Evaluation of nicotinic acetylcholine receptor subunit expression levels for the improvement of diagnostic approaches and treatment in endometrial hyperplasia and cancer
Levakov S.A., Gvazava E.N., Gromova T.A., Petrosyan E.G., Mazur D.V., Rezekina A.I., Gondarenko E.A., Antipova N.V.
Objective: To determine and compare the expression levels of genes encoding nicotinic acetylcholine receptors (nAChRs) in hyperplastic and oncological endometrial processes.
Materials and methods: The study included 120 women of reproductive age divided into groups according to histological examination results: endometrial hyperplasia (EH) (n=58), endometrioid adenocarcinoma of the endometrium (n=14), and those with unchanged endometrium (n=48). The expression levels of genes encoding nAChRs (α1, α2, α3, α4, α5, α6, α7, α9, α10, β1, β2, β3, and β4) were determined by real-time reverse transcription polymerase chain reaction using specific primers for the corresponding nAChRs.
Results: Among the patients in the first group, increased expression was observed for the α1, α4, β1, and β2 subunits, with the greatest diagnostic significance attributed to the α4 subunit of the nAChRs. In the endometrium of patients in the second group, gene expression was detected for the α1, α3, and α6 subunits, with the α3 subunit of the nAChRs showing the greatest diagnostic significance. In the third group, pronounced expression was noted for the α3, α5, α6, and α7 subunits of the nAChRs.
Conclusion: The observed expression of nAChRs in EH suggests their influence on the differentiation of unchanged endometrium, potentially leading to the development of EH. The expression of these subunits in endometrial adenocarcinoma indicates that they may serve as potential predictors of endometrial neotransformation.
Authors' contributions: Levakov S.A. – conception and design of the study; Gvazava E.N., Antipova N.V., Petrosyan E.G., Mazur D.V., Rezekina A.I. – material collection and processing; Gvazava E.N., Antipova N.V., Gonadrenko E.A., Rezekina A.I. – statistical analysis; Gromova T.A., Gvazava E.N. – drafting of the manuscript; Gromova T.A. – editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Levakov S.A., Gvazava E.N., Gromova T.A., Petrosyan E.G., Mazur D.V., Rezekina A.I., Gondarenko E.A., Antipova N.V. Evaluation of nicotinic acetylcholine receptor subunit expression levels for the improvement of diagnostic approaches and treatment in endometrial hyperplasia and cancer.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (12): 108-116 (in Russian)
https://dx.doi.org/10.18565/aig.2024.50
Keywords
Endometrial hyperplasia (EH) is a non-invasive abnormal proliferation of the uterine mucosa, characterized by an increase in the glandular-stromal ratio and proliferation of the glands [1, 2]. According to various sources, the probability of transformation of this condition into endometrial cancer ranges from 10% to 40% [3, 4]. According to estimates by the World Health Organization in 2020, the prevalence of endometrial cancer was 35, 915 in women under 44 years of age, 14,203 in women under 39 years of age, and 2,232 in women under 29 years of age [5]. In the Russian Federation, since 2012, there has been a 31.8% increase in the prevalence of endometrial cancer, and by 2022, the incidence rate is expected to be 195.6 cases per 100,000 people [6]. A possible manifestation of EH and endometrial cancer may be abnormal uterine bleeding, which is characterized by excessive duration, volume of blood loss, and/or frequency. In most cases, this is the reason for patients to seek medical help, which can contribute to the timely detection of EH and endometrial cancer at the initial stages [1].
Potential risk factors that can provoke the development of EH and endometrial cancer include imbalances in the ratio between the levels of progestins and estrogens, with a predominance of the latter. Such conditions include the perimenopausal period, polycystic ovary syndrome, presence of granulosa cell tumors, use of estrogens alone as part of hormone replacement therapy, intake of phytoestrogens, tamoxifen, and obesity. Additionally, possible risk factors include genetic conditions such as Cowden syndrome and hereditary nonpolyposis colorectal cancer as well as the presence of diabetes mellitus, immunosuppression, and infection [7–10]. On the contrary, smoking is associated with a lower risk of endometrial cancer, possibly due to its antiestrogenic effects. However, the biological mechanisms underlying this association remain unclear [11–13].
To date, diagnostic methods for the early detection of endometrial cancer (such as ultrasound examination of the pelvic organs, hysteroscopy, and endometrial biopsy with subsequent histological examination) are mainly invasive and have insufficient specificity. This necessitates the identification of specific noninvasive markers for the early detection of possible malignant EH with subsequent development of endometrial cancer.
The cholinergic system is one of the most important neural pathways in the human body. The key molecules that transmit acetylcholine are muscarinic metabotropic receptors and ionotropic neuronal nicotinic acetylcholine receptors (nAChRs). The latter belongs to the superfamily of ligand-gated ion channels and consists of five different types of subunits (α, β, δ, ε, or γ); α and β are the major subunits. Neuronal subunits are divided into alpha (α2–α7, α9, and α10) and beta subunits (β2–β4). The α8 subunit was not detected in mammalian tissues. Nicotinic receptors have been extensively studied for their electrophysiological functions at neuromuscular junctions and in central and peripheral nervous systems. Currently, there is increasing evidence of the presence of nAChRs in non-neuronal cells and tissues, suggesting a role for these receptors in biological processes other than synaptic transmission [14]. Several studies have shown a close relationship between tumor development and nAChRs as key molecules involved in tumor growth, angiogenesis, metastasis, and apoptosis, suggesting that the regulation of nAChR activity may be a promising part of antitumor therapy [15, 16]. It has also been shown that tissues without electrophysiological functions, such as epithelium, express some of the nAChR subunits. Candidates for the function of nAChRs in epithelial cells may include intracellular signaling pathways that lead from receptor-mediated ion flux to subsequent biological effects [14, 15]. According to several studies, the expression of α7 nAChRs is associated with the development of bladder cancer, colon cancer, stomach cancer, squamous cell cancer of the head and neck, and pancreatic cancer [17–21]. In lung cancer, hyperexpression of the α7 nAChR subunit is associated with nicotine-mediated proliferation, proangiogenesis, and metastasis, which likely contribute to the early phase of oncogenesis in non-small cell lung cancer and can be eliminated by α7-nAChR-specific inhibitors [22]. As the disease progresses, the expression of this subunit decreases [23, 24]. It was also found that downregulation of α9-nAChR expression in human breast cancer cells by RNA interference significantly reduced nicotine-induced cell proliferation in this cancer type and inhibited tumor growth [25, 26].
Treatment resistance is one of the biggest challenges in cancer therapy as it limits the effectiveness of antitumor treatments and slows recovery. Research has found that exposure to tobacco smoke and nicotine is associated with increased resistance to chemotherapy, generalized through nAChR-dependent effects on chemotherapeutic drugs. Thus, nAChR antagonist-based therapy in combination with traditional chemotherapy may represent an improved approach, as it renders tumors susceptible to lower doses of chemotherapeutic agents, thereby avoiding their toxicity. This strategy may be particularly useful for the treatment of tumors with nAChR overexpression [27]. However, the understanding of the role of nAChRs in the development and progression of EH and endometrial cancer is currently limited. Therefore, studying the differential expression of nAChRs in these two conditions will help to determine which nAChR subunits play a key role in the pathogenesis of their development and may potentially become targets for the creation of targeted drugs.
This study aimed to compare the expression levels of genes encoding nAChR subunits in EH and uterine cancer to optimize the prediction of the development of these conditions.
Materials and methods
As part of the study, 120 patients of reproductive age were selected based on their clinical complaints (menstrual cycle disorders and abnormal uterine bleeding) and pelvic organ ultrasound examination data. Ultrasound findings included endometrial thickening from 10 to 20 mm with a heterogeneous structure, increased echogenicity of the endometrium, and the presence of uneven and fuzzy contours and multiple chaotic color loci, as noted in 9.3% of cases using color Doppler mapping.
All the patients underwent hysteroscopy and separate diagnostic curettage of the uterine and cervical cavities. Three study groups were identified based on the histological findings. Group 1 included patients with EH without atypia (n=58), Group 2 included patients with endometrial cancer (Endometrioid adenocarcinoma) (n=14), and Group 3, the control group, included patients with an unaltered endometrium (n=48).
The inclusion criteria for groups 1 and 2 were as follows: patients aged 18–49 years, with a histologically confirmed diagnosis of EH or Endometrioid adenocarcinoma of the uterine body, and the absence of acute gynecological pathology and significant pathomorphological disorders of the reproductive system.
The inclusion criteria for group 3 were patients aged 18–49 years, absence of acute gynecological pathology, and absence of significant pathomorphological disorders of the reproductive system.
Exclusion criteria included patients under 18 years of age or in the postmenopausal period, presence of acute inflammatory diseases of the pelvic organs, other structural pathologies of the uterine cavity, severe extragenital pathology in the decompensation stage, and any oncological diseases in the medical history or at the time of the study.
After hysteroscopy and fractional diagnostic curettage of the uterus, the obtained endometrium was used for this study. During sample collection, 1 ml of TRIzol solution was added to inhibit RNase activity, and the samples were placed in a freezer for 1 h. Subsequently, the postoperative material was transported to the M.M. Shemyakin and Yu.A. Ovchinnikov Institute of Bioorganic Chemistry of the Russian Academy of Sciences, where homogenization and rapid freezing of the samples were performed at at temperature of -80°C were performed. RNA was isolated from the samples using the ExtractRNA reagent (Eurogen) according to the manufacturer's instructions, after which cDNA was synthesized using the MMLV RT kit for reverse transcription (Eurogen) according to the manufacturer's guidelines. cDNA synthesis was conducted for 5 min at 10°C, followed by 25 min at 37°C and 42 °C, and at 70°C for 10 min to inactivate the enzyme. The expression levels of genes encoding nAChR subunits in the uterine epithelium (α1, α2, α3, α4, α5, α6, α7, α9, α10, β1, β2, β3, and β4) were determined by real-time PCR using a LightCycler 96 (Roche) with the qPCRmix-HS SYBR reagent (Eurogen). The PCR program included pre-incubation at 95°C for 150 s, followed by 45 cycles at 95°C for 20 s, 60°C for 20 s, and 72°C for 20 s. Data were collected using the LightCycler software (version 4.1).
Real-time PCR data were processed using the 2-ΔCT method in Microsoft Excel. The same PCR melting peaks were observed in triplicate for each pair of primers across all samples. The obtained Ct (cycle threshold) value obtained for each sample did not exceed 36. The 18S RNA gene served as an internal control sample.
Statistical analysis
Statistical analysis was performed using STATISTICA version 13.0 and Microsoft Excel (Excel 2016 (16) – Microsoft Office 2016). Continuous variables showing a normal distribution were expressed as mean (M) and standard deviation (SD) and presented as M (SD). If the data were not normally distributed, the median (Me) with an interquartile range of Me [Q25; Q75] was reported. Categorical variables are presented as counts (n) and percentages (%). Differences between the groups were considered statistically significant at p<0.05. Receiver operating characteristic (ROC) analysis was used to evaluate the predictive (diagnostic) value of the markers.
Results
In accordance with the results obtained, the clinical and demographic characteristics of the patients studied were summarized (Table 1). The median ages of the patients with unaltered endometrium and those with hyperplasia were comparable; however, an older age was observed in patients with endometrial cancer than in those with unaltered endometrium (41 [36; 46] years vs. 37 [31; 43] years, p=0.024). The group of patients with EH had significantly more grade 1 obesity (body mass index 30–34.9 kg/m²) than the group with unaltered endometrium (25% vs. 37.9%, p=0.018). In addition, the number of smokers was significantly higher in the group with endometrial adenocarcinoma than in the groups with unaltered and hyperplastic endometrium (28.6% vs. 16.7% and 17.2%, respectively; p<0.05). The group of patients with EH had an older age at first birth than the groups with unaltered endometrium and endometrial cancer (28 [25; 31] years versus 25 [22; 29] years and 26 [23; 29] years, respectively, p<0.05). Other indicators did not differ significantly between the groups.
The group of patients with EH also had an older age at first birth than the groups with unaltered endometrium and endometrial cancer (28 [25; 31] years versus 25 [22; 29] years and 26 [23; 29] years, respectively, p<0.05). The studied groups did not differ significantly in other indicators.
The evaluation of gynecological anamnesis and parameters of ultrasound examination of the uterus (uterine body volume, m-echo) among the patients in the study groups are presented in Table 2. Among patients with EH and endometrial cancer, heavy menstrual bleeding (46.6% and 57.1% vs. 20.8%, respectively, p<0.05), painful menstrual bleeding (34.5% and 42.9% vs. 20.8%, respectively, p<0.05), and irregular menstrual cycle (17.2% and 28.5% vs. 4.2%, respectively, p<0.05) were significantly more frequent.
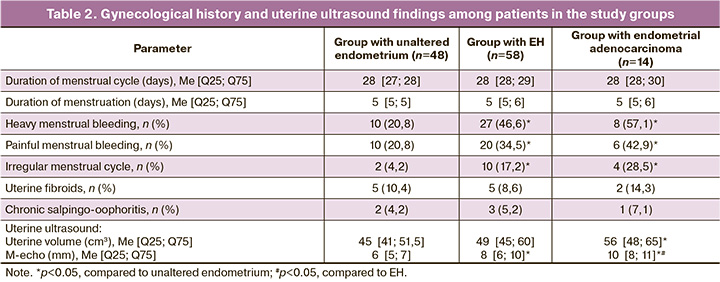
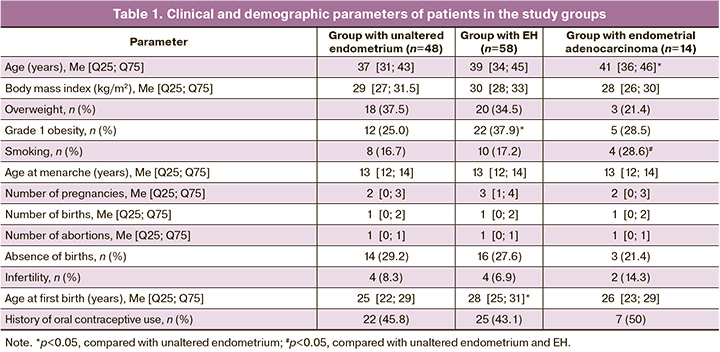
Based on the results of the identified expression levels of genes encoding nAChR subunits, the expression of the studied genes in the endometrium of patients in the EH group showed increased expression of α1, α4, β1, and β2 subunits. The expression of all studied nAChR subunits was also present in the group of patients with unaltered endometrium; however, the most pronounced expression was noted for the α3, α5, α6, and α7 subunits. The expression of the α9 subunit was the same in both the study groups (Figs. 1–5). Furthermore, the expression of α3-nAChR subunits was 2.7 times higher (p<0.001) (Fig. 1), α5-nAChR – 2.2 times higher (p<0.001) (Fig. 2), α6-nAChR – 2.6 times higher (p<0.001) (Fig. 3), α7-nAChR – 1.6 times higher (p=0.014) (Fig. 3) among patients with unaltered endometrium compared to the EH group. On the contrary, the expression of the α4-nAChR subunit, on the contrary, was 72.4 times higher (p<0.001) (Fig. 2), β1-nAChR subunits was 2.9 times higher (p<0.001) (Fig. 4), β2-nAChR was 1.6 times higher (p=0.018) (Fig. 5) among patients with EH, compared with the unaltered endometrium group.
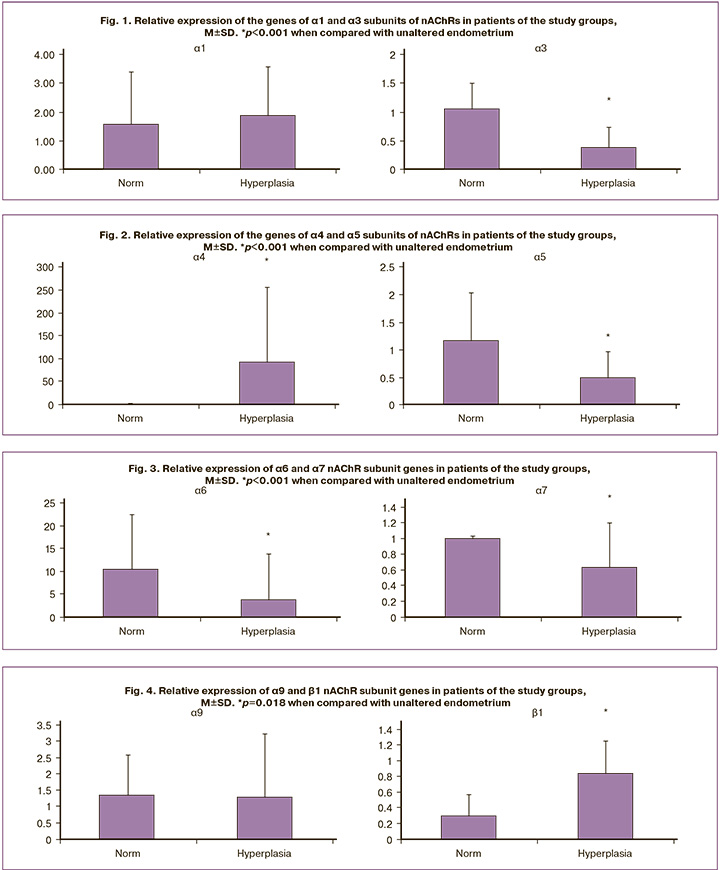
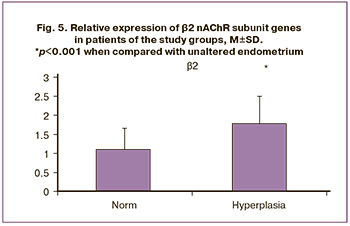
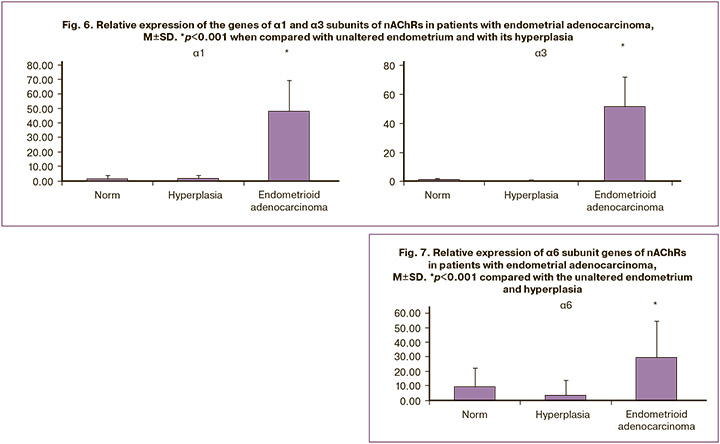
According to the obtained results, in patients from the Endometrioid adenocarcinoma group, a significant increase in the expression level of genes encoding nAChR subunits was detected for the α1, α3, and α6 subunits (relative expression increased by 25.9 times, 135.4 times, and 8.0 times, respectively) (Figs. 6, 7). At the same time, the absence of expression was noted in endometrial cancer for the genes α4, α5, α7, α9, α10, β1, β2, β3, and β4 (Figs. 2–5). The results were statistically significant (p<0.001). It is important to note that in the group of patients with Endometrioid adenocarcinoma, the expression of these subunits was significantly higher than that in patients with an unaltered endometrium (p<0.001).
ROC analysis was performed to differentiate between a normal endometrium and EH (Table 3). Among all the nAChR subunits studied in these patient groups, the relative expression levels of the α4 and α6 subunits were the most effective for the differential diagnosis between EH and normal endometrium. Statistical analysis indicated that the expression level of the α4 subunit provided the highest probability for this differentiation, with a relative expression greater than 8.31, sensitivity of 81%, specificity of 70%, AUC=0.86, 95% CI: 0.78–0.94, and p<0.0001, since the expression level of the α6 subunit showed lower diagnostic significance (relative expression less than 6.25 with a sensitivity of 59%, specificity of 60%, AUC=0.66; 95% CI: 0.55–0.77; p=0.013).
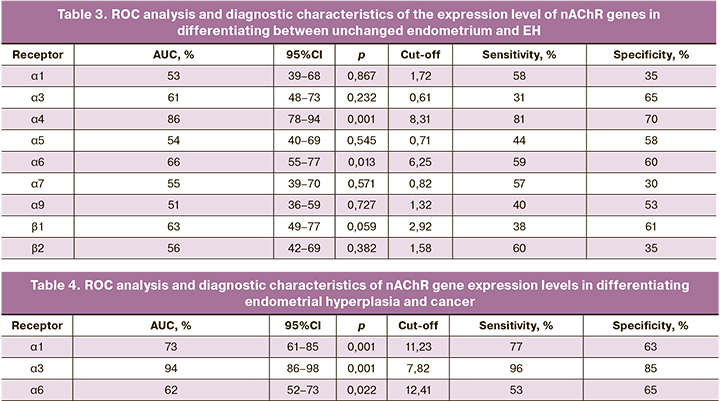
In contrast, the expression level of the α6 subunit showed a lower diagnostic significance, with a relative expression of <6.25, sensitivity of 59%, specificity of 60%, AUC=0.66, 95% CI: 0.55–0.77, and p=0.013.
Endometrioid adenocarcinoma can be differentiated from EH based on the relative expression levels of α1, α3, and α6 nAChR subunits (Table 4). The α3 nAChR subunit had the greatest diagnostic significance, with a relative expression level exceeding 7.82, providing the highest probability of diagnosing Endometrioid adenocarcinoma (sensitivity, 96%; specificity, 85%; AUC, 0.94; 95% CI: 0.86–0.98; p=0.001). The expression levels of the α1 and α6 nAChR subunits demonstrated lower diagnostic significance in differentiating between these groups. For the α1 nAChR subunit, sensitivity was 77%, specificity was 63%, AUC=0.73, 95% CI: 0.61–0.85, and p=0.001. For the α6 nAChR subunit, sensitivity was 53%, specificity was 65%, AUC=0.62, 95% CI: 0.52–0.73, and p=0.022.
Conclusion
Based on the results of the study and the comparison of the expression levels of genes encoding nAChR subunits in the study groups, it was revealed that in the group with endometrial hyperplasia, there was an increased expression of genes encoding the α1, α4, β1, and β2 subunits compared to the control group. The data obtained suggest that the increased expression levels of these nAChR subunits may affect the differentiation of the unchanged endometrium with the potential development of endometrial hyperplasia. Conversely, in the control group, increased expression of other subunits α3, α5, α6, and α7 was observed compared to the group of patients with endometrial hyperplasia and endometrial adenocarcinoma, which may potentially confirm this assumption. In this context, the assessment of the level of relative expression of the α4 nAChR subunit, which, according to the results of the study, showed greater diagnostic significance than the expression levels of other subunits, suggests that differential diagnosis between the endometrium with and without hyperplasia is possible. These results indicate the involvement of these subunits in protective mechanisms aimed at suppressing pathological proliferation and transformation of endometrial cells. In the group of patients with endometrial adenocarcinoma, increased expression of the α1, α3, and α6 nAChR subunits was identified, which allows for a differential diagnosis between this disease and endometrial hyperplasia. In this case, the greatest diagnostic significance was observed for the expression of the α3 nAChR subunit. These results suggest a potential impact of the overexpression of these subunits, particularly the α3 nAChR subunit, on the development of endometrial adenocarcinoma, and indicate the possibility of considering them as predictors of potential neotransformation in endometrial hyperplasia.
Thus, the results of this study indicate that the expression of nAChR subunits is regulated and varies depending on the stage of endometrial cell transformation. Combinations of these subunits form numerous homo- and heteropentameric receptors with different structural, functional, and pharmacological properties, which potentially opens up a complex area of research for understanding the biological processes in both unchanged and tumor endometrial cells.
Based on the characteristics of nAChR gene expression in the endometrium of patients of reproductive age, it can be suggested that the endometrium in a state of hyperplasia and adenocarcinoma is influenced by cholinergic signaling. This allows the identification of a spectrum of possible therapeutic targets that could be based on inhibitors of cholinergic signaling. Thus, there is a need for further functional studies on the effect of cholinergic signaling on the epithelium of the female genital tract, in terms of maintaining homeostasis and its role in carcinogenesis. Furthermore, the development of specific blockers targeting the expression of nAChRs in tumor cells may improve the efficacy and safety of antitumor treatments.
References
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Гиперплазия эндометрия. 2021. [Ministry of Health of the Russian Federation. Clinical guidelines. Endometrial hyperplasia. 2021.(in Russian)].
- Nees L.K., Heublein S., Steinmacher S., Juhasz-Böss I., Brucker S., Tempfer C.B. et al. Endometrial hyperplasia as a risk factor of endometrial cancer. Arch. Gynecol. Obstet. 2022; 306(2): 407-21. https://dx.doi.org/10.1007/s00404-021-06380-5.
- Chandra V., Kim J.J., Benbrook D.M., Dwivedi A., Rai R. Therapeutic options for management of endometrial hyperplasia. J. Gynecol. Oncol. 2016; 27(l): e8. https://dx.doi.org/10.3802/jgo.2016.27.e8.
- Kadirogullari P., Atalay C.R., Ozdemir O., Sari M.E. Prevalence of co-existing endometrial carcinoma in patients with preoperative diagnosis of endometrial hyperplasia. J. Clin. Diagn. Res. 2015; 9(10): 10-4. https://dx.doi.org/10.7860/JCDR/2015/12484.6618.
- Roh H.J., Yoon H.J., Jeong D.H., Lee T.H., Kwon B.S., Suh D.S. et al. Prognostic factors of oncologic outcomes after fertility-preservative management with progestin in early-stage of endometrial cancer. J. Res. Med. Sci. 2021; 26: 48. https://dx.doi.org/10.4103/jrms.jrms_103_20.
- Каприн А.Д., Старинский В.В., Шахзадова А.О., ред. Состояние онкологической помощи населению России в 2022 году. М.: МНИОИ им. П.А. Герцена, филиал ФГБУ «НМИЦ радиологии» Минздрава России; 2022. 239 с. [Kaprin A.D., Starinsky V.V., Shakhzadova A.O., ed. The state of oncological care for the population of Russia in 2022. Moscow: P.A. Herzen MSROI, branch of the FSBI "NMRC of Radiology" of the Ministry of Health of Russia; 2022. 239 p. (in Russian)].
- Tzortzatos G., Andersson E., Soller M., Askmalm M.S., Zagoras T., Georgii-Hemming P. et al. The gynecological surveillance of women with Lynch syndrome in Sweden. Gynecol. Oncol. 2015; 138(3): 717-22. https://dx.doi.org/10.1016/j.ygyno.2015.07.016.
- van der Meer A.C., Hanna L.S. Development of endometrioid adenocarcinoma despite Levonorgestrel-releasing intrauterine system: a case report with discussion and review of the RCOG/BSGE Guideline on the Management of Endometrial Hyperplasia. Clin. Obes. 2017; 7(1): 54-7. https://dx.doi.org/10.1111/cob.12168.
- Brinton L.A., Trabert B., Anderson G.L., Falk R.T., Felix A.S., Fuhrman B.J. et al. Serum estrogens and estrogen metabolites and endometrial cancer risk among postmenopausal women. Cancer Epidemiol. Biomarkers Prev. 2016; 25(7): 1081-9. https://dx.doi.org/10.1158/1055-9965.EPI-16-0225.
- Wu Y., Sun W., Liu H., Zhang D. Age at menopause and risk of developing endometrial cancer: a meta-analysis. Biomed. Res. Int. 2019; 2019: 8584130. https://dx.doi.org/10.1155/2019/8584130.
- Raglan O., Kalliala I., Markozannes G., Cividini S., Gunter M.J., Nautiyal J.et al. Risk factors for endometrial cancer: an umbrella review of the literature. Int. J. Cancer. 2019; 145(7): 1719-30. https://dx.doi.org/10.1002/ijc.31961.
- Kwon J.Y., Park K., Song J.M., Pyeon S.Y., Lee S.H., Chung Y.S. et al. Risk factors and prognosis of stroke in gynecologic cancer patients. Cancers (Basel). 2023; 15(19): 4895. https://dx.doi.org/10.3390/cancers15194895.
- Юренева С.В., Ермакова Е.И. Менопауза и климактерическое состояние женщины. Акушерство и гинекология. 2018; 7: 32-8. [Yureneva S.V., Ermakova E.I. Menopause and menopausal condition of a woman. Obstetrics and Gynecology. 2018; (7): 32-8. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.7.32-38.
- Шулепко М.А., Бычков М.Л., Кульбацкий Д.С., Люкманова Е.Н. Никотиновые ацетилхолиновые рецепторы человека. Часть II: не-нейрональная холинергическая система. Биоорганическая химия. 2019; 45(3): 227-37. [Shulepko M.A., Bychkov M.L., Kulbaczkii D.S., Lyukmanova E.N. Human nicotinic acetylcholine receptors. Part II: Non-neuronal cholinergic system. Russian Journal of Bioorganic Chemistry. 2019; 45(3): 227-37.(in Russian)]. https://dx.doi.org/10.1134/S0132342319020131.
- Khodabandeh Z., Valilo M., Velaei K., Pirpour Tazehkand A. The potential role of nicotine in breast cancer initiation, development, angiogenesis, invasion, metastasis, and resistance to therapy. Breast Cancer. 2022; 29(5): 778-89. https://dx.doi.org/10.1007/s12282-022-01369-7.
- Li X., Tae H.S., Chu Y., Jiang T., Adams D.J., Yu R. Medicinal chemistry, pharmacology, and therapeutic potential of α-conotoxins antagonizing the α9α10 nicotinic acetylcholine receptor. Pharmacol. Ther. 2021; 222: 107792. https://dx.doi.org/10.1016/j.pharmthera.2020.107792.
- He Z., Xu Y., Rao Z., Zhang Z., Zhou J., Zhou T. et al. The role of α7-nAChR-mediated PI3K/AKT pathway in lung cancer induced by nicotine. Sci. Total Environ. 2024; 912: 169604. https://dx.doi.org/ 10.1016/j.scitotenv.2023.169604.
- Bele T., Turk T., Križaj I. Nicotinic acetylcholine receptors in cancer: Limitations and prospects. BBA. 2024; 1870(1): 166875. https://dx.doi.org/10.1016/j.bbadis.2023.166875.
- Ferlay J., Colombet M., Soerjomataram I., Parkin D.M., Piñeros M., Znaor A. et al. Cancer statistics for the year 2020: An overview. Int. J. Cancer. 2021; 149(4): 778-89. https://dx.doi.org/10.1002/ijc.33588.
- Nieh S., Jao S.W., Yang C.Y., Lin Y.-S., Tseng Y.H., Liu C.L. et al. Regulation of tumor progression via the Snail-RKIP signaling pathway by nicotine exposure in head and neck squamous cell carcinoma. Head Neck. 2015; 37(12): 1712-21. https://dx.doi.org/10.1002/hed.23820.
- Jin T., Hao J., Fan D. Nicotine induces aberrant hypermethylation of tumor suppressor genes in pancreatic epithelial ductal cells. Biochem. Biophys. Res. Commun. 2018; 499(4): 934-40. https://dx.doi.org/10.1016/j.bbrc.2018.04.022.
- Medjber K., Freidja M.L., Grelet S., Lorenzato M., Maouche K., Nawrocki-Raby B. et al. Role of nicotinic acetylcholine receptors in cell proliferation and tumour invasion in broncho-pulmonary carcinomas. Lung Cancer. 2015; 87(3): 258-64. https://dx.doi.org/10.1016/j.lungcan.2015.01.001.
- Wang S., Hu Y. α7 nicotinic acetylcholine receptors in lung cancer. Oncol. Lett. 2018; 16(2): 1375-82. https://dx.doi.org/10.3892/ol.2018.8841.
- Schaal C., Chellappan S. Nicotine-mediated regulation of nicotinic acetylcholine receptors in non-small cell lung adenocarcinoma by E2F1 and STAT1 transcription factors. PloS One. 2016; 11(5): e0156451. https://dx.doi.org/10.1371/journal.pone.0156451.
- Huang L.C., Lin C.L., Qiu J.Z., Lin C.Y., Hsu K.W., Tam K.W. et al. Nicotinic acetylcholine receptor subtype alpha-9 mediates triple-negative breast cancers based on a spontaneous pulmonary metastasis mouse model. Front. Cell. Neurosci. 2017; 3(11): 336. https://dx.doi.org/10.3389/fncel.2017.00336.
- Fararjeh A.S., Tu S.H., Chen L.C., Cheng T.C., Liu Y.R., Chang H.L. et al. Long-term exposure to extremely low-dose of nicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) induce non-malignant breast epithelial cell transformation through activation of the a9-nicotinic acetylcholine receptor-mediated signaling pathway. Environ. Toxicol. 2019; 34(1): 73-82. https://dx.doi.org/10.1002/tox.22659.
- Afrashteh Nour M., Hajiasgharzadeh K., Kheradmand F., Asadzadeh Z., Bolandi N., Baradaran B. Nicotinic acetylcholine receptors in chemotherapeutic drugs resistance: аn emerging targeting candidate. Life Sci. 2021; 278: 119557. https://dx.doi.org/10.1016/j.lfs.2021.119557.
Received 16.09.2024
Accepted 05.12.2024
About the Authors
Sergey A. Levakov, Dr. Med. Sci., Professor, Head of the Department of Obstetrics and Gynecology, N.V. Sklifosovsky ICM, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia (Sechenov University), 119991, Russia, Moscow, Trubetskaya str., 8, bld. 2, +7(495)609-14-00, levakoff@yandex.ru,https://orcid.org/0000-0002-4591-838X
Ekaterina N. Gvazava, PhD student at the Department of Obstetrics and Gynecology, N.V. Sklifosovsky ICM, I.M. Sechenov First Moscow State Medical University,
Ministry of Health of Russia (Sechenov University), 119991, Russia, Moscow, Trubetskaya str., 8, bld. 2, +7(495)609-14-00, https://orcid.org/0000-0001-9062-5351
Tatyana A. Gromova, PhD, Teaching Assistant at the Department of Obstetrics and Gynecology, N.V. Sklifosovsky ICM, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia (Sechenov University), 119991, Russia, Moscow, Trubetskaya str., 8, bld. 2, tgromova928@yandex.ru, https://orcid.org/0000-0001-6104-9842
Ellina G. Petrosyan, Engineer-Researcher at the Laboratory of Membrane and Bioenergetic Systems, Shemyakin–Ovchinnikov Institute of Bioorganic Chemistry,
Russian Academy of Sciences, 117997, Russia, Moscow, Miklukho-Maklaya str., 16/10, +7(495)335-01-00.
Diana V. Mazur, Engineer-Researcher at the Laboratory of Membrane and Bioenergetic Systems, Shemyakin–Ovchinnikov Institute of Bioorganic Chemistry,
Russian Academy of Sciences, 117997, Russia, Moscow, Miklukho-Maklaya str., 16/10, +7(495)335-01-00, dianamazur@yahoo.com,
https://orcid.org/0009-0007-8655-9081
Anastasiya I. Rezekina, Engineer-Researcher at the Laboratory of Membrane and Bioenergetic Systems, Shemyakin–Ovchinnikov Institute of Bioorganic Chemistry,
Russian Academy of Sciences, 117997, Russia, Moscow, Miklukho-Maklaya str., 16/10, +7(495)335-01-00.
Elena A. Gondarenko, Engineer-Researcher at the Laboratory of Membrane and Bioenergetic Systems, Shemyakin–Ovchinnikov Institute of Bioorganic Chemistry,
Russian Academy of Sciences, 117997, Russia, Moscow, Miklukho-Maklaya str., 16/10, +7(495)335-01-00, gondarenkoea@gmail.com,
https://orcid.org/0009-0003-5148-1182
Nadezhda V. Antipova, PhD, Senior Researcher at the Laboratory of Membrane and Bioenergetic Systems, Shemyakin–Ovchinnikov Institute of Bioorganic Chemistry,
Russian Academy of Sciences, 117997, Russia, Moscow, Miklukho-Maklaya str., 16/10, +7(495)335-01-00; National Research University "Higher School of Economics",
101000, Russia, Moscow, Myasnitskaya str., 20, +7(495)771-32-32, https://orcid.org/0000-0002-5799-7767
Corresponding author: Tatyana A. Gromova, tgromova928@yandex.ru



