Manopausal hormonal therapy and clinical and morphological features of endometrial cancer in women with metabolic disorders
Objective: To study clinical and morphological features of endometrial cancer in postmenopausal women with obesity (BMI≥30 kg/m2) depending on the use of menopausal hormone therapy (MHT).Klyukina L.A., Sosnova E.A., Ischenko A.A.
Materials and methods: A prospective study included 214 postmenopausal patients with verified endometrial cancer. The patients underwent surgical treatment in the Department of Gynecology of the Medical and Rehabilitation Center, Ministry of Health of Russia and in the Department of Oncogynecology in the University Clinical Hospital No. 4 of I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia, in the period 2019–2021. The stage of uterine cancer was diagnosed in accordance with TNM classification (UICC, the 8th edition, 2016) and international FIGO classification (2009). Endometrial cancer (EC) was differentiated by grades: G1 – well differentiated; G2 – moderately differentiated; G3 – poorly differentiated cancer. The main group included 140 postmenopausal women with BMI≥30 kg/m2; 58 patients took hormonal therapy (HT). The comparison group comprised 74 women without cancer with BMI 17.6–24.9 kg/m2, including 20 patients receiving HT. All patients used estrogen/gestagen combination preparations for 12 months and longer.
Results: Endometrial cancer with myometrial invasion less than 50% (Т1а according to TNM classification (UICC, the 8th edition, 2016) was found significantly more often in the subgroup of women with normal body weight, while later stages of EC with advanced invasion were significantly more often in the main group of women with obesity (p=0.012). Assessment of differentiation grades also demonstrated significant differences: in the comparison group the rate of well differentiated EC (G1) was significantly higher compared with the main group, where moderately differentiated EC was observed more often (p=0.001). The comparative analysis of the studied parameters in the subgroups of women who did not take HT, showed no significant differences (р=0.383; р=0.745).
Conclusion: The study demonstrated that in the subgroup of patients with obesity, who used HT, the incidence of later stages of moderately and poorly differentiated (G2–G3) EC was significantly higher. The results of the study proved importance of BMI monitoring and timely correction of hormonal-metabolic disorders in menopausal women prior to HT, aimed both to reduce EC rates, and to minimize the risk of development of later stages of moderately and poorly differentitated EC.
Keywords
Malignant tumors of the female reproductive system are a relevant subject for discussion due to the annual increase in the incidence of these diseases and the high mortality rate. According to the International Agency for Research on Cancer (IARC) GLOBOCAN, the global resource of cancer epidemiology, in 2020 a total of 7,848,742 cases of cancer of various localizations were registered worldwide among women over 45 years old; endometrial carcinoma accounted for 4.9% (381,452 cases), and in the Russian Federation the number of this type of cancer reached 533,762 cases (5.3%), while the higher incidence was noted only for breast cancer. The incidence of endometrial carcinoma (EC) in the group of women older than 45 years shows a dramatic 3-fold increase with the highest rate in women at the age of 55-65. The fact that more than 50% of postmenopausal women have excessive body weight contributes to the alert for cancer in this age group [1].
EC is a hormone-dependent tumor, and the most common morphological type of EC is endometrioid adenocarcinoma, which is diagnosed in 80–85% of cases [2]. There are two pathogenetic types of EC [3].
Type 1 tumors are more common and develop in women with signs of persistent hyperestrogenism and endometrial hyperplasia; patients with Type 1 tumors tend to have obesity, diabetes mellitus, hypertension, estrogen-producing ovarian tumors, polycystic ovary syndrome.
Type 2 tumors are usually poorly differentiated, have high risk of poor prognosis, are more common for older women with endometrial atrophy and with no signs of hyperestrogenism.
Only about 5% of cases of EC are caused by hereditary diseases, in particular, by Lynch syndrome [4]. There are also a number of factors that are associated with a high risk of EC: hyperestrogenism, early menarche, nulliparity, late menopause, age over 55 years, long-term tamoxifen therapy, menopausal hormone therapy (MHT) with progestogen intake less than 12–14 days, and a family history of EC [5–7].
Obesity is strongly associated with an increased risk of EC; therefore, adipose tissue should be considered as a complex, multifactorial component of oncogenesis. Adipose tissue functions as an endocrine organ. Thus, obesity leads to impaired secretion of inflammatory cytokines, adipocytes, dysfunction and infiltration of immune cells, inducing deoxyribonucleic acid (DNA) damage, angiogenesis, cell proliferation, and mutagenesis [8]. According to a meta-analysis by the American Institute for Cancer Research, for every increase of five BMI units, there is a 50% increase in the risk of developing endometrioid adenocarcinoma [9]. A significant increase in risk was also stated by Bianchini F. et al.: the authors revealed a linear increase in the risk of EC by 200–400% in women with BMI over 25 kg/m2 [10].
However, long-term estrogen overexposure can be not only endogenous, like in women with obesity, but also exogenous, for example, like in women taking hormone therapy during menopause. The climax is manifested by a gradual restructuring of the body functioning while the ovarian activity decreases and then finally stops. About 85% of women during the menopause have specific complaints which require the prescription of MHT.
Thus, the high incidence of obesity among postmenopausal women and common use of MHT make it especially relevant to study the clinical and morphological features of EC in women of this age group.
The aim of this study was to determine clinical and morphological features of the endometrioid type of EC in postmenopausal women with obesity (BMI over 30 kg/m2) depending on the intake of MHT.
Materials and methods
Prospective study included 214 postmenopausal women with diagnosed endometrioid carcinoma who underwent surgical treatment at the Department of Gynecology of the Medical Rehabilitation Center of the Ministry of Health of the Russian Federation and at the Division of Gynecologic Oncology of the University Clinical Hospital No. 4 of the I.M. Sechenov First Moscow State Medical University in the period 2019–2021. All patients underwent a standard preoperative examination, nerve-sparing total hysterectomy including the upper third of the vagina, and pelvic lymph node dissection followed by histological examination of surgical specimen. The stage of endometrial carcinoma was determined according to the TNM system (UICC, 8th edition, 2016) and the FIGO international classification (2009). Based on to the degree of differentiation, EC was divided into three groups: G1 – well-differentiated, G2 – moderately differentiated, G3 – poorly differentiated. The class of obesity was assessed in accordance with the clinical recommendations “Obesity” (2021), which used BMI to diagnose overweight and obesity, and to define its grade: Obese Class I (30.0–34.9 kg/m2); Obese Class II (35.0–39.9 kg/m2); Obese Class III (≥40 kg/m2) [11].
All patients were divided into 2 groups according to their BMI: the study group included women with BMI from 30.0 kg/m2 to 42.6 kg/m2 (140/214; 65.4%); the control group included 74 women with BMI from 17.6 kg/m2 to 24.9 kg/m2 (74/214; 34.6%). The mean age of women was M (SD): in the study group – 62.9 (7) years; in the control group – 62.2 (9) years. Each group was divided into two subgroups depending on the use of MHT. In the study group, 58 (58/140; 41.4%) women received MHT for an average of 6 years 6 months, 82 (82/140; 58.6%) women did not take any MHT. In the control group, 20 (20/74; 27%) women took MHT for an average of 8 years 2 months, 54 (54/74; 73%) did not use this therapy. All women had been taking combined estrogen-gestagen drugs for 12 months or more. The mean age of women taking MHT was M (SD): in the study group – 61.6 (3) years; in the control group – 65.9 (8) years. All women participating in the study signed an informed consent. The inclusion criteria for the study group were as follows: age over 45 years, BMI 30.0 kg/m2 or more, indication for receiving MHT for 12 months or more, histologically confirmed endometrioid type of EC, no signs of other localized malignancy, no indications for tamoxifen intake. The inclusion criteria for the control group were as follows: age over 45 years, BMI up to 24.9 kg/m2, indication for receiving MHT for 12 months or more, histologically confirmed endometrioid type of EC, no signs of other localized malignancy, no indications for tamoxifen intake. Exclusion criteria: age under 45 years, normal menstrual cycle, MHT for less than 12 months, histologically confirmed non-endometrioid type of EC (serous, clear cell, malignant mesenchymoma, mixed epithelial tumors), history of other localized malignancy, long-term use of tamoxifen, refusal of a woman to participate in the study.
Statistical analysis
Statistical analysis was performed using MS EXCEL and IBM SPSS 23 software packages. Distribution parameters (frequency analysis) were calculated for all studied parameters in each group. Significance of differences between the studied groups and subgroups was assessed using frequency analysis and Pearson's chi-squared test (χ2 test). Differences were considered to be statistically significant at p≤0.05.
The study was approved by the local Ethics Committee of the I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia (Ref. No. 03-21 of February 11, 2021). All patients signed an informed consent for participation in the study and publication of their medical data.
Results and discussion
The results of comparative analysis of the EC stages according to the TNM system (UICC, 8th edition, 2016), FIGO international classification (2009), and the degree of differentiation between the studied groups are presented in Table 1.
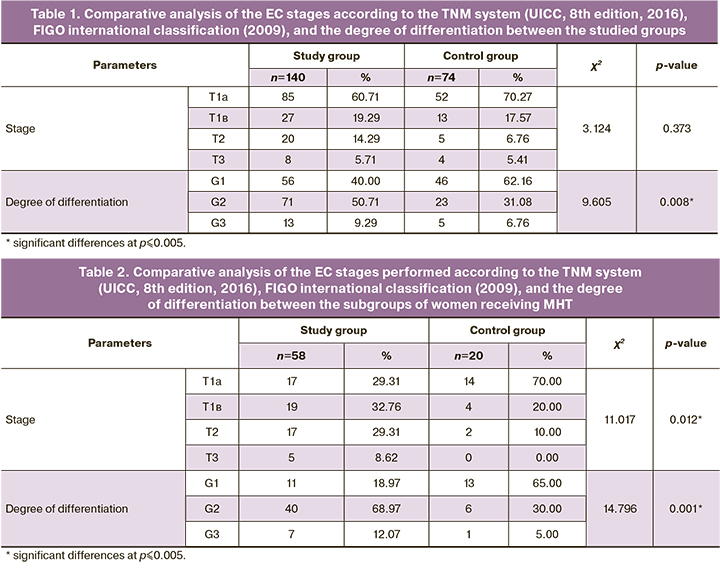
The table shows statistically significant differences in the degree of differentiation: high degree of differentiation (G1) was significantly more common for the control group, while moderate degree of differentiation (G2) was more common for the study group (p=0.008).
A comparative analysis of the EC stages performed according to the TNM system (UICC, 8th edition, 2016), FIGO international classification (2009), and the degree of differentiation between the subgroups of women receiving MHT revealed significant differences (Table 2).
This table shows statistically significant differences in the studied parameters. The stage with less than 50% myometrial invasion (T1a according to the TNM system (UICC, 8th edition, 2016) was more common for subgroup including women with normal body weight, and the later stages of EС with a higher degree of invasion were more common for women with obesity from the study group (p=0.012). The analysis of the degree of differentiation also showed significant differences: the incidence of well-differentiated forms (G1) of EC was significantly higher in the control group than in the study group, which was characterized by a higher incidence of moderately differentiated forms of EC (p=0.001).
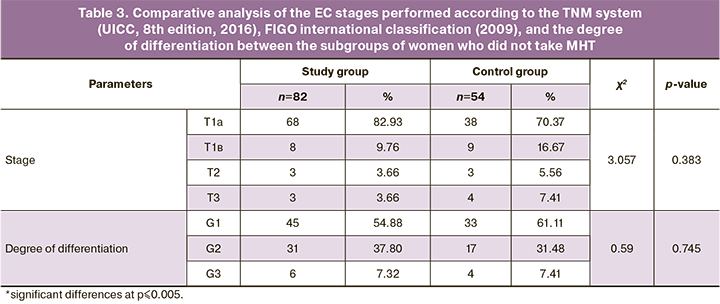
A comparative analysis of the subgroups of women who did not take MHT did not reveal any significant differences in the studied parameters (Table 3).
Today, the role of hyperestrogenism in the process of neoplastic transformation of the endometrium is fully confirmed by numerous studies. Obesity is not only the main risk factor for the development of EC of Type I, but also a key factor affecting the prognosis [12]. Metabolic obesity (BMI over 30 kg/m2), but not an excess body weight, is the main etiological factor of EC; moreover, impaired glucose tolerance and chronic tissue insulin resistance have a synergetic effect that dramatically increase the risk of EC [13].
The molecular mechanisms of obesity that may be associated with the risk of EC development are complex and not fully understood. Adipose tissue not only induce proliferation via complex signaling pathways, receptors, and gene expression, but also contribute to the invasion and modulation of cancer cells [8]. Obesity increases the aromatase production, which catalyzes the endogenous conversion of androgen to estrogen, which in turn leads to a decrease in the amount of sex hormone binding globulin, a hormone that binds and transports estrogen [14]. Thus, the level of biologically active estrogen increases even more. The oncogenic estrogen signaling is mediated by estrogen receptors that modulate transcriptional nuclear signaling [15], indicating a possible relation between estrogen receptor overexpression, invasion, and metastasis of endometriod adenocarcinoma [16]. Yang et al. found that estrogen-induced regulation of prohibitin expression is an important part of the oncogenic transformation of the endometrium, the signaling pathway of estrogen and its receptors; moreover, high levels of prohibitin are associated with a poor prognosis of EC [17]. Obesity is associated with a number of metabolic disorders, which include: chronic adipose tissue inflammation with the production of specific inflammatory adipocytokines, oxidative stress, peripheral insulin resistance with hyperinsulinemia and dyslipidemia [8]. Insulin and insulin-like growth factor 1 can influence the degree of tumor differentiation [18]. Adiponectin was considered to be a key mediator between obesity and malignancy development [19]. Most epidemiological data show that hypoadiponectinemia is associated with an increased risk of obesity-associated malignancies and poor cancer prognosis [19].
Specific course of EC associated with metabolic obesity is being widely studied. According to Russian studies, a moderate degree of differentiation (in 71.8% of cases) and tumor invasion depth up to ½ of the myometrium (in 65.3%) are considered to be a specific feature of EC associated with obesity [20]. Our study confirmed that a moderately differentiated tumor was significantly more common in women with obesity (p=0.008), which agrees with the findings of A. Tawfik et al. [21].
The association between MHT and the risk of EС is also widely discussed, however the results of the studies do not provide a clear answer. A number of researchers stated that MHT increases the risk of EC, while other studies do not confirm this statement [22–26]. A study by Swiss researcher J. Simin et al. shows a 12% increase in the risk of EC in women who have ever taken combined MHT (standardised incidence rates, SIR=1.12, 95% confidence interval (CI) 1.06–1.19) [27]. The data on the association of MHT use with an increased risk of EC do not clearly indicate whether the use of combined MHT can eliminate the excess risk of EC [28].
Conclusion
The analysis of our study indicates that obese women who received MHT had a higher rate of severe stages of EC with a poor and moderate degree of differentiation (G2–G3). The results of the studies indicate the necessity of BMI control and timely correction of hormonal and metabolic disorders in menopausal women before prescribing MHT, both to reduce the incidence of EC and to minimize the risks of severe stages of EC with a poor and moderate degree of differentiation.
References
- Reeves G.K., Pirie K., Beral V., Green J., Spencer E., Bull D.; Million Women Study Collaboration. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007; 335(7630): 1134. https://dx.doi.org/10.1158/10.1136/bmj.39367.495995.AE.
- Silverberg S.G., Mutter G.L., Kurman R.J. Tumors of the uterine corpus: epithelial tumors and related lesions. In: Tavassoli F.A., Devilee P., eds. WHO Classification of tumors: pathology and genetics of tumors of the breast and female genital organs. Lyon, France: IARC Press; 2003.
- Бохман Я.В. Руководство по онкогинекологии. М.: Медицина; 1989. 325 с. [Bokhman Ya.V. Guide to oncogynecology. M.: Medicine; 1989. 325 p. (in Russian)].
- Resnick K.E., Hampel H., Fishel R., Cohn D.E. Current and emerging trends in Lynch syndrome identification in women with endometrial cancer. Gynecol. Oncol. 2009; 114(1): 128-34. https://dx.doi.org/10.1016/j.ygyno.2009.03.003.
- Van den Bosch T., Coosemans A. Morina M., Timmerman D., Amant F. Screening for uterine tumours. Best Pract. Res. Clin. Obstet. Gynaecol. 2012; 26(2):257-66. https://dx.doi.org/10.1016/j.bpobgyn.2011.08.002.
- Dinkelspiel H.E., Wright J.D., Lewin S.N., Herzog T.J. Contemporary clinical management of endometrial cancer. Obstet. Gynecol. Int. 2013; 2013: 583891. https://dx.doi.org/10.1155/2013/583891.
- Felix A.S., Brinton L.A. Cancer progress and priorities: uterine cancer. Cancer Epidemiol. Biomarkers Prev. 2018; 27(9): 985-94. https://dx.doi.org/10.1158/1055-9965.EPI-18-0264.
- Kiesel L., Eichbaum C., Baumeier A., Eichbaum M. Obesity epidemic – the underestimated risk of endometrial cancer. Cancers (Basel). 2020; 12(12): 3860. https://dx.doi.org/10.3390/cancers12123860.
- World Cancer Research Fund/American Institute for Cancer Research: Continuous update project report. Food: nutrition, physical activity, and the prevention of endometrial cancer. 2013. Available at: http://www.dietandcancerreport.org Accessed 1 November 2020.
- Bianchini F., Kaaks R., Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002; 3(9): 565-74. https://dx.doi.org/10.1016/s1470-2045(02)00849-5.
- Дедов И.И., Мокрышева Н.Г., Мельниченко Г.А., Трошина Е.А., Мазурина Н.В., Ершова Е.В., Комшилова К.А., Андреева Е.Н., Анциферов М.Б., Бирюкова Е.В., Бордан Н.С., Вагапова Г.Р., Волкова А.Р., Волкова Н.И., Волынкина А.П., Дзгоева Ф.Х., Киселева Т.П., Неймарк А.Е., Романцова Т.И., Руяткина Л.А., Суплотова Л.А., Халимов Ю.Ш., Яшков Ю.И. Ожирение. Клинические рекомендации. Consilium Medicum. 2021; 23(4): 311-25. [Dedov I.I., Mokrysheva N.G., Melnichenko G.A., Troshina E.A., Mazurina N.V., Ershova E.V. et al. Obesity. Clinical Guidelines. Consilium Medicum. 2021; 23(4): 311-25. (in Russian)]. https://dx.doi.org/10.26442/20751753.2021.4.200832.
- Лапина И.А., Гаврилов М.В., Таранов В.В., Доброхотова Ю.Э., Кольтинова Т.Г. Метаболический синдром как фактор риска сосудистых осложнений у больных раком эндометрия. Акушерство и гинекология. 2019; 6: 132-8. [Lapina I.A., Gavrilov M.V., Taranov V.V., Dobrokhotova Yu.E., Koltinova T.G. Metabolic syndrome as a risk factor for vascular complications in patients with endometrial cancer. Akusherstvo i ginekologiya/ Obstetrics and Gynecology. 2019; 6: 132-8 (in Russian)]. https://dx.doi.org/10.18565/aig.2019.6.132-138.
- Khandekar M.J., Cohen P., Spiegelman B.M. Molecular mechanisms of cancer development in obesity. Nat. Rev. Cancer. 2011; 11(12): 886-95. https://dx.doi.org/10.1038/nrc3174.
- Simpson E.R., Mahendroo M.S., Means G.D., Kilgore M.W., Hinshelwood M.M., Graham-Lorence S. et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr. Rev. 1994; 15(3): 342-55. https://dx.doi.org/10.1210/edrv-15-3-342.
- Rodriguez A.C., Blanchard Z., Maurer K.A., Gertz J. Estrogen signaling in endometrial cancer: A key oncogenic pathway with several open questions. Horm. Cancer. 2019; 10: 51-63. https://dx.doi.org/10.1007/s12672-019-0358-9.
- Hou X., Zhao M., Wang T., Zhang G. Upregulation of estrogen receptor mediates migration, invasion and proliferation of endometrial carcinoma cells by regulating the PI3K/AKT/mTOR pathway. Oncol. Rep. 2014; 31(3): 1175-82. https://dx.doi.org/10.3892/or.2013.2944.
- Yang B., Chen R., Liang X., Shi J., Wu X., Zhang Z., Chen X. Estrogen enhances endometrial cancer cells proliferation by upregulation of prohibitin. J. Cancer. 2019; 10(7): 1616-21. https://dx.doi.org/10.7150/jca.28218.
- Khandwala H.M., McCutcheon I.E., Flyvbjerg A., Friend K.E. The effects of insulin-like growth factors on tumorigenesis and neoplastic growth. Endocr. Rev. 2000; 21: 215-44. https://dx.doi.org/10.1210/edrv.21.3.0399.
- Tumminia A., Vinciguerra F., Parisi M., Graziano M., Sciacca L., Baratta R., Frittitta L. Adipose tissue, obesity and adiponectin: role in endocrine cancer risk. Int. J. Mol. Sci. 2019; 20(12): 2863. https://dx.doi.org/10.3390/ijms20122863.
- Кишкина А.Ю., Коломиец Л.А., Юнусова Н.В. Клинические варианты метаболического синдрома у больных раком эндометрия. Сибирский онкологический журнал. 2019; 18(5): 38-44. [Kishkina A.Yu., Kolomiets L.A., Yunusova N.V. Clinical options for metabolic syndrome in patients with endometrial cancer. Sibirskij onkologicheskij zhurnal/ Siberian Oncological Journal. 2019; 18(5): 38-44 (in Russian)]. https://dx.doi.org/10.21294/1814-4861-2019-18-5-38-44.
- Tawfik A., Bassma M., Elsabaa Dalia A., Sally S., El-Tawab, Heba A. The impact of metabolic syndrome on the clinical profile and tumor characteristics of endometrial carcinoma. J. Reprod. Contracept. Obstet. Gynecol. 2016; 5(11): 3696-703. https://dx.doi.org/10.18203/2320-1770.
- Razavi P., Pike M.C., Horn-Ross P.L., Templeman C., Bernstein L., Ursin G. Long-term postmenopausal hormone therapy and endometrial cancer. Cancer Epidemiol. Biomark. Prev. 2010; 19(2): 475-83. https://dx.doi.org/10.1158/1055-9965.EPI-09-0712.
- Sponholtz T.R., Palmer J.R., Rosenberg L.A., Hatch E.E., Adams-Campbell L.L., Wise L.A., Lucile A.-C. Exogenous hormone use and endometrial cancer in U.S. Black Women. Cancer Epidemiol. Biomark. Prev. 2018; 27(5): 558-65. https://dx.doi.org/10.1158/1055-9965.EPI-17-0722.
- Jaakkola S., Lyytinen H.K., Dyba T., Ylikorkala O., Pukkala E. Endometrial cancer associated with various forms of postmenopausal hormone therapy: A case control study. Int. J. Cancer. 2011; 128(7): 1644-51. https://dx.doi.org/10.1002/ijc.25762.
- Phipps A.I., Doherty J.A., Voigt L.F., Hill D.A., Beresford S.A.A., Rossing M.A. et al. Long-term use of continuous-combined estrogen-progestin hormone therapy and risk of endometrial cancer. Cancer Causes Control. 2011; 22(12): 1639-46. https://dx.doi.org/10.1007/s10552-011-9840-6.
- McCullough M.L., Patel A.V., Patel R., Rodriguez C., Feigelson H.S., Bandera E.V. et al. Body mass and endometrial cancer risk by hormone replacement therapy and cancer subtype. Cancer Epidemiol. Biomark. Prev. 2008; 17: 73-9. https://dx.doi.org/10.1158/1055-9965.EPI-07-2567.
- Simin J., Tamimi R., Lagergren J., Adami H.-O., Brusselaers N. Menopausal hormone therapy and cancer risk: An overestimated risk? Eur. J. Cancer. 2017; 84: 60-8. https://dx.doi.org/10.1016/j.ejca.2017.07.012.
- Nelson H.D., Humphrey L.L., Nygren P., Teutsch S.M., Allan J.D. Postmenopausal hormone replacement therapy: scientific review. JAMA. 2002; 288(7): 872e81. https://dx.doi.org/10.1001/jama.288.7.872.
Received 07.02.2022
Accepted 26.03.2022
About the Authors
Lidia A. Klyukina, PhD student of the Department of Obstetrics and Gynecology No. 1, N.V. Sklifosovsky Institute of Clinical Medicine,I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia (Sechenov University), lidiaklyukina@mail.ru, https://orcid.org/0000-0001-7602-4584, 119991, Moscow, Russia, Trubetskaya str., 8-2.
Elena A. Sosnova, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology No. 1, N.V. Sklifosovsky Institute of Clinical Medicine,
I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia (Sechenov University), sosnova-elena@inbox.ru, https://orcid.org/0000-0002-1732-6870, 119991, Moscow, Russia, Trubetskaya str., 8-2.
Anton A. Ishchenko, PhD, Head of the Center for Gynecology and New Reproductive Technologies, Medical and Rehabilitation Center,
Ministry of Health of the Russian Federation, ra2001_2001@mail.ru, https://orcid.org/0000-0001-6673-3934, 125367, Moscow, Russia, Ivankovskoe highway, 3.
Corresponding author: Lidia A. Klyukina, lidiaklyukina@mail.ru
Authors' contributions: Sosnova E.A., Isсhenko A.A., Klyukina L.A. – the concept and design of the study, material collection and processing, statistical data processing; Klyukina L.A. – writing the article; Sosnova E.A., Ischenko A.A. – editing the article.
Conflicts of interest: The authors declare that they have no conflicts of interest.
Funding: The study was conducted without any sponsorship.
Ethical Approval: The study was approved by the local Ethics Committee of I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia (Ref. No. 03-21 of February 11, 2021).
Patients’ Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citiation: Klyukina L.A., Sosnova E.A., Ischenko A.A. Manopausal hormonal therapy and clinical and morphological features of endometrial cancer in women with metabolic disorders.
Акушерство и гинекология/Obstetrics and Gynecology. 2022; 4: 141-147 (in Russian)
https://dx.doi.org/10.18565/aig.2022.4.141-147



