Expression of SMAD-dependent pathway components in the pathogenesis of hyperplastic processes of the endometrium in benign uterine diseases
Sarkisyan R.M., Gavrilova T.Yu., Asaturova A.V., Adamyan L.V.
Hyperplastic processes of the endometrium, including endometrial polyps and hyperplasia, are among the most common disorders of the female reproductive system. Modern studies on the pathogenesis of hyperplastic changes have focused on the molecular mechanisms that regulate cell proliferation and apoptosis. Among the key signaling pathways, the transforming growth factor (TGF-β) pathway plays a crucial role in regulating cell growth, differentiation, and apoptosis through both SMAD-dependent and SMAD-independent cascades.
Objective: To clarify the pathogenetic aspects of hyperplastic processes of the endometrium in conjunction with benign lesions of the uterine body (leiomyoma of the uterine body and adenomyosis) by studying the expression of components of the SMAD-dependent TGF-β signaling pathway.
Materials and methods: A comprehensive examination was conducted on 90 patients of reproductive age with various conditions: endometrial hyperplasia (group 1, n=15), endometrial polyps (group 2, n=15), endometrial polyps combined with uterine leiomyoma (group 3, n=15), endometrial polyps combined with adenomyosis (group 4, n=15), endometrial hyperplasia combined with uterine leiomyoma (group 5, n=15), and endometrial hyperplasia combined with adenomyosis (group 6, n=15). Clinical, laboratory, and instrumental data, along with the morphological and immunohistochemical characteristics of the endometrium before and after surgical treatment, were assessed.
Results: The expression of TGF-β signaling pathway components was found to be significantly increased in hyperplastic processes of the endometrium, particularly in cases of adenomyosis and uterine leiomyoma. A correlation was established between the expression levels of these pathway components and clinical and morphological characteristics, underscoring the importance of TGF-β in the pathogenesis of hyperplastic processes.
Conclusion: This study demonstrated that the TGF-β signaling pathway, especially its SMAD-dependent cascade, plays a vital role in the pathogenesis of endometrial hyperplastic processes. These findings highlight the potential of TGF-β signaling pathway components as diagnostic markers and emphasize the need for further research to develop effective targeted approaches for the treatment and prevention of the recurrence of endometrial hyperplastic processes.
Authors' contributions: Adamyan L.V., Asaturova A.V., Sarkisyan R.M. – conception and design of the study; Sarkisyan R.M., Gavrilova T.Yu., Asaturova A.V. – data acquisition and processing; Sarkisyan R.M., Asaturova A.V. – statistical analysis; Sarkisyan R.M., Asaturova A.V., Gavrilova T.Yu. – drafting of the manuscript; Adamyan L.V. – editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no finding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Sarkisyan R.M., Gavrilova T.Yu., Asaturova A.V., Adamyan L.V. Expression of SMAD-dependent pathway components in the pathogenesis of hyperplastic processes of the endometrium in benign uterine diseases.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (3): 102-112 (in Russian)
https://dx.doi.org/10.18565/aig.2025.14
Keywords
Endometrial hyperplastic processes, including endometrial polyps (EP) and endometrial hyperplasia (EH), are among the most common pathologies of the female reproductive system and are often detected in women of reproductive age [1]. These conditions not only diminish the quality of life of patients, causing heavy uterine bleeding, intermenstrual spotting, and anemiabut also increase the risk of malignant transformation, particularly in cases of complex hyperplasia. The presence of concomitant benign uterine diseases, such as leiomyoma and adenomyosis, further complicates this situation by disrupting the homeostasis of endometrial tissues and exacerbating pathological processes [2–5]. Recent studies on the pathogenesis of hyperplastic changes have focused on the molecular mechanisms that regulate cell proliferation and apoptosis. Among the key signaling pathways, the transforming growth factor β (TGF-β) signaling pathway plays a crucial role in regulating cell growth, differentiation, and apoptosis through SMAD-dependent and SMAD-independent cascades [6]. Under normal conditions, this pathway exhibits antiproliferative and pro-apoptotic functions; however, pathological conditions can alter its regulatory role, leading to disturbances in cellular homeostasis and proliferative processes within the endometrium [7].
A particularly important aspect of this research is the examination of the expression of components of the SMAD-dependent signaling pathway, including SMAD2, SMAD3, and SMAD4, in the hyperplastic processes of the endometrium. Several studies have indicated that the expression levels of these proteins increase with hyperplastic changes, suggesting a compensatory mechanism in response to pathological proliferation [8, 9]. However, it remains unclear whether this increase in expression is the primary cause or merely a consequence of the activation of other signaling pathways, such as the AKT-dependent pathway or SMAD-independent TGF-β cascades. An additional complicating factor in pathogenesis is the interaction of TGF-β with the estrogen signaling pathway [10]. It is known that the activation of the estrogen receptor through TGF-β can enhance estrogen signaling, creating a feedback loop that normally promotes self-regulation. However, in instances where endometrial hyperplastic processes coexist with leiomyoma or adenomyosis, this mechanism may be disrupted, leading to further destabilization of proliferative processes and an increase in pathological changes.
Thus, this study aimed to investigate the expression of components of the SMAD-dependent TGF-β signaling pathway in endometrial hyperplastic processes, both in isolation and in conjunction with benign uterine diseases. This study encompasses a comprehensive analysis of molecular markers and their correlation with the clinical and morphological features of the disease, thereby facilitating the identification of the underlying mechanisms of pathogenesis. The study findings will enhance the understanding of the role of the TGF-β signaling pathway in regulating hyperplastic processes and aid in developing targeted therapeutic strategies to improve women's reproductive health.
Materials and methods
A comprehensive examination of 90 patients with endometrial hyperplastic processes was conducted at the Department of Gynecology at V.I. Kulakov NMRC for OG&P of the Ministry of Health of the Russian Federation. The patients were divided into the following groups: EP group (group 1, n=15), EH group (group 2, n=15), EP with adenomyosis (group 3, n=15), EP with myoma (group 4, n=15), EH with adenomyosis (group 5, n=15), and EH with myoma (group 6, n=15). Preoperative counseling included collection of medical history, complaints, and assessment of clinical, laboratory, and instrumental data, followed by intraoperative evaluation of the histological material. This evaluation was conducted in the 1st Department of Anatomical Pathology at V.I. Kulakov NMRC for OG&P using standard methods combined with immunohistochemical studies. A postoperative assessment of the endometrium was performed using instrumental methods and histological and immunohistochemical studies to evaluate the expression of TGF-β, SMAD2, SMAD3, and SMAD4.
The inclusion criteria were as follows: reproductive age between 18 and 45 years; the presence of endometrial hyperplastic processes (EP or EH) identified during ultrasound, confirmed intraoperatively, and verified through pathological examination; the presence of uterine fibroids or adenomyosis detected via gynecological and ultrasound examination confirmed intraoperatively and verified during pathological examination; and informed consent to participate in the study.
The exclusion criteria included malignant neoplasms of any localization, hormonal therapy within six months before surgery, inflammatory diseases of the pelvic organs in the acute stage, severe somatic pathology, and the use of antibacterial drugs one month prior to the study.
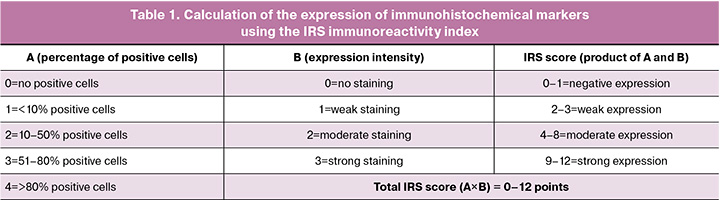
Immunohistochemical markers were assessed using a semi-quantitative Immunoreactive index (the (immunoreactivity index IRS) score (Table 1).
Statistical analysis
Statistical processing of the data was performed using Microsoft Office Excel 2015 and MedCalc version 12. The normality of the distribution was assessed using the Kolmogorov–Smirnov test. Continuous variables with a normal distribution were described using arithmetic mean (M) and standard deviation (SD), expressed as M (SD). For continuous variables lacking a normal distribution, the median (Me) and interquartile range (Q1, Q3) were used and expressed as Me (Q1, Q3). Categorical variables were described using counts and percentages. When comparing more than two groups, the Bonferroni correction for multiple pairwise comparisons was applied. The traditional level of type I error (p<0.05) was divided by the number of pairwise comparisons according to the formula m=n(n-1)/2, where n is the number of groups. For the six study groups, the number of comparisons was 15; therefore, the new critical significance level was set at p<0.0033. Kruskal–Wallis test and Spearman correlation analysis were employed to assess the relationship between the expression of TGF-β signaling pathway components. The values of the correlation coefficients were interpreted according to the Chaddock scale: 0–0.3 – weak, 0.3–0.5 – moderate, 0.5–0.7 – noticeable; 0.7–0.9 – high, 0.9–1.0 – very high.
Results
Patients in the study groups did not differ significantly in age, which averaged 35.1 (5.5) years. Obesity was found in 18/90 (20%) of the women. We found no statistically significant differences in the onset of menarche, duration of the menstrual cycle, or duration of menstruation (Table 2).
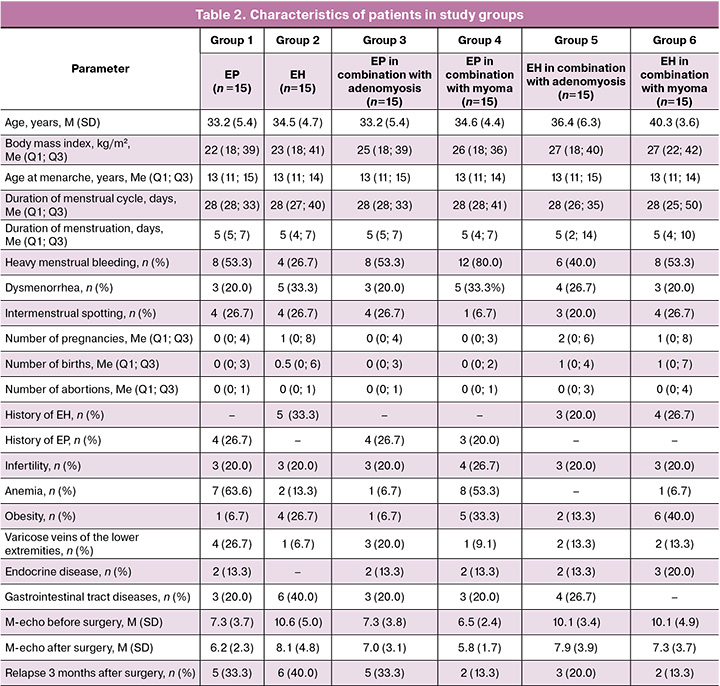
The main complaint of the patients was heavy menstrual bleeding: 8/15 (53.3%) in groups 1, 3, and 6, 4/15 (26.7%) in group 2, 12/15 (80%) in group 4, and 6/15 (40.0%) in group 5. Dysmenorrhea was noted in 5/15 (33.3%) in groups 2 and 4, 3/15 (20.0%) in groups 1, 3, and 6, and 4/15 (26.7%) in group 5. In addition, patients more frequently complained of intermenstrual spotting and anemia (Table 2).
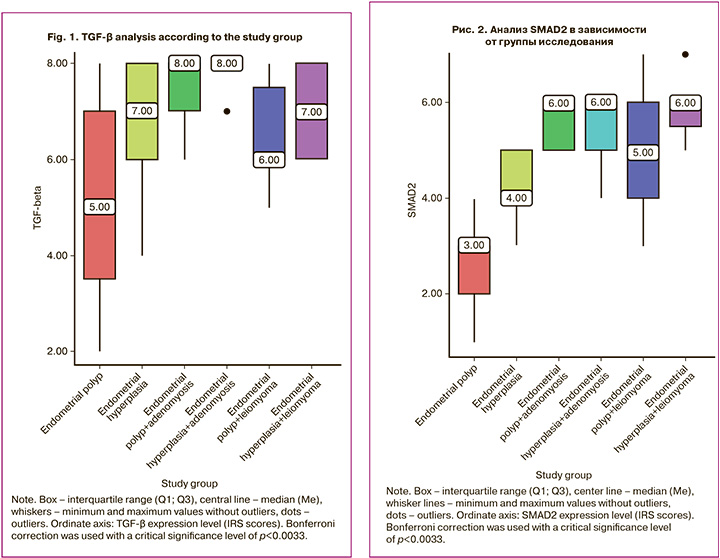
According to the immunohistochemical study, TGF-β expression was 5.00 (3.5; 7.0) in group 1, 7.00 (6.0; 8.0) in group 2, 8.0 (7.0; 8.0) in group 3, 6.0 (6.0; 7.5) in group 4, 8.0 (8.0; 8.0) in group 5, 7.0 (6.0; 8.0) in group 6 (the variables are expressed as the Me (Q1; Q3)). Statistically significant differences were found between groups 1 and 3 (p=0.001), groups 5 and 1 (p=0.001), and groups 4 and 5 (p=0.034) (Kruskal–Wallis test) (Fig. 1).
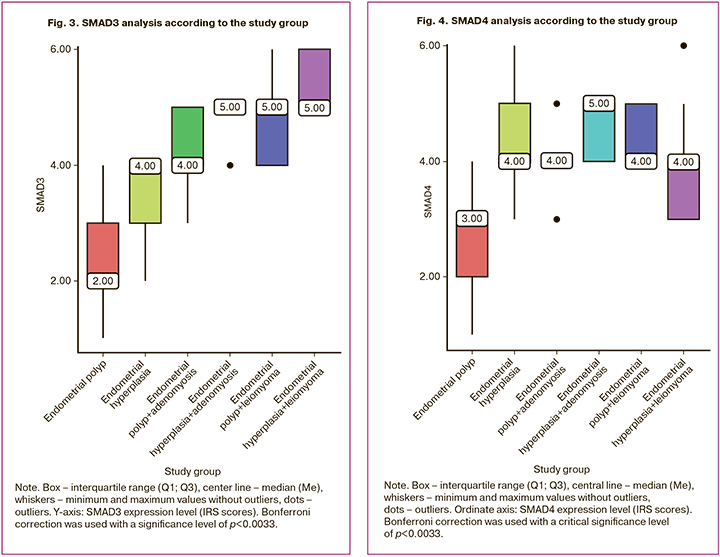
According to the immunohistochemical study, SMAD2 expression was 3.0 (2.0; 3.0) points in group 1, 4.0 (4.0; 5.0) points in group 2, 6.0 (5.0; 6.0) points in group 3, 5.0 (4.0; 6.0) points in group 4, 6.0 (5.0; 6.0) points in group 5, 6.0 (5.5; 6.0) points in group 6 (the variables are presented as Me (Q1; Q3)). Statistically significant differences were found between groups 1 and 3 (p=0.001), groups 5 and 1 (p=0.001), groups 4 and 1 (p=0.001), groups 6 and 1 (p=0.001), groups 3 and 2 (p=0.025), groups 5 and 2 (p=0.011), and between groups 6 and 2 (p=0.001) (Kruskal–Wallis test) (Fig. 2).
According to immunohistochemical study, SMAD3 expression was 2.0 (2.0; 3.0) points in group 1, 4.0 (3.0; 4.0) points in group 2, 4.0 (4.0; 5.0) points in group 3, 5.0 (4.0; 5.0) points in group 4, 5.0 (5.0; 5.0) points in group 5, 5.0 (5.0; 6.0) points in group 6 (where the variables are expressed as Me (Q1; Q3)). Statistically significant differences were found between groups 1 and 3 (p=0.001), groups 5 and 1 (p=0.001), groups 4 and 1 (p=0.001), groups 6 and 1 (p=0.001), groups 5 and 2 (p=0.001), groups 4 and 2 (p=0.006), groups 6 and 2 (p=0.001), and groups 6 and 3 (p=0.029) (Kruskal–Wallis test) (Fig. 3).
According to the immunohistochemical study, SMAD4 expression was 3.0 (2.0; 3.0) points in group 1, 4.0 (4.0; 5.0) points in group 2, 4.0 (4.0; 4.0) points in group 3, 4.0 (4.0; 5.0) points in group 4, 5.0 (4.0; 5.0) points in group 5, 5.0 (3.0; 4.0) points in group 6 (the indicators are presented as Me (Q1; Q3)). A statistically significant difference was found between groups 1 and 2 (p=0.001), groups 3 and 1 (p=0.002), groups 5 and 1 (p=0.001), groups 4 and 1 (p=0.001), groups 6 and 1 (p=0.011) (Kruskal–Wallis test) (Fig. 4).
A significant correlation was found between the expression of TGF-β and SMAD2, and a moderate correlation was found between the expression of TGF-β and SMAD3, as well as between the expression of TGF-β and SMAD4 (Table 3). A high correlation was found between the expression of SMAD2 and SMAD3, and a moderate correlation was found between the expression of SMAD2 and SMAD4 and between SMAD3 and SMAD4 (the strength of the correlation was assessed using the Chaddok scale).
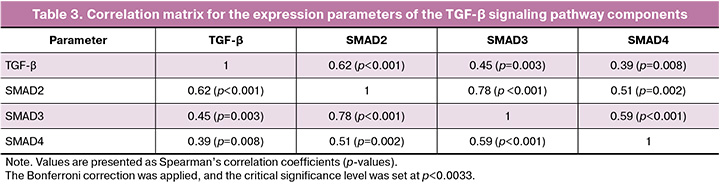
To illustrate the obtained results, micrographs of the expression of the studied markers in EH alone and in combination with adenomyosis are shown (Fig. 5).
Discussion
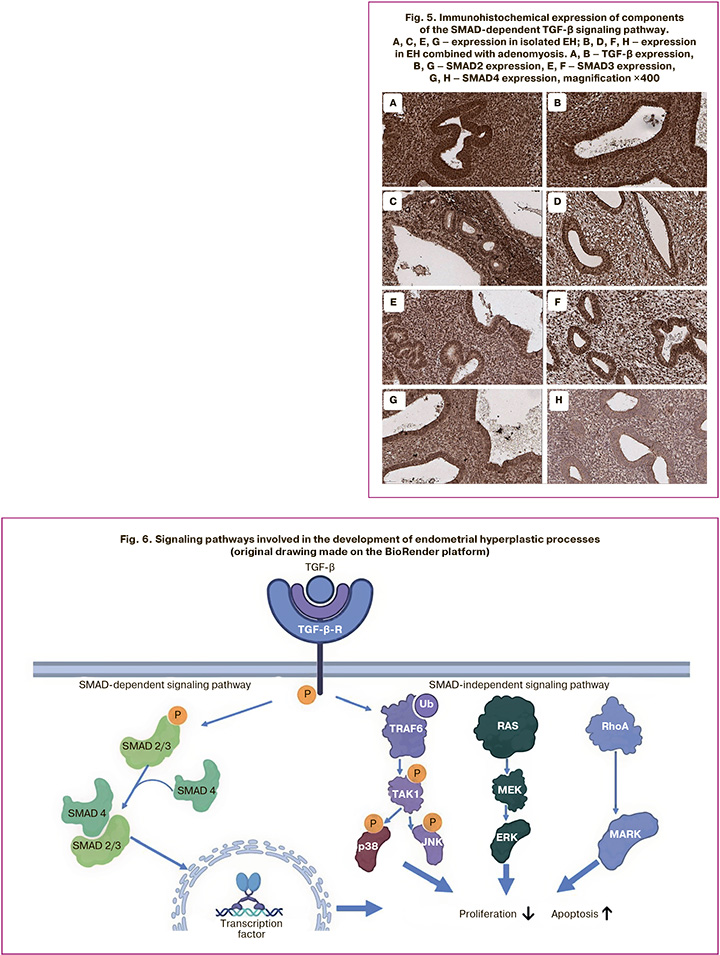
The role of the regulation of signaling pathway components in the development of endometrial hyperplastic processes has been extensively studied over the past 20 years. Notably, the AKT-dependent signaling pathway has garnered significant attention, as it has been identified as a central pathway in the pathogenesis of EH, the immediate precursor of endometrioid adenocarcinoma of the uterine mucosa [11–13]. However, subsequent research has revealed that other signaling pathways also contribute to the development of endometrial hyperplastic processes, with the TGF-β-dependent signaling pathway being particularly important (Fig. 6).
TGF-β-dependent signaling pathway can be divided into two main branches: SMAD-dependent and SMAD-independent signaling pathways. The latter group includes key proteins from the JNK, ERK, and MAPK families. Although the role of SMAD-independent pathways has been well studied [14–16], there is limited understanding of the regulation of the SMAD-dependent pathway in the pathogenesis of endometrial hyperplastic processes. This knowledge gap motivated our study to examine the expression of components of the SMAD-dependent signaling pathway. This study not only aimed to clarify their role in the formation of EP and EH, but also to compare the degree of activation of the SMAD-dependent signaling pathway in combined benign uterine diseases, such as adenomyosis and leiomyoma.
Considering the pathogenesis of endometrial hyperplastic processes, it is crucial to recognize that the uterine mucosa is subjected to cyclic influences from steroid hormones, estrogen, and progesterone, and can undergo numerous regeneration cycles throughout a woman's reproductive life. This regenerative potential is facilitated by the presence of unique stem and progenitor cells dispersed throughout the uterine mucosa, which aids in rapid recovery following menstruation [17, 18]. The inhibition of the TGF-β signaling pathway plays a vital role in sustaining the proliferation and regeneration of endometrial mesenchymal stem cells and endometrial epithelial organoids, highlighting the critical function of the TGF-β signaling pathway in endometrial regeneration and restoration [19, 20]. However, the mechanisms regulated by the TGF-β signaling pathway in the endometrium remain poorly understood.
The TGF-β signaling pathway encompasses various secreted ligands, inhibitors, cell surface receptor kinases, and the transcription factors SMAD2/SMAD3 and SMAD4, which are activated through phosphorylation and subsequently translocated to the nucleus to regulate gene expression (Fig. 6). This signaling pathway governs essential developmental processes, including cell migration, differentiation, and proliferation, and is often dysregulated in various cancer subtypes. Mutations in TGF-β pathway components have been identified across different tumor types and are correlated with genes associated with metastasis and reduced patient survival [21]. In the female reproductive system, TGF-β plays a crucial role in maintaining the integrity and development of the uterus and ovaries, controlling processes such as endometrial receptivity, decidualization, and placentation during early pregnancy and throughout gestation [22, 23]. Additionally, components of the TGF-β signaling pathway function as tumor suppressors. Mouse models have demonstrated that conditional deletion of the TGF-β receptor ALK5 or the transcription factors SMAD2 and SMAD3 in the endometrial epithelium and stroma results in an aggressive metastatic form of endometrial cancer [24, 25]. Furthermore, mouse models of endometrial cancer with conditional inactivation of the PTEN gene, as well as the ARID1A gene, exhibit abnormal TGF-β signaling, further confirming that TGF-β pathway inactivation contributes to the metastatic potential of endometrial tumors.
Our study demonstrated that the expression of TGF-β was significantly different in the endometrium of patients with isolated EP, in combination with adenomyosis, and in the endometrium of patients with EH and adenomyosis. From these results, it can be concluded that adenomyosis affects the level of TGF-β expression in both EH and EP. It can also be concluded that TGF-β expression was higher in EH than in EP; however, a statistically significant difference was found only in the groups of patients with EP and EH in combination with adenomyosis. As already noted, among the published studies, there are few observations of TGF-β expression in hyperplastic changes of the endometrium. However, in a study by Gold L.I. et al. A statistically significant progressive increase in immunoreactivity was found for all three TGF-β isoforms in endometrial glands from the normal proliferative state to simple hyperplasia, and from simple hyperplasia to complex hyperplasia. The most noticeable increase was observed when comparing normal proliferative endometrium with complex hyperplasia; the increases were 5.1-, 3.4-, and 2.6-fold for TGF-β1, TGF-β2, and TGF-β3, respectively. However, no further increase was observed during the progression from preneoplastic complex hyperplasia to endometrial carcinoma [8]. In a study by Faraji A. et al., TGF-β expression in the polyp area was significantly lower than that in the unchanged endometrium, but the authors did not report data on the expression of the factor in the adjacent endometrium, which does not allow a comparative analysis of the results of this study with our results [9]. In addition, TGF-β expression in the endometrium of patients with uterine leiomyoma and adenomyosis was studied. In a study by Inagaki N. et al., TGF-β expression was higher in both leiomyomas and adenomyoses than in the control group [26]. In studies by Kishi Y. et al. and Jacobo A. et al., no difference was found in TGF-β expression in ectopic adenomyosis foci and eutopic endometrium of the control group, but the eutopic endometrium of patients with adenomyosis has not been studied [27, 28].
Regarding the study of combined benign lesions of the uterine body and TGF-β expression, we did not find such data in the available literature.
We also studied the expression of components of the SMAD-dependent pathway (SMAD2, SMAD3, and SMAD4). These interconnected components work synergistically to implement proapoptotic and antiproliferative potential. We found an increase in the expression of these proteins in the group of patients with isolated EH compared with the isolated EP group, as well as when comparing the expression in the EP group combined with adenomyosis, compared with isolated polyps, and when comparing the EH group combined with adenomyosis, compared with isolated EH. These proteins have been studied in EH patients. Liu et al. studied the expression of SMAD4 in EH and the development of endometrial carcinoma. A positive expression was detected in all EH cases [29]. Although SMAD family proteins have been actively studied in adenomyosis, no emphasis has been placed on the eutopic endometrium, although increased expression has been reported in adenomyosis foci compared with the control group [27, 30].
In patients with uterine leiomyoma, the expression of SMAD2/3/4 proteins has been studied mainly in the tumor tissue itself. It was shown to be higher than in the normal myometrium, and it was noted that the SMAD-independent pathway of activation of the TGF-β signaling pathway also plays a role in the development of smooth muscle tumors of the uterine body [31, 32]. We also did not find any studies devoted to the combination of benign lesions of the uterine body and expression of proteins of the SMAD family. Thus, in our study, an increase in the components of the TGF-β signaling pathway (namely, its SMAD-dependent cascade, since we studied the expression of the TGF-β protein itself, as well as the SMAD2, SMAD3, and SMAD4 proteins) was observed in various pathological processes of the endometrium. Normally, this pathway performs important functions such as suppression of cell division, induction of apoptosis, and regulation of immune processes. In our study, we have shown that the development of endometrial hyperplastic processes enhances the expression of the components of this pathway, and concomitant benign lesions of the uterine body aggravate this regulatory mechanism, further increasing the expression of both TGF-β and the components of the signaling pathway located lower in the hierarchy of proteins of the SMAD family. Since the function of the TGF-β signaling pathway is antiproliferative and proapoptotic, one would expect a greater decrease in the expression of one or more components of this signaling pathway if its implementation was the main factor in the pathogenesis of endometrial hyperplastic processes and/or benign lesions of the uterine body, since all these conditions are somehow associated with increased proliferation and decreased apoptosis of cellular elements. Nevertheless, in the endometrium of patients with EP and EH, both isolated and in combination with adenomyosis and leiomyoma, we observed an increase in the expression of TGF-β and SMAD2/3/4, although in some cases this was only a trend, and in others, statistically significant differences between the groups. In our opinion, this is due to the fact that the main initiating mechanism of the pathogenesis of endometrial hyperplastic processes is associated with the implementation of SMAD-independent signaling pathways, as well as with the AKT-dependent signaling pathway, the implementation of which generally occurs in parallel with TGF-β regulated signaling pathways. Therefore, the increase in the expression of components of the TGF-β signaling pathway revealed in our study is most likely compensatory, responding to increased proliferation and decreased apoptosis under the influence of parallel signaling pathways. This assumption is supported by studies focusing on precancerous and malignant lesions of the uterine mucosa, which did not show a decrease in the expression of TGF-β signaling pathway components [33, 34]. Numerous studies on the disruption of SMAD-independent signaling pathways have also indirectly confirmed this, as they lead to an imbalance between proliferation and apoptosis.
Another possible mechanism regulating the TGF-β signaling pathway is its connection with estrogen. It has been shown that activation of the estrogen receptor (ER) through the TGF-β signaling pathway can create a feedback mechanism, where TGF-β enhances the estrogen signaling pathway, leading to its faster and more effective inhibition. When the TGF-β signaling pathway is suppressed, ER activity returns to normal levels [35]. However, we did not observe such an inhibition in our study. It is likely that the combination of hyperplastic processes in the endometrium and benign lesions of the uterine body disrupts the feedback mechanism, which leads to the suppression of TGF-β expression owing to the activation of other signaling pathways and the presence of external estrogen-dependent tissues (such as smooth muscle tumors and foci of adenomyosis). This situation prevents the achievement of a balance in regulating proliferative processes.
Conclusion
The results of this study demonstrate the key role of the TGF-β signaling pathway, particularly its SMAD-dependent cascade, in the pathogenesis of hyperplastic processes in the endometrium, such as polyps and hyperplasia, including their association with benign diseases of the uterine body (adenomyosis and leiomyoma). The increased expression of TGF-β and SMAD components (SMAD2, SMAD3, and SMAD4) in these conditions indicates compensatory activation of the signaling pathway in response to increased cell proliferation and impaired apoptosis. These findings underscore the complexity of regulatory mechanisms in the endometrium and the significance of the interaction between the TGF-β pathway and other molecular processes, including estrogen-dependent signaling pathway. These results support the feasibility of further investigation of TGF-β pathway components as potential diagnostic markers and therapeutic targets for the targeted treatment of endometrial hyperplastic processes. An integrated approach, including the immunohistochemical study of key markers, not only enhances diagnostics but also reduces the risk of relapse, representing an important step towards improving the quality of life for women and maintaining their reproductive health.
References
- Адамян Л.В., ред. Сочетанные доброкачественные опухоли и гиперпластические процессы матки (миома, аденомиоз, гиперплазия эндометрия). Клинические рекомендации по ведению больных. М.; 2015. [Adamyan L.V., ed. Combined benign tumors and hyperplastic processes of the uterus (fibroids, adenomyosis, endometrial hyperplasia). Clinical guidelines for patient management. Moscow; 2015. (in Russian)].
- Собивчак М.С., Протасова А.Э., Раскин Г.А., Кавун А.М. Клинико-морфологические особенности гиперпластических процессов эндометрия у пациенток разных возрастных групп. Онкогинекология. 2021; 4: 27-34. [Sobivchyak M.S., Protasova A.E., Raskin G.A., Kavun A.M. Clinical and morphological features of hyperplastic processes of the endometrium in patients of different age groups. Oncogynecology. 2021; (4): 27-34. (in Russian)]. https://dx.doi.org/10.52313/22278710_2021_4_27.
- Думановская М.Р., Чернуха Г.Е., Табеева Г.И., Асатурова А.В. Гиперплазия эндометрия: поиск оптимальных решений и стратегий. Акушерство и гинекология. 2021; 4: 23-31. [Dumanovskaya M.R., Chernukha G.E., Tabeeva G.I., Asaturova A.V. Endometrial hyperplasia: search for optimal solutions and strategies. Obstetrics and Gynecology. 2021; (4): 23-31 (in Russian)]. https://dx.doi.org/10.18565/aig.2021.4.23-31.
- Liu J., Liang Y., Ouyang J., Yang S. Analysis of risk factors and model establishment of recurrence after endometrial polypectomy. Ann. Palliat. Med. 2021; 10(11): 11628-34. https://dx.doi.org/10.21037/apm-21-2747.
- Ciscato A., Zare S.Y., Fadare O. The significance of recurrence in endometrial polyps: a clinicopathologic analysis. Hum. Pathol. 2020; 100: 38-44. https://dx.doi.org/10.1016/j.humpath.2020.03.005.
- Eritja N., Felip I., Dosil M.A., Vigezzi L., Mirantes C., Yeramian A. et al. A Smad3-PTEN regulatory loop controls proliferation and apoptotic responses to TGF-β in mouse endometrium. Cell. Death Differ. 2017; 24(8): 1443-58. https://dx.doi.org/10.1038/cdd.2017.73.
- Aashaq S., Batool A., Mir S.A., Beigh M.A., Andrabi K.I., Shah Z.A. TGF-β signaling: A recap of SMAD-independent and SMAD-dependent pathways. J. Cell Physiol. 2022; 237(1): 59-85. https://dx.doi.org/10.1002/jcp.30529.
- Gold L.I., Saxena B., Mittal K.R., Marmor M., Goswami S., Nactigal L. et al. Increased expression of transforming growth factor beta isoforms and basic fibroblast growth factor in complex hyperplasia and adenocarcinoma of the endometrium: evidence for paracrine and autocrine action. Cancer Res. 1994; 54(9): 2347-58.
- Faraji A., Shamsadinimoghadam R., Jahromi M.A., Namazi N. TGF-β1 role in uterine leiomyoma and endometrial polyp: an insight to drug-based treatment instead of surgical techniques. Obstet. Gynecol. Sci. 2021; 64(1): 107-13. https://dx.doi.org/10.5468/ogs.20191.
- Ito I., Hanyu A., Wayama M., Goto N., Katsuno Y., Kawasaki S. et al. Estrogen inhibits transforming growth factor beta signaling by promoting Smad2/3 degradation. J. Biol. Chem. 2010; 285(19): 14747-55. https://dx.doi.org/10.1074/jbc.M109.093039.
- Wilson M.R., Reske J.J., Holladay J., Wilber G.E., Rhodes M., Koeman J. et al. ARID1A and PI3-kinase pathway mutations in the endometrium drive epithelial transdifferentiation and collective invasion. Nat. Commun. 2019; 10(1): 3554. https://dx.doi.org/10.1038/s41467-019-11403-6.
- Pavlidou A., Vlahos N.F. Molecular alterations of PI3K/Akt/mTOR pathway: a therapeutic target in endometrial cancer. ScientificWorldJournal. 2014; 2014: 709736. https://dx.doi.org/10.1155/2014/709736.
- Кондриков Н.И., Силакова А.В. Гиперпластические изменения и предраковые состояния эндометрия: вопросы терминологии и классификации. Архив патологии. 2010; 1: 60-2. [Kondrikov N.I., Silakova A.V. Hyperplastic changes and precancerous endometrial: issues of terminology and classification. Arkhiv Pathologii. 2010; 1: 60-2. (in Russian)].
- Mohamed M.Z., Baky M.A.E., Hassan O.A., Mohammed H.H., Abdel-Aziz A.M. PTEN/PI3K/VEGF signaling pathway involved in the protective effect of xanthine oxidase inhibitor febuxostat against endometrial hyperplasia in rats. Hum. Exp. Toxicol. 2020; 39(9): 1224-34. https://dx.doi.org/10.1177/0960327120914977
- Supriya A., Kiran A.V.V.V.R., Krishnamurthy P.T. Adipokine modulation in endometrial hyperplasia by polyunsaturated fatty acids. Journal of Pharmacology and Pharmacotherapeutics. 2024; 15(3): 237-52. https://dx.doi.org/10.1177/0976500X241259578.
- Zhao J., Hu Y., Zhao Y., Chen D., Fang T., Ding M. Risk factors of endometrial cancer in patients with endometrial hyperplasia: implication for clinical treatments. BMC Womens Health. 2021; 21(1): 312. https://dx.doi.org/10.1186/s12905-021-01452-9.
- Cousins F.L., Filby C.E., Gargett C.E. Endometrial stem/progenitor cells-their role in endometrial repair and regeneration. Front. Reprod. Health. 2022; 3: 811537. https://dx.doi.org/10.3389/frph.2021.81153.
- Tempest N., Maclean A., Hapangama D.K. Endometrial stem cell markers: current concepts and unresolved questions. Int. J. Mol. Sci. 2018; 19(10): 3240. https://dx.doi.org/10.3390/ijms19103240.
- Gurung S., Ulrich D., Sturm M., Rosamilia A., Werkmeister J.A., Gargett C.E. Comparing the effect of TGF-β receptor inhibition on human perivascular mesenchymal stromal cells derived from endometrium, bone marrow and adipose tissues. J. Pers. Med. 2020; 10(4): 261. https://dx.doi.org/10.3390/jpm10040261.
- Lucciola R., Vrljicak P., Gurung S., Filby C., Darzi S., Muter J. et al. Impact of sustained transforming growth factor-β receptor inhibition on chromatin accessibility and gene expression in cultured human endometrial MSC. Front. Cell. Dev. Biol. 2020; 8: 567610. https://dx.doi.org/10.3389/fcell.2020.567610.
- Korkut A., Zaidi S., Kanchi R.S., Rao S., Gough N.R., Schultz A. et al.; Cancer Genome Atlas Research Network; Weinstein J.N., Mishra L., Akbani R. A pan-cancer analysis reveals high-frequency genetic alterations in mediators of signaling by the TGF-β superfamily. Cell. Syst. 2018; 7(4): 422-437.e7. https://dx.doi.org/10.1016/j.cels.2018.08.010.
- Akimoto Y., Fujii W., Naito K., Sugiura K. The effect of ACVR1B/TGFBR1/ACVR1C signaling inhibition on oocyte and granulosa cell development during in vitro growth culture. J. Reprod. Dev. 2023; 69(5): 270-8. https://dx.doi.org/10.1262/jrd.2023-041.
- Li Q., Agno J.E., Edson M.A., Nagaraja A.K., Nagashima T., Matzuk M.M. Transforming growth factor β receptor type 1 is essential for female reproductive tract integrity and function. PLoS Genet. 2011; 7(10): e1002320. https://dx.doi.org/10.1371/journal.pgen.1002320.
- Soyal S.M., Mukherjee A., Lee K.Y., Li J., Li H., DeMayo F.J. et al. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis. 2005; 41(2): 58-66. https://dx.doi.org/10.1002/gene.20098.
- Kriseman M., Monsivais D., Agno J., Masand R.P., Creighton C.J., Matzuk M.M. Uterine double-conditional inactivation of Smad2 and Smad3 in mice causes endometrial dysregulation, infertility, and uterine cancer. Proc. Natl. Acad. Sci. U. S. A. 2019; 116(9): 3873-82. https://dx.doi.org/10.1073/pnas.1806862116.
- Inagaki N., Ung L., Otani T., Wilkinson D., Lopata A. Uterine cavity matrix metalloproteinases and cytokines in patients with leiomyoma, adenomyosis or endometrial polyp. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003; 111(2): 197-203. https://dx.doi.org/10.1016/s0301-2115(03)00244-6.
- Kishi Y., Shimada K., Fujii T., Uchiyama T., Yoshimoto C., Konishi N. et al. Phenotypic characterization of adenomyosis occurring at the inner and outer myometrium. PLoS One. 2017; 12(12): e0189522. https://dx.doi.org/10.1371/journal.pone.0189522.
- Jacobo A., Borges R.F., de Souza C.A.B., Genro V.K., Cunha-Filho J.S. Transforming growth factor beta-1 (TGF-β1) expression in patients with adenomyosis. Rev. Bras. Ginecol. Obstet. 2024; 46: e-rbgo31. https://dx.doi.org/10.61622/rbgo/2024rbgo31.
- Liu F.S., Chen J.T., Hsieh Y.T., Ho E.S., Hung M.J., Lu C.H. et al. Loss of Smad4 protein expression occurs infrequently in endometrial carcinomas. Int. J. Gynecol. Pathol. 2003; 22(4): 347-52. https://dx.doi.org/10.1097/01.pgp.0000092131.88121.0a.
- Richards E.G., El-Nasharb S.A., Schoolmeester J.K., Hopkins M.R., Famuyide A.O., Daftary G.S. Adenomyosis is associated with diminished endometrial expression of bone morphogenetic proteins BMPR1B and SMAD4. Fertil. Steril. 2016; 106(3): e211. https://dx.doi.org/10.1016/j.fertnstert.2016.07.608.
- Salama S.A., Diaz-Arrastia C.R., Kilic G.S., Kamel M.W. 2-Methoxyestradiol causes functional repression of transforming growth factor β3 signaling by ameliorating Smad and non-Smad signaling pathways in immortalized uterine fibroid cells. Fertil. Steril. 2012; 98(1): 178-84. https://dx.doi.org/10.1016/j.fertnstert.2012.04.002.
- Chegini N., Luo X., Ding L., Ripley D. The expression of Smads and transforming growth factor beta receptors in leiomyoma and myometrium and the effect of gonadotropin releasing hormone analogue therapy. Mol. Cell. Endocrinol. 2003; 209: 9-16. https://dx.doi.org/10.1016/j.mce.2003.08.007.
- Dai J.L., Schutte M., Bansal R.K., Wilentz R.E., Sugar A.Y., Kern S.E. Transforming growth factor-beta responsiveness in DPC4/SMAD4-null cancer cells. Mol. Carcinog. 1999; 26(1): 37-43. https://dx.doi.org/10.1002/(sici)1098-2744(199909)26:1<37::aid-mc5>3.0.co;2-6.
- Hocevar B.A., Brown T.L., Howe P.H. TGF-beta induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway. EMBO J. 1999; 18(5): 1345-56. https://dx.doi.org/10.1093/emboj/18.5.1345.
- Matsuda T., Yamamoto T., Muraguchi A., Saatcioglu F. Cross-talk between transforming growth factor-beta and estrogen receptor signaling through Smad3. J. Biol. Chem. 2001; 276(46): 42908-14. https://dx.doi.org/10.1074/jbc.M105316200.
Received 27.01.2025
Accepted 26.02.2025
About the Authors
Rita M. Sarkisyan, PhD student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, ritamisakovna@mail.ru, https://orcid.org/0000-0002-4097-5537Tatyana Yu. Gavrilova, Dr. Med. SCi., obstetrician-gynecologist at the Gynecological Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, t_gavrilova@oparina4.ru, https://orcid.org/0000-0001-7424-4292
Alexandra V. Asaturova, Dr. Med. Sci., Head of the 1st Pathological Anatomical Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, a.asaturova@gmail.com, https://orcid.org/0000-0001-8739-5209
Leila V. Adamyan, Academician of the Russian Academy of Sciences, Dr. Med. Sci., Professor, Deputy Director for Research, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4; Chief Specialist in Gynecology
of the Ministry of Health of Russia; Head of the Department of Reproductive Medicine and Surgery of the Faculty of Postgraduate Education, Russian University of Medicine, Ministry of Health of Russia, adamyanleila@gmail.com, https://orcid.org/0000-0002-3253-4512
Corresponding author: Rita M. Sarkisyan, ritamisakovna@mail.ru



