.A., Andreeva E.N. Genetic aktekty gynecological diseases. M: GEOTAR-media; 2008. 215 p. (in Russian)]
4. Torres-de la Roche L.A., Becker S., Cezar C., Hermann A., Larbig A., Leicher L. et al. Pathobiology of myomatosis uteri: the underlying knowledge to support our clinical practice. Arch. Gynecol. Obstet. 2017; 296(4): 701-7. doi: 10.1007/s00404-017-4494-6.
5. Подзолкова Н.М., Колода Ю.А., Коренная В.В., Кайибханова К.Н. Эффективность вспомогательных репродуктивных технологий при миоме матки. М.: ГБОУ ДПО Российская медицинская академия последипломного образования Минздрава России; 2015: 60-4. [Podzolkova N.M., KolodaYu.A., Korennaya V.V., Kayibhanova K.N. The effectiveness of assisted reproductive technologies for uterine myoma. Moscow: Russian Medical Academy of Postgraduate Education, Ministry of Health of Russia; 2015: 60-4. (in Russian)]
6. Yang Q., Mas A., Diamond M.P., Al-Hendy A. The mechanism and function of epigenetics in uterine leiomyoma development. Reprod. Sci. 2016; 23(2):163-75.
7. Ligon A.H., Morton C.C. Leiomyoma: heritability and cytogenetic studies. Hum. Reprod. Update. 2001; 7(1): 8-14.
8. Zhang K., Wiener H., Aissani B. Admixture mapping of genetic variants for uterine fibroids. J. Hum. Genet. 2015; 60(9): 533-8.
9. Mäkinen N., Mehine M., Tolvanen J., Kaasinen E., Li Y., Lehtonen H.J. et al. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science. 2011; 334(6053): 252-5.
10. Markowski D.N., Bartnitzke S., Löning T., Drieschner H., Helmke B., Bulle J. MED12 mutations in uterine fibroids – their relationship to cytogenetic subgroups. Int. J. Cancer. 2012; 131(7): 1528-36. doi: 10.1002/ijc.27424.
11. McGuire M.M., Yatsenko A., Hoffner L., Jones M., Surti U., Rajkovic A. Whole exome sequencing in a random sample of North American women with leiomyomas identifies MED12 mutations in majority of uterine leiomyomas. PLoS One. 2012; 7(3): e33251. doi: 10.1371/journal.pone.0033251.
12. Heinonen H.R., Pasanen A., Heikinheimo O., Tanskanen T., Palin K., Tolvanen J. et al. Multiple clinical characteristics separate MED12-mutation-positive and -negative uterine leiomyomas. Sci. Rep. 2017; 7: 1015. doi: 10.1038/s41598-017-01199-0.
13. Mittal P., Shin Y.H., Yatsenko S.A., Castro C.A., Surti U., Rajkovic A. MED12 gain-of-function mutation causes leiomyomas and genomic instability. J. Clin. Invest. 2015; 125(8): 3280-4.
14. Осиновская Н.С., Иващенко Т.Э., Долинский А.К., Султанов И.Ю., Гимбовская С., Хоффман Э., Беженарь В.Ф., Баранов В.С. Мутации гена MED-12 в клетках лейомиомы у женщин Северо-Западного региона РФ. Генетика. 2013; 49(12): 1426-31. [Osinovskaya N.S., Ivashchenko T.E., Dolinsky A.K. Mutation of the MED-12 gene in cells of the leiomyoma in women of the North-Western region of the Russian Federation. Genetics. 2013; 49 (12): 1426-31.(in Russian)]
15. Кузнецова М.В., Трофимов Д.Ю., Тихончук Е.Ю., Согоян Н.С., Адамян Л.В., Сухих Г.Т. Молекулярные механизмы патогенеза миомы матки: анализ мутаций гена MED12 в российской популяции. М.; 2016. [Kuznetsova M.V, Trofimov D.Yu., Tikhonchuk E.Yu., Sogoyan N.S., Adamyan L.V., Sukhikh G.T., Molecular mechanisms of the pathogenesis of uterine fibroids: analysis of mutations in the MED12 gene in the Russian population, M.; 2016. (in Russian)]
16. Di Tommaso S., Massari S., Malvasi A., Vergara D., Maffia M., Greco M., Tinelli A. Selective genetic analysis of myoma pseudocapsule and potential biological impact on uterine fibroid medical therapy. Expert Opin. Ther. Targets. 2015; 19(1): 7-12. doi: 10.1517/14728222.2014.975793.
17. Yatsenko S.A., Mittal P., Wood-Trageser M.A., Jones M.W., Surti U., Edwards R.P. et al. Highly heterogeneous genomic landscape of uterine leiomyomas by whole exome sequencing and genome-wide arrays. Fertil. Steril. 2017; 107(2): 457-66. e9.
18. Osinovskaya N.S., Malysheva O.V., Shved N.Yu., Ivashchenko T.E.,Sultanov I.Yu., Efimova O.A., Yarmolinskaya M.I., Bezhenar V.F., Baranov V.S. Frequency and spectrum of MED12 Exon 2 mutations in multiple versus solitary uterine, leiomyomas from Russian patients. Int. J. Gynecol. Pathol. 2016; 35(6): 509-15. doi: 10.1097/PGP.0000000000000255.
Received 25.04.2018
Accepted 21.09.2018
About the Authors
Sogoyan, Nelly S., resident of Academician V. I. Kulakov Research Center of Obstetrics, Gynecology and Perinatology.119997, Russia, Moscow, Ac. Oparina str. 4. Е-mail: sogoyan.n@mail.ru
Kuznetsova, Maria V., PhD, research scientist, laboratory of molecular-genetic methods, of Academician V. I. Kulakov Research Center of Obstetrics, Gynecology and Perinatology. 119997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954381341. Е-mail: mkarja@mail.ru
Asaturova, Alexanda V., PhD, pathologist, senior scientific assistant of Academician V. I. Kulakov Research Center of Obstetrics, Gynecology and Perinatology.
119997, Russia, Moscow, Ac. Oparina str. 4. E-mail: a_asaturova@oparina4.ru
Trofimov, Dmitry Yu., DSci, professor RAS, Head of Laboratory of molecular-genetic methods, of Academician V. I. Kulakov Research Center of Obstetrics, Gynecology and Perinatology. 119997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954381341. ORCID.org/0000-0003-0001-1765ology
Adamyan, Leila V., Academician of RAS, MD, PhD, Professor RAS, Honored Master of Science of the Russian Federation, Head Specialist in Obstetrics and Gynecology of Ministry of Healthcare of Russia, Head of the Department of Surgical Gynecology of Academician V. I. Kulakov Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Healthcare of Russia, Head of the Department of Reproductive Medicine and Surgery, Faculty of Postgraduate Education, Moscow State University of Medicine and Dentistry. 119997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954384068. E-mail: l_adamyan@oparina4.ru
For citations: Sogoyan N.S., Kuznetsova M.V., Asaturova A.V., Adamyan L.V., Trofimov D.Yu. Somatic mutations in MED12 gene exon 2 in women with a single uterine fibroid or multiple ones. 2018; (12): 63-70. (in Russian)
http://dx.doi.org/10.18565/aig.2018.12.63-70



