The role of growth factor genes in the development of uterine fibroids combined with endometrial hyperplasia
Objective. To study the association of polymorphic variants of growth factor genes with the development of uterine fibroids combined with endometrial hyperplasia.Altukhova O.B., Radzinsky V.E., Polyakova I.S., Churnosov M.I.
Materials and methods. The study included 982 women: 193 women with uterine fibroids combined with endometrial hyperplasia and 789 healthy controls. Five polymorphic loci of growth factor genes were chosen for the study: EGF c.-382А>G rs4444903, ТGFβ-1 c.-1347Т>С rs1800469, IGF1 c.*2716G>А rs6214, VEGF c.-958С>Т rs833061, FGFR2 c.109+906Т>С rs2981582. The study was carried out using the polymerase chain reaction method.
Results. Polymorphic loci were found to be associated with the development of uterine fibroids combined with endometrial hyperplasia. Genotype CC rs2981582 FGFR2 increases the risk of developing uterine fibroids combined with endometrial hyperplasia (OR=1.38). The combination of alleles A rs4444903 EGF and C rs1800469 TGFß-1 is also a risk factor for the development of the disease (OR=1.50). The combination of G rs4444903 EGF, C rs1800469 TGFß-1, G rs6214 IGF1, and T rs2981582 FGFR2 can play a protective role in the development of the disease (OR=0.59). These polymorphisms have a significant regulatory potential and affect the expression level of eight different genes; they are located in the areas of exonic splicing enhancers and they are the binding sites of transcription factors.
Conclusion. Polymorphic loci of the genes РGR c.1415-11113G>Т rs1042838, ESR1 c.*1029Т>С rs3798577, ESR1 c.453-397Т>С rs2234693, ESR1 c.453-351А>G rs9340799 are associated with the development of uterine fibroids combined with endometrial hyperplasia.
Keywords
Uterine fibroids and endometrial hyperplasia are common disorders in modern gynecology [1, 2]. These diseases occur in women of all age groups [1, 3]. The incidence of uterine fibroids in reproductive-aged women is 25%, and the incidence of endometrial hyperplasia ranges from 10 to 50% [1–5]. Uterine fibroids combined with endometrial hyperplasia are revealed in 30–35% of reproductive–aged patients, and in 72.7–100% of patients after menopause [6]. The problem of proliferative diseases of the uterus is of great medical and social importance due to the complexity of their treatment, reproductive disorders, severe consequences for patients, the risk for the development of malignant endometrial tumors [7, 8], and recurrence of the diseases.
There is still no consensus on the mechanisms responsible for developing proliferative diseases of the uterus [5, 9]. The role of genetic factors in the development of these diseases has been proven by the achievements of molecular genetics [10, 11]. Growth factor genes play a significant role in the development of proliferative diseases of the uterus. Due to the inflammatory processes, the germ of uterine fibroids is infiltrated by macrophages that secrete tumor necrosis factors, interleukins [12]. These substances alter the expression of granulocyte-macrophage colony-stimulating factor (GM-CSF), which stimulates the hyperproduction of transforming growth factor (TGF)-β by tumor cells. TGF-β may increase the effector mechanism of GM-CSF. When TGF-β affects fibroblasts, it stimulates the expression of a growth factor that causes the production of extracellular matrix (CTGF). Fibroblasts under the influence of TGF-β become myofibroblasts, which are involved in the development of connective tissue components. Tumor necrosis factor (TNF)-α, synthesized by macrophages, can competitively block the signaling pathway between TGF-β and CTGF, as there is a common binding site for TNF-α and TGF-β with the CTGF promoter [13, 14].
The aim of the study is to reveal the association of polymorphic variants of growth factor genes with the development of uterine fibroids combined with endometrial hyperplasia.
Materials and Methods
The study included women with uterine fibroids combined with endometrial hyperplasia (n=193) and healthy controls (n=789) who live in the Central Black Earth Region of the Russian Federation and are not related to each other [15]. The study samples were taken in Gynecology Department of the Belgorod Regional Clinical Hospital of St. Joasaph. The patients who were eligible for inclusion in the study group had uterine fibroids combined with endometrial hyperplasia, verified by echographic and hysteroscopic methods. The diagnosis was confirmed morphologically. The control group included healthy women who underwent routine medical examination in the Department of the Perinatal Center. Each woman gave a written consent to participate in the study.
Genomic DNA isolated by the standard method of phenol-chloroform extraction from peripheral blood leukocytes was used for molecular genetic testing. Five polymorphic loci of growth factor genes were chosen for the study: EGF c.-382А>G rs4444903 (epidermal growth factor), ТGFβ-1 c.-1347Т>С rs1800469 (transforming growth factor β-1), IGF1 c.*2716G>А rs6214 (insulin-like growth factor 1), VEGF c.-958С>Т rs833061 (vascular endothelial growth factor), FGFR2 c.109+906Т>С rs2981582 (fibroblast growth factor receptor). The choice of these single nucleotide polymorphisms (SNPs) for the study is due to their significant regulatory potential [16].
The study of polymorphic loci of growth factor genes was carried out using the polymerase chain reaction method with oligonucleotide primers and probes synthesized by LLC Syntol (Russia).
Statistical analysis
The frequencies of alleles and genotypes were compared in the study and control groups. The analysis of this comparison was carried out in the two-way contingency tables using the χ2 criterion. The associations of allelic variants with the development of the disease were evaluated using the odds ratio (OR) and corresponding 95% confidence interval (CI). Statistical processing of the obtained data was performed using the program STATISTICA for Windows 10.0.
The combinations of genetic variants (alleles and genotypes) of the above-mentioned loci and their association with the development of comorbidities were studied using the APSampler program (https://sourceforge.net/projects/apsampler/), the Monte-Carlo–Markov chain method and Bayesian nonparametric statistics [17, 18]. The level of pperm< 0.05 was considered as statistically significant.
The effect of polymorphisms on gene expression was studied using the GTExportal database (https://www.gtexportal.org/). The study included materials with p<8×10-5, FDR≤0.05. The effect of the allele on changes in gene expression was determined using the β criterion [10]. Regulatory effects of polymorphic loci were detected according to the SNPinfo program (https://npinfo.niehs.nih.gov/).
Results and Discussion
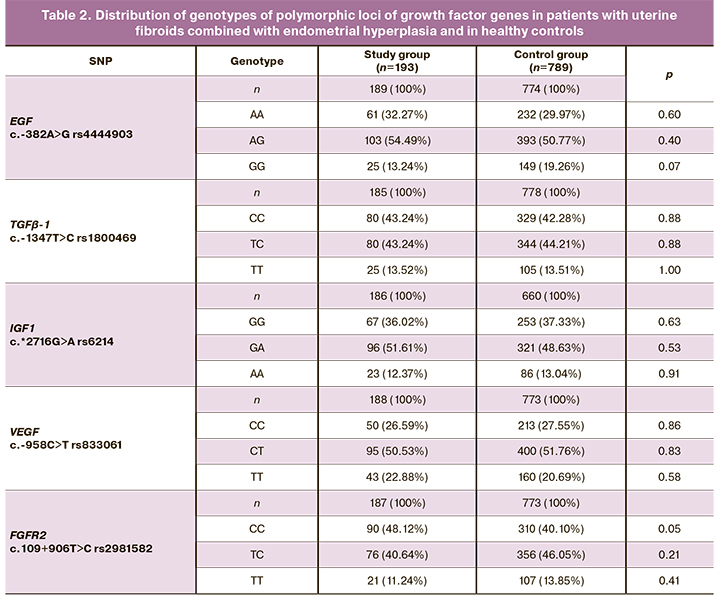
The data on the frequencies of alleles and genotypes in the studied samples are presented in Tables 1 and 2. The distribution of frequencies of alleles and genotypes of all genes corresponds to Hardy–Weinberg equilibrium.
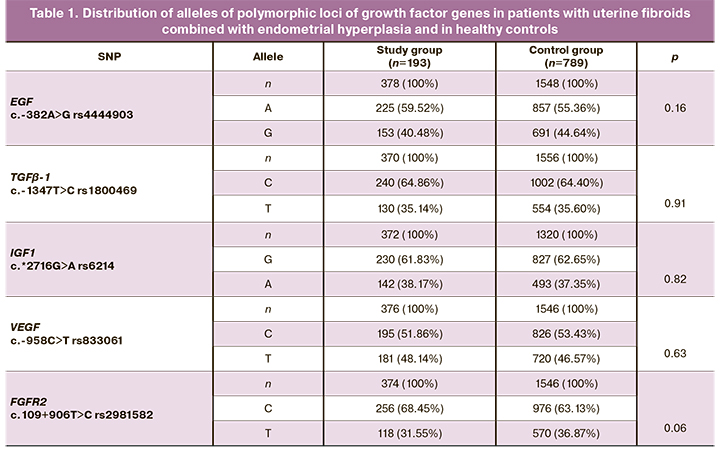
The analysis of the frequencies of alleles and genotypes of the polymorphic markers of growth factor genes in the study and control groups showed that women with uterine fibroids combined with endometrial hyperplasia had genotype CC rs2981582 FGFR2 in 48.12% of cases, which is higher than the same indicator in the control group, where the frequency of this genotype was 40.11% (OR=1.38, 95% CI 0.99–1.93, p=0.05, pperm=0.05).
Other studied polymorphisms of growth factor genes in the sample of patients showed no significant differences in the prevalence of alleles and genotypes (p>0.05).
It was found that the combination of genetic variants G rs4444903 EGF, C rs1800469 ТGFβ-1, G rs6214 IGF1 and T rs2981582 FGFR2 in the study group (22.85%) and in the control group (33.07%) was statistically significantly different. This combination of alleles acts as a protective factor in the development of uterine fibroids combined with endometrial hyperplasia (OR=0.59, 95% CI 0.40–0.88, p=0.005, pperm=0.08).
It was revealed that the combination of allele A rs4444903 EGF and allele C rs1800469 TGFß-1 was observed in 77.91% of women with uterine fibroids combined with endometrial hyperplasia, which is significantly higher than the same indicator in the control group, 70.06%. This combination of alleles is a risk factor for the development of uterine fibroids combined with endometrial hyperplasia (OR=1.50, 95% CI 1.02–2.21, p=0.02, pperm=0.03).
According to the studies, the FGFR2 gene regulates cell proliferation, differentiation, and cell migration [19]. The analysis of the literature shows that rs2981582 FGFR2 genetic polymorphism is associated with the development of breast cancer [20] and pancreatic cancer [19].
The use of the online program GTExportal (http://www.gtexportal.org/) helped to establish that 3 studied polymorphisms were significantly associated (p<8×10-5, FDR≤0.05) with the level of mRNA expression (cis-eQTL) of 8 different genes in different tissues and organs.
The polymorphic variant A rs4444903 EGF was found to be associated with increased expression of the EGF gene in the pituitary gland (β=0.65, p=1.8e-22, FDR≤0.05), skeletal muscles (β=0.11, p=0.000046, FDR≤0.05), and cultivated fibroblast cells (β=0.20, p=0.000051, FDR≤0.05) (Figure).
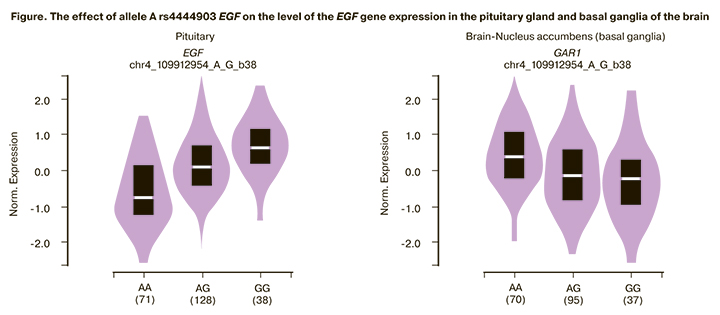
Polymorphic variant A rs4444903 EGF is associated with a low level of expression of the GAR1 gene in the basal ganglia of the brain (β=-0.27, p=0.000018, FDR≤0.05).
The TGFß-1 rs1800469 polymorphism is associated with the low expression of the B9D2 gene in cultivated fibroblast cells (β =-0.15, p=0.000021, FDR≤0.05); it also reduces the level of EXOSC5 gene expression in cardiac tissue (β =-0.23, p=1.4e-9, FDR≤0.05), skeletal muscles (β =-0.11, p=5.5 e-8, FDR≤0.05) and the TMEM91 gene in cultivated fibroblast cells (β =-0.092, p=0.0000027, FDR≤0.05).
The polymorphic variant C rs833061 VEGFA is associated with a high level of VEGFA gene expression in thyroid tissue (β=0.11, p=0.00015, FDR≤0.05).
The data of the GTExportal database indicate a significant regulatory potential of the studied polymorphisms.
The polymorphic locus rs4444903 EGF is placed in the area of the exon splicing enhancer (ESE) of the serine-arginine rich protein (spr55) (score=3.78) and in the area of the exon splicing enhancer of the serine-arginine splicing factor (SC35) (score=2.94). Allele A rs4444903 EGF is the binding site of transcription factors AP4_01 (Core Match Score=1), CACD_01 (Core Match Score=0.850), CEBP_Q3 (Core Match Score=0.986), DSP_Q6 01 (Core Match Score=0.914), FAC1_01 (Core Match Score=0.808), etc. The regulatory potential of A rs4444903 EGF is 0.12.
According to the SNPinfo database, the regulatory potential (RegPotential) of the locus rs1800469 is 0.22. Allele A rs1800469 TGFß-1 is the binding site of transcription factors AIRE_02 (Core Match Score=0.648), AP4_01 (Core Match Score=0.778), AREB6_02 (Core Match Score=1.0), SARNT_01 (Core Match Score=0.792), etc.
Polymorphism C rs6214 IGF1 is the binding site of the micro-RNAs hsa-miR-542-3p (score=140) and hsa-miR-570 (score=140).
The regulatory potential (RegPotential) of rs833061 c.-958C>T VEGF is 0.215. Allele C rs833061 VEGF is the binding site of the transcription factors AP2_Q6 (Core Match Score=0.953), ATF6_01 (Core Match Score=0.8), BRCA_01 (Core Match Score=0.994).
The TGFß-1 gene plays an important role in the regulation of cell proliferation, differentiation, and cell migration [21, 22]; therefore, it can influence the development of uterine fibroids combined with endometrial hyperplasia. According to the literature data, TGFß-1 serves as an important factor in regulating the expression of other growth factors [23]. TGFß-1 is synthesized by monocytes, macrophages, T- and B- lymphocytes. An increase in TGFß-1 is observed in the secretory phase of the cycle [24]. According to the literature, the polymorphic variant T rs1800469 TGFß-1 leads to an increase in the expression level of TGFß-1 [23, 25].
The genetic polymorphism rs4444903 c.-382A>G EGF, which is a part of the hazardous combination, can affect the interaction of EGF with its receptor and thus stimulate the processes of intracellular protein phosphorylation, affecting the proliferation and differentiation of endometrial cells [26, 27].
The literature data suggest that IGF1 is associated with increased proliferation, growth of endometrial tumors, myomas, and leiomyosarcomas [28]. Some studies indicate an increase in IGF1 in the hyperplastic endometrium [29]. Allelic variant A rs6214 IGF1 causes a decrease in protein expression, and allelic variant G of this gene, which is a part of the hazardous combination, on the contrary, causes an increase in protein expression.
Conclusion
Therefore, the results of the study indicate the importance of polymorphic loci of the genes of growth factors EGF rs4444903 c.-382A>G, TGFß-1 rs1800469 c.-1347T>C, IGF1 rs6214 c.*2716G>A, VEGF rs833061 c.-958C>T, FGFR2 rs2981582 c.109+906T>C in the development of uterine fibroids combined with endometrial hyperplasia. CC genotype rs2981582 FGFR2 is a risk factor for the development of uterine fibroids combined with endometrial hyperplasia (OR=1.38). The combination of alleles A rs4444903 EGF and C rs1800469 TGFß-1 (OR=1.50) is also a risk factor for the development of the disease. The combination of four genetic markers G rs4444903 EGF, C rs1800469 TGFß-1, G rs6214 IGF1, and T rs2981582 FGFR2 (OR=0.59) has protective significance for the development of uterine fibroids combined with endometrial hyperplasia. The studied SNPs have significant regulatory potential, and they are located in the areas of exon splicing enhancers; moreover, they are the binding sites of transcription factors, and they are associated with the influence of eight genes on the mRNA expression level in different tissues and organs.
References
- Адамян Л.В., ред. Cочетанные доброкачественные опухоли и гиперпла-стические процессы матки (миома, аденомиоз, гиперплазия эндометрия). Проект клинических рекомендаций по ведению больных. М.; 2015. 92 с. [Adamyan L.V., ed. Combined benign tumors and hyperplastic processes of the uterus (fibroids, adenomyosis, endometrial hyperplasia). Draft clinical guidelines for the management of patients. M.; 2015. 92p. (in Russian)].
- Киселев В.И., Сидорова И.С., Унанян А.Л., Муйжнек Е.Л. Гиперпластические процессы органов женской репродуктивной системы: теория и практика. М.: МЕДПРАКТИКА-М; 2010. 468 с. [Kiselev V.I., Sidorova I.S., Unanyan A.L., Muizhnek E.L. Hyperplastic processes of the organs of the female reproductive system: theory and practice. M.: MEDPRAKTIKA-M; 2010. 468 p. (in Russian)].
- McWilliams M.M., Chennathukuzhi V.M. Recent advances in uterine fibroid etiology. Semin. Reprod. Med. 2017; 35(2): 181-9. https://dx.doi.org/10.1055/s-0037-1599090.
- Пономаренко И.В., Полоников А.В., Чурносов М.И. Гиперпластические процессы эндометрия: этиопатогенез, факторы риска, полиморфизм генов-кандидатов. Акушерство и гинекология. 2019; 1: 13-8. [Ponomarenko I.V., Polonikov A.V., Churnosov M.I. Endometrial hyperplastic processes: etiopathogenesis, risk factors, polymorphism of candidate genes. Akusherstvo I Ginekologiya/Obstetrics and Gynecology. 2019; 1: 13-8. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.1.13-18.
- Пономаренко И.В., Чурносов М.И. Современные представления об этиопатогенезе и факторах риска лейомиомы матки. Акушерство и гинекология. 2018; 8: 27-32. [Ponomarenko I.V., Churnosov M.I. Current views on the etiopathogenesis and risk factors of uterine leiomyoma. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2018;8: 27-32 (in Russian)]. https://dx.doi.org/10.18565/aig.2018.8.27-32.
- Гарашова М.А. Частота встречаемости и сочетанности неопластических процессов гениталий в постменопаузальном периоде. Вестник современной клинической медицины. 2019; 12(2): 28-32. [Garashova M.A. The frequency of occurrence and combination of neoplastic genital processes in the postmenopausal period. Bulletin of modern clinical medicine. 2019; 12(2): 28-32 (in Russian)]. https://dx.doi.org/10.20969/VSKM.2019.12(2).28-32.
- Ørbo A., Arnes M., Vereide A., Straume B. Relapse risk of endometrial hyperplasia after treatment with the levonorgestrel‐impregnated intrauterine system or oral progestogens. BJOG. 2016; 123(9): 1512-9. https://dx.doi.org/10.1111/1471-0528.13763.
- Kim J.J., Kurita Т., Bulun S.E. Рrogesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr. Rev. 2013; 34(1): 130-62. https://dx.doi.org/10.1210/er.2012-1043.
- Krivoshei I.V., Altuchova O.B., Golovchenko O.V., Polonikov A.V., Churnosov M.I. Genetic factors of hysteromyoma. Research Journal of Medical Sciences. 2015; 9(4): 182-5. https://dx.doi.org/10.3923/rjmsci.2015.182.185.
- Пономаренко И.В., Полоников А.В., Чурносов М.И. Полиморфные локусы гена LHCGR ассоциированы с развитием миомы матки. Акушерство и гинекология. 2018; 10: 86-91. [Ponomarenko I.V., Polonikov A.V., Churnosov M.I. Polymorphic loci of the LHCGR gene are associated with the development of uterine fibroids. Akusherstvo I Ginekologiya/Obstetrics and Gynecology. 2018; 10: 86-91 (in Russian)]. https://dx.doi.org/10.18565/aig.2018.10.86-91.
- Пономаренко И.В., Полоников А.В., Чурносов М.И. Ассоциация полиморфизма rs4986938 гена ESR2 с развитием гиперплазии эндометрия. Акушерство и гинекология. 2019; 4: 66-72. [Ponomarenko I.V., Polonikov A.V., Churnosov M.I. Association of ESR2 rs4986938 polymorphism with the development of endometrial hyperplasia. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; (4): 66-72. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.4.66-72.
- Churnosov M.I., Altuchova O.B., Demakova N.A., Batlutskaya I.V., Polonikov A.V. Associations of cytokines genetic variants with myomatous knots sizes. Research Journal of Pharmaceutical, Biological and Chemical Sciences. 2014; 5(6): 1344-7.
- Лицова А.О., Малышкина А.И., Воронин Д.Н. Особенности системной и локальной продукции цитокинов у женщин с миомой матки различных темпов роста. Российский иммунологический журнал. 2012; 6(2): 105-6. [Litsova A.O., Malishkina A.I., Voronin D.N. Features of systemic and local production of cytokines in women with uterine fibroids of various growth rates. Russian immunological journal. 2012; 6(2): 105-6 (in Russian)].
- Сhuang Т.D., Luo X., Panda H. miR-93/106b and their host gene, МСМ7 are differentially expressed in leiomyomas and functionally target F3 and IL-8. Мol. Endocrinol. 2012; 26(6): 1028-42. https://dx.doi.org/10.1210/me.2012-1075.
- Сорокина И.Н., Рудых Н.А., Безменова И.Н., Полякова И.С. Популяционно-генетические характеристики и генетико-эпидемиологическое исследование ассоциаций генов-кандидатов с мультифакториальными заболеваниями. Научные результаты биомедицинских исследований. 2018; 4(4): 20-30. [Sorokina I.N., Rudykh N.A., Bezmenova I.N., Polyakova I.S. Population genetic characteristics and genetic epidemiological research of candidate genes associations with multifactorial diseases. Research Results in Biomedicine. 2018; 4(4): 20-30 (in Russian)]. https://dx.doi.org/10.18413/2313-8955-2018-4-4-0-3.
- Пономаренко И.В. Отбор полиморфных локусов для анализа ассоциаций при генетико-эпидемиологических исследованиях. Научный результат. Медицина и фармация. 2018; 4(2): 40-54. [Ponomarenko I.V. Selection of polymorphic loci for association analysis in genetic-epidemiological studies. Research Result. Medicine and Pharmacy. 2018; 4(2): 40-54 (in Russian)]. https://dx.doi.org/10.18413/2313-8955-2018-4-2-0-5.
- Favorov A.V., Andreewski T.V., Sudomoina M.A., Favorova O.O., Parmigiani M.F. A Markov chain Monte Carlo technique for identification of combinations of allelic variants underlying complex diseases in humans. Genetics. 2005; 171(4): 2113-21. https://dx.doi.org/10.1534/genetics.105.048090.
- Lvovs D., Favorova O.O., Favorov A.V. A polygenic approach to the study of polygenic diseases. Acta Naturae. 2012; 4(3): 59-71.
- Гнатенко Д.А., Копанцев Е.П., Свердлов Е.Д. Роль сигнального пути FGR/FGFR в канцерогенезе поджелудочной железы. Биомедицинская химия. 2016; 62(6): 622-9. [Gnatenko D.A., Kopantsev E.P., Sverdlov E.D. The role of the signaling pathway FGR/FGFR in pancreatic cancer. Biomedical chemistry. 2016; 62(6): 622-9. (in Russian)]. https://dx.doi.org/10.18097/PBMC20166206622.
- Cui F. Variants of FGRF2 and their associations with breast cancer risc: a HUGE systematic rewiew and meta-analysis. Breast Cancer Res. Treat. 2016; 155(2): 313-35. https://dx.doi.org/10.1007/s10549-015-3670-2.
- Lecanda J. Тransforming growth factor-β, estrogen, and progesterone converge on the regulation of p27Kip1 in the normal and malignant endometrium. Сancer Res. 2007; 67(3): 1007-18. https://dx.doi.org/10.1158/0008-5472.CAN-06-0235.
- Fragoso J.М. Тhe Т29С (rs1800470) polymorphism of the transforming growth factor-β1 (ТGF-β1) gene is associated with restenosis after coronary stenting in Мexican patients. Exp. Мol. Рathol. 2015; 98(1): 13-7. https://dx.doi.org/10.1016/j.yexmp.2014.11.007.
- Бурменская О.В., Байрамова Г.Р., Непша О.С., Донников А.Е. Цитокиновый профиль иммунокомпетентных клеток влагалища при хроническом рецидивирующем вульвовагинальном кандидозе. Уральский медицинский журнал. 2011; 3: 44-9. [Burmenskaya O.V., Bayramova G.R., Nepsha O.S., Donnikov A.E. Cytokine mRNA profiles of immunocompetent vaginal cells of women with chronic recurrent vulvovaginal candidiasis. Uralskiy Medical journal. 2011; 3: 44-9. (in Russian)].
- Солодовникова Н.Г., Ниаури Д.А. Роль цитокинов в развитии наружного генитального эндометриоза (обзор литературы). Вестник Санкт-Петербургского университета. Серия: Медицина. 2006; 2: 115-22. [Solodovnikova N.G., Niauri D.A. The role of cytokines in the development of external genital endometriosis: (literature review). Bulletin of St. Petersburg University. Series Medicine. 2006; 2: 115-22. (in Russian)].
- Меньшикова Н.С. Функциональный полиморфизм генов иммуносупрессорных цитокинов при наружном генитальном эндометриозе. Мать и дитя в Кузбассе. 2013; 1: 24-6. [Menshikova N.S. Functional polymorphism of immunosuppressive cytokine genes in case of external genital endometriosis. Mother and child in Kuzbass. 2013; 1: 24-6. (in Russian)].
- Привалова Е.Е. Процессы цитокин- и нитроксидергической регуляции при наружном генитальном эндометриозе, ассоциированным с бесплодием. Вестник Южно-Уральского государственного университета. Серия: Образование, здравоохранение, физическая культура. 2009; 20: 58-61. [Privalova E.E. The processes of cytokinnitroxergic regulation in external genital endometriosis associated with infertility. Bulletin of SUSU. 2009; 20: 58-61. (in Russian)].
- Адамян Л.В., Салимова Д.Ф., Кондратович Л.М. Патогенетические аспекты эндометриоз-ассоциированного бесплодия. Проблемы репродукции. 2015; 21(6): 90-6. [Adamyan L.V., Salimova D.F., Kondratovich L.M. Pathogenetic aspects of endometriosis-associated infertility. Reproduction problems. 2015; 21(6): 90-6. (in Russian)].
- Гуляева Л.Ф., Красильников С.Э. Молекулярные механизмы канцерогенеза эндометрия. Бюллетень Восточно-Сибирского научного центра СО РАМН. 2012; 3(1): 110-5. [Gulyaeva L.F., Krasilnikov S.E. Molecular mechanisms of carcinogenesis of the endometrium. Bulletin of the East Siberian Scientific Center of the SB RAMS. 2012; 3(1): 110-5. (in Russian)].
- Крылова Ю.С., Кветной И.М. Айлмазян Э.К. Рецептивность эндометрия: молекулярные механизмы регуляции имплантации. Журнал акушерства и женских болезней. 2013; 62(2): 63-74. [Krylova Yu.S., Kvetnoy I.M. Ailmazyan E.K. Endometrial receptivity: molecular mechanisms for the regulation of implantation. Journal of Obstetrics and Women's Diseases. 2013; 62(2): 63-74. (in Russian)].
Received 11.06.2020
Accepted 19.11.2020
About the Authors
Oksana B. Altukhova, MD, associate professor, Department of obstetrics and gynecology, Medical Faculty, Belgorod State National Research University.Tel.: +7(4722)30-13-83. E-mail: kristalinka@yandex.ru. 308015, Russia, Belgorod, Pobedy str., 85.
Viktor E. Radzinsky, MD, professor, Head of the Department of obstetrics and gynecology, Medical Faculty, Peoples' Friendship University of Russia. Tel.: +7(495)360-46-69. E-mail: radzinskiy-ve@rudn.ru. ORCID: 0000-0003-4956-0466. 117198, Russia, Moscow, Miklukho-Maklaya str., 6.
Mikhail I. Churnosov, MD, professor, Head of the Department of Biomedical Disciplines, Medical Faculty, Belgorod State National Research University.
Tel.: +7(4722)30-13-83. E-mail: churnosov@bsu.edu.ru. ORCID: 0000-0003- 1254-6134. 308015, Russia, Belgorod, Pobedy str., 85.
Irina S. Polyakova, associate professor, Department of Biomedical Disciplines, Medical Faculty, Belgorod State National Research University.
Tel.: +7(4722)30-13-83. E-mail: polyakovairina@bsu.edu.ru. 308015, Russia, Belgorod, Pobedy str., 85.
For citation: Altukhova O.B., Radzinsky V.E., Polyakova I.S., Churnosov M.I. The role of growth factor genes in the development of uterine fibroids combined with endometrial hyperplasia.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2021; 4: 104-110 (in Russian)
https://dx.doi.org/10.18565/aig.2021.4.104-110



