Genes of estrogen and progesterone receptors and characteristics of uterine damage in myoma
Altukhova O.B., Radzinsky V.E., Sirotina S.S., Efremova O.A., Batlutskaya I.V., Orlova V.S., Belousova O.N., Rudyh N.A., Churnosov M.I.
Relevance: A uterine myoma is a hormone-dependent tumor composed of muscle and connective tissue elements. It occupies a special place in the spectrum of tumors affecting the female reproductive system, accounting for 10–20% of all gynecological pathologies, and is diagnosed in 10–30% of women of reproductive age. One important aspect of the pathogenesis of uterine fibroids is the imbalance between progesterone and estrogen levels, which creates conditions conducive to the rapid growth of fibroid nodules. Polymorphisms in the genes of estrogen and progesterone receptors may influence their expression and, therefore, be significant in the pathophysiology of large uterine myomas.
Objective: To investigate the associations between combined polymorphic variants of estrogen and progesterone receptor genes and the development of large uterine myomas.
Materials and methods: A total of patients (n=380) diagnosed with uterine fibroids were divided into three groups based on the size of the largest nodule. Five polymorphic loci of estrogen and progesterone receptors were selected for the study: rs2234693 ESR1 c453-397T>C, rs3798577 ESR1 c.1029T>C, rs9340799 ESR1 c.453-351A>G, rs484389 PGR c.38T>C, and rs1042838 PGR c.1415-11113G>T. The analysis was performed by polymerase chain reaction (PCR) on a CFX-96 Real-Time System thermal cycler. Odds ratios (OR) and 95% confidence intervals (CI) were calculated to determine associations. The APSampler program (https://sourceforge.net/projects/apsampler/) was used to evaluate the associations of allele and genotype combinations of the analyzed genes with the occurrence of large myomas. The online programs HaploReg and Gtex Portal were used to assess the functional effects of SNPs associated with the formation of large myomas.
Results: This study demonstrated a significant role of ESR1 rs3798577, ESR1 rs9340799, PGR rs484389, and PGR rs1042838 in the characteristics of uterine fibroids. A combination of polymorphic variants C rs3798577 ESR1 and AA rs9340799 ESR1 should be considered a risk factor for the development of large uterine fibroids (OR = 2.30-2.42), while a combination of markers GG rs1042838 PGR, C rs484389 PGR, and G rs9340799 ESR1 should be regarded as a protective factor against the formation of large nodules (OR=0.27–0.28).
Conclusion: The polymorphic loci ESR1 rs3798577, ESR1 rs9340799, PGR rs484389, and PGR rs1042838 were associated with the development of large uterine fibroids. The combinations of allelic variants of estrogen and progesterone receptor genes identified for the first time, which are associated with an increased risk of developing large uterine fibroids, underscores the importance of the interaction of polymorphic gene variants in the formation of this pathology. These findings may have practical applications in identifying risk groups for preventive measures.
Authors' contributions: Churnosov M.I., Altukhova O.B., Radzinsky V.E. – conception and design of the study; Belousova O.N., Churnosov M.I., Batlutskaya I.V. – material collection and analysis; Sirotina S.S., Efremova O.A., Rudyh N.A. – drafting of the manuscript; Churnosov M.I., Altukhova O.B., Orlova V.S. – editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the Belgorod State National Research University (Ref. No: 6 of 23.04.2021).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Altukhova O.B., Radzinsky V.E., Sirotina S.S., Efremova O.A., Batlutskaya I.V., Orlova V.S.,
Belousova O.N., Rudyh N.A., Churnosov M.I. Genes of estrogen and progesterone receptors and
characteristics of uterine damage in myoma.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (3): 113-119 (in Russian)
https://dx.doi.org/10.18565/aig.2024.304
Keywords
A uterine myoma is a hormone-dependent tumor composed of muscle and connective tissue elements [1, 2]. It holds a significant position among tumors affecting the female reproductive system, accounting for 10–20% of all gynecological pathologies, and is diagnosed in 10–30% of women of reproductive age [3, 4]. It is estimated that every fourth or fifth woman worldwide suffers from uterine fibroids. Approximately 80% of women aged 30–40 years are at risk of developing uterine myoma, with 20-25% already diagnosed, leading to an increase in surgical interventions among women of reproductive age [5, 6]. The hormonal status of a woman plays a crucial role in the development of large myomatous nodes [7, 8]. Increased expression of extracellular matrix components, along with estrogen and progesterone receptors in uterine fibroids, disrupts the balance between progesterone and estrogen levels, creating conditions conducive to the rapid growth of fibroid nodules [9–11].
The interactions of polymorphic gene variants in the development and progression of uterine fibroids have been actively discussed by researchers both domestically and internationally [12–14]. For instance, Bondagji N.S. et al. (2016) demonstrated that the polymorphic locus rs12484776 significantly contributes to the formation of uterine fibroids in women in Saudi Arabia, both independently and in interaction with the polymorphic loci rs1056836, rs2280543, and rs7913069 [15]. Rafnar T. et al. (2018) presented a meta-analysis of two genome-wide association studies of uterine fibroids among European women, involving 16,595 cases and 523,330 controls, which identified 21 variants across 16 polymorphic loci that contribute to the development of large uterine fibroids [16]. However, the interactions between estrogen and progesterone receptor genes in the development of large uterine fibroids have not yet been studied, highlighting the relevance of this research.
This study aimed to investigate the association between combined polymorphic variants of estrogen and progesterone receptor genes and the development of large uterine fibroids.
Materials and methods
The study sample included 380 women with confirmed diagnoses of uterine fibroids. Patients were selected using a continuous method in the gynecological department of the Belgorod Regional Clinical Hospital of St. Joasaph. The women included in the study were native residents of the Central Black Earth Region of Russia with no family ties to each other. Exclusion criteria were the presence of diagnosed malignant diseases of the female reproductive system and pregnancy. The study was conducted with written informed consent of the patients and was reviewed and approved by the Research Ethics Committee of Belgorod State University (Ref. No: 6 of 23.04.2021).
To assess the type of uterine damage in fibroids, the size of myomatous nodules was used as an indicator [17]. The patients (n=380) were divided into three groups. Group 1 included women with the largest nodule size greater than 60 mm (n=205, 53.94%). Group 2 included women with the largest nodule size ranging from 25 to 60 mm (n=125, 32.89%). Group 3 included women with the largest nodule size of up to 25 mm (n=50; 13.16%).
For the polymerase chain reaction (PCR), DNA was isolated from peripheral blood leukocytes using the phenol-chloroform extraction method. DNA samples were genotyped by allele-specific PCR in real-time using TaqMan probes, and amplification was conducted on a CFX96 detection system (Bio-Rad, USA) [18]. The following DNA markers were included in this study: rs2234693 ЕSR1 с453-397Т>С, rs3798577 ЕSR1 c.1029Т>С, rs9340799 ЕSR1 с.453-351А>G, rs484389 РGR c.38Т>С, and rs1042838 РGR c.1415-11113G>Т.
Statistical analysis
Statistical analysis was performed using STATISTICA 10.0. Categorical variables were presented as numbers and percentages. Differences in genotype/allele frequencies between the study groups were determined using standard methods (c2 test, 2×2 contingency table). Variables such as body mass index, patient age, and age at menarche were assessed using the Shapiro–Wilk test. Continuous variables with a normal distribution were expressed as mean (M) and standard deviation (SD). The Mann–Whitney test was used to compare continuous variables and the χ2 test was used for categorical variables. The results were considered statistically significant at p<0.05. Interaction analysis of polymorphic variants of genes was performed using the AP Sampler software (https://sourceforge.net/projects/apsampler/), which uses the Monte Carlo Markov chain method and Bayesian nonparametric statistics, with the calculation of the odds ratio (OR) and its 95% confidence interval (95% CI) [19]. A permutation test (pperm) was used to adjust for the effects of false positives in the multiple comparisons. Since the three groups were compared with each other, pperm<0.016 (0.05/3) was considered statistically significant [20]. The functional significance of the polymorphic loci under consideration was studied using the HaploReg program (v4.1) (http://archive.broadinstitute.org/mammals/haploreg/haploreg.php). When assessing the change in interaction with transcription factors of gene structure variants, the Δ LOD Score was calculated using the formula: LOD Score of the alternative (alt) allele – LOD Score of the reference (ref) allele. If the Δ LOD Score was <0, a conclusion was made regarding the reduced affinity of DNA motifs to transcription factors; if the value was >0, a relative increase was made. The GTExportal tool (https://www.gtexportal.org/home/snp) was used to establish the role of polymorphisms in gene transcription changes.
Results and discussion
Analysis of clinical and demographic characteristics (Table 1) showed no significant differences between the study groups (p>0.05).
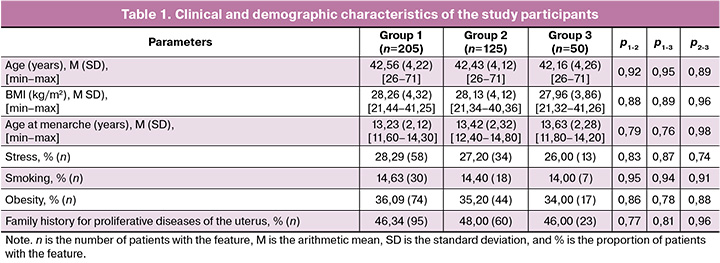
For all studied polymorphic loci, the observed distribution of genotypes corresponded to that expected under Hardy–Weinberg equilibrium (p>0.05).
Comparative analysis of the allele and genotype frequencies of estrogen and progesterone receptor polymorphic loci among the three groups of women with fibroids, categorized by fibroid size, found no significant differences (p>0.05) (Table 2).
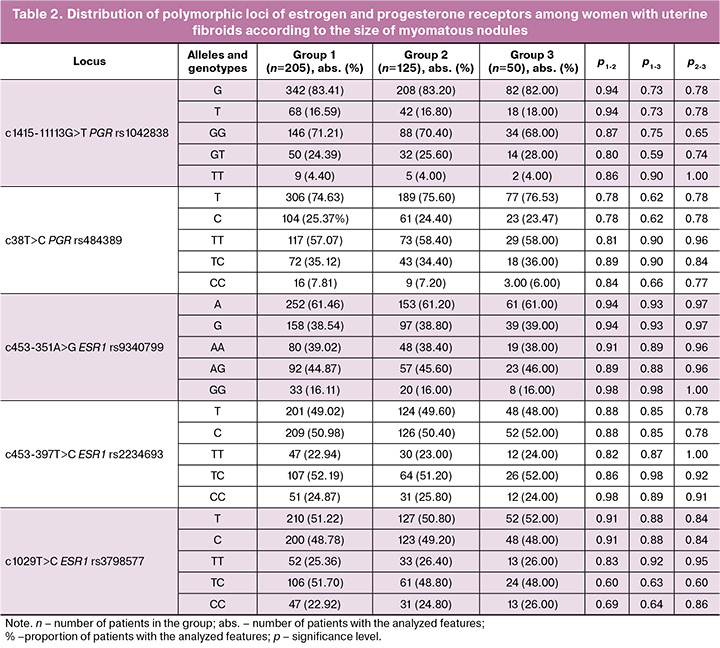
Analysis of the interaction between the studied polymorphic loci revealed that in group 1, the combination of the ESR1 C allele (rs3798577) and the ESR1 AA genotype (rs9340799) was present in 35.12% (75/205) of patients, significantly differing from group 2 (22.40% (24/125), pperm=0.003, OR=2.42, 95% CI 1.43–4.11) and group 3 (20.00% (10/50), pperm=0.003, OR=2.30, 95% CI 1.09–4.88) (Table 3). This established combination of polymorphic loci serves as a marker of the risk of developing uterine fibroids with large myomatous nodules.
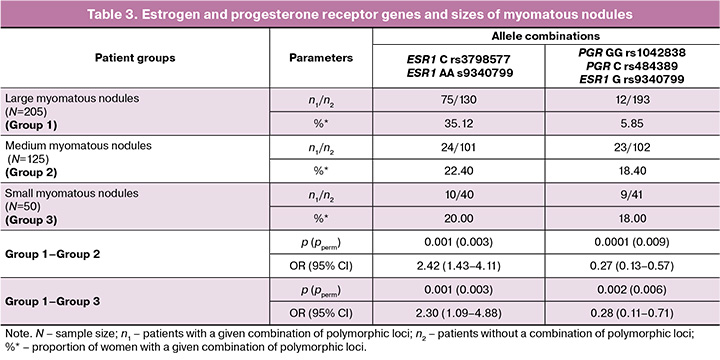
It was also found that in group 1, the combination of molecular genetic markers GG РGR (rs1042838), C РGR (rs484389) and G ЕSR1 (rs9340799) occurs less frequently (5.85% (12/205)) than in group 2 (18.40% (23/125), pperm=0.009, OR=0.27, 95% CI 0.13–0.57), and in group 3 (18.00% (9/50), pperm=0.006, OR=0.28, 95% CI 0.11–0.71). This combination of polymorphic loci indicates a minimal risk of developing large myomatous nodules. The data regarding the role of polymorphic loci of estrogen and progesterone receptors in the development of large myomatous nodules in patients with uterine myoma can be explained by the medical and biological effects of the protein products of these genes. According to the literature, myomatous nodules are sensitive to progesterone and estrogens [21, 22]. It has been shown that a significant increase in estrogens is detected in myomatous nodules, while normal myometrium has a limited response to estrogens and remains intact. Many researchers have established the stimulatory effect of progesterone on the proliferative activity of myomatous nodules [23, 24]. Progesterone influences the growth of myomatous nodules through local growth factors, extracellular matrix, and its corresponding receptors. Research indicates that sex hormones, particularly progesterone, induce the expression of Bcl-2 via their receptors, blocking apoptosis, and thereby extending the life cycle of the cell [24–26], which promotes the growth of myomatous nodules. In a study by Pechkovskij E.V. et al. [27], it was established that the T allele of rs1042838 PGR and the C allele of rs484389 PGR were associated with a decrease in the number of progesterone receptors in mammary gland cells, reducing the likelihood of a cellular response to progesterone. Progesterone has an antiproliferative effect not only in the mammary gland but also in the uterus, which helps explain the protective effects of the combination of polymorphic loci G rs1042838 PGR, C rs484389 PGR, and allele G rs9340799 ESR1 in the development of large myomatous nodules. According to Golovchenko O. et al., the latter allele is responsible for increased estrogen expression in the myometrium [28].
To evaluate the medical and biological effects of the combination of markers C rs3798577 ESR1 and AA rs9340799 ESR1, which are risk factors for the formation of large myomatous nodules, we conducted an in-silico analysis. The HaploReg database indicates that the rs9340799 polymorphism, located in the intron region of ESR1, is situated in an enhancer region and affects the affinity for transcription factors. The G allele of rs9340799 ESR1 increases the sensitivity of the DNA in this region to the transcription factors Ets (Δ LOD=4.3) and Hand1 (Δ LOD=11.8) in breast and adipose tissues, potentially regulating the expression of this gene.
The rs3798577 polymorphism, located in the 3'-UTR region of ESR1, also possesses significant regulatory potential. It is located in a region modified by histone proteins (H3K4me3 and H3K9ac), marking promoters in adipocytes, female skeletal muscle tissue, muscle satellite cell cultures, and adipose tissue precursors, among others. This polymorphic locus is also found in a region modified by histone proteins (H3K4me1 and H3K27ac), marking enhancers in over 30 different cell cultures, tissues, and organs (mesenchymal stem cells, female muscle tissue, adipocytes, ovaries, etc.) [29]. The functional effects of polymorphic loci rs3798577 and rs9340799 ESR1 confirmed their association with hormone-dependent diseases of the female reproductive system.
It should be noted that the roles of the polymorphic loci rs3798577 ESR1, rs9340799 ESR1, rs484389 PGR, and rs1042838 PGR in the development of various diseases are actively studied by various scientific teams. The PubMed Central database (https://www.ncbi.nlm.nih.gov/pmc) contains more than 190 articles related to medical and biological studies of rs9340799 ESR1, over 40 publications examining the significance of rs3798577 ESR1, and more than 50 studies on rs484389 PGR and rs1042838 PGR. These articles demonstrated associations between these polymorphic loci and the development of uterine fibroids, miscarriage, infertility, tumors of the female reproductive system, and cardiovascular diseases. [24, 25, 27, 28]. However, the literature primarily addresses the monoeffects of polymorphic variants of estrogen and progesterone receptor genes in uterine myoma or combinations of these polymorphic loci in other gynecological pathologies.
Conclusion
This study established a significant role for the genes rs3798577 ESR1, rs9340799 ESR1, rs484389 PGR, and rs1042838 PGR in the nature of uterine damage in myoma. The combination of markers C rs3798577 ESR1 and AA rs9340799 ESR1 (OR=2.30–2.42) should be considered a risk factor for the development of large myomatous nodules, whereas the combination of markers G rs9340799 ESR1, C rs484389 PGR, and GG rs1042838 PGR (OR=0.27–0.28) should be viewed as a protective factor against the development of large nodules. The combinations of polymorphic loci of estrogen and progesterone receptors identified in this study are crucial for the development of large uterine fibroids. Based on these findings, it is possible to develop preventive measures aimed at identifying patients at risk of this pathology.
References
- Пономаренко И.В., Чурносов М.И. Современные представления об этиопатогенезе и факторах риска лейомиомы матки. Акушерство и гинекология. 2018; 8: 27-32. [Ponomarenko I.V., Churnosov M.I. Current views on the etiopathogenesis and risk factors of uterine leiomyoma. Obstetrics and Gynecology. 2018; (8): 27-32. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.8.27-32.
- Ludovisi M., Moro F., Pasciuto T., Di Noi S., Giunchi S., Savelli L. et al. Imaging in gynecological disease (15): clinical and ultrasound characteristics of uterine sarcoma. Ultrasound Obstet. Gynecol. 2019; 54(5): 676-87. https://dx.doi.org/10.1002/uog.20270.
- Alsudairi H.N., Alrasheed A.T., Dvornyk V. Estrogens and uterine fibroids: an integrated view. Research Results in Biomedicine. 2021; 7(2): 156-63. https://dx.doi.org/10.18413/2658-6533-2021-7-2-0-6.
- Aninye I.O., Laitner M.H. Uterine fibroids: assessing unmet needs from bench to bedside. J. Womens Health (Larchmt). 2021; 30(8): 1060-7. https://dx.doi.org/10.1089/jwh.2021.0280.
- Giuliani E., As-Sanie S., Marsh E.E. Epidemiology and management of uterine fibroids. Int. J. Gynaecol. Obstet. 2020; 149(1): 3-9. https://dx.doi.org/10.1002/ijgo.13102.
- Baranov V.S., Osinovskaya N.S., Yarmolinskaya M.I. Pathogenomics of uterine fibroids development. Int. J. Mol. Sci. 2019; 20(24): 6151. https://dx.doi.org/10.3390/ijms20246151.
- McWilliams M.M., Chennathukuzhi V.M. Recent advances in uterine fibroid etiology. Semin. Reprod. Med. 2017; 35(2): 181-9. https://dx.doi.org/10.1055/s-0037-1599090.
- Shen Z., Li S.,Sheng B., Shen Q., Sun L.Z., Zhu H., Zhu X. The role of atorvastatin in suppressing tumor growth of uterine fibroids. J. Transl. Med. 2018; 16(1): 53. https://dx.doi.org/10.1186/s12967-018-1430-x.
- Ulin M., Ali M., Chaudhry Z.T., Al-Hendy A., Yang Q. Uterine fibroids in menopause and perimenopause. 2020; 27(2): 238-42. https://dx.doi.org/10.1097/GME.0000000000001438.
- Алтухова О.Б., Радзинский В.Е., Полякова И.С., Чурносов М.И. Вовлеченность полиморфизма генов рецепторов эстрогенов и прогестерона в развитие миомы матки. Акушерство и гинекология. 2020; 3: 127-32. [Altukhova O.B., Radzinsky V.E., Polyakova I.S., Churnosov M.I. Involvement of estrogen and progesterone receptor gene polymorphisms in the development of uterine fibroids. Obstetrics and Gynecology. 2020; (3): 127-32. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.3.127-132.
- Yang Q., Mas A., Diamond M.P., Al-Hendy A. The mechanism and function of epigenetics in uterine leiomyoma development. Reprod. Sci. 2016; 23(2): 163-75. https://dx.doi.org/10.1177/1933719115584449.
- Пономаренко И.В., Полоников А.В., Чурносов М.И. Полиморфные локусы гена LHCGR, ассоциированные с развитием миомы матки. Акушерство и гинекология. 2018; 10: 86-91. [Ponomarenko I.V., Polonikov A.V., Churnosov M.I. Polymorphic LHCGR gene loci associated with the development of uterine fibroids. Obstetrics and Gynecology. 2018; (10): 86-91. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.10.86-91.
- Machado-Lopez A., Simón C., Mas A. Molecular and cellular insights into the development of uterine fibroids. Int. J. Mol. Sci. 2021; 22(16): 8483. https://dx.doi.org/10.3390/ijms22168483.
- Пономаренко И.В., Полоников А.В., Чурносов М.И. Ассоциация полиморфизма rs4986938 гена ESR2 с развитием гиперплазии эндометрия. Акушерство и гинекология. 2019; 4: 66-72. [Ponomarenko I.V., Polonikov A.V., Churnosov M.I. Association of ESR2 rs4986938 polymorphism with the development of endometrial hyperplasia. Obstetrics and Gynecology. 2019; (4): 66-72. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.4.66-72.
- Bondagji N.S., Morad F.A., Al-Nefaei A.A., Khan I.A., Elango R., Abdullah L.S. et al. Replication of GWAS loci revealed the moderate effect of TNRC6B locus on susceptibility of Saudi women to develop uterine leiomyomas. J. Obstet. Gynaecol. Res. 2017; 43(2): 330-8. https://dx.doi.org/10.1111/jog.13217.
- Rafnar T., Gunnarsson B., Stefansson O.A., Sulem P., Ingason A., Frigge M.L. et al. Variants associating with uterine leiomyoma highlight genetic background shared by various cancers and hormone-related traits. Nat. Commun. 2018; 9(1): 3636. https://dx.doi.org/10.1038/s41467-018-05428-6.
- Можейко Л. Миома матки: классификация, диагностика, современные методы лечения. Наука и инновации. 2019; 10(200): 79-84. [Mozhejko L. Uterine fibroids: classification, diagnosis, modern methods of treatment. Science and Innovation. 2019; 10(200): 79-84. (in Russian)].
- Головченко И.О. Генетические детерминанты уровня половых гормонов у больных эндометриозом. Научные результаты биомедицинских исследований. 2023; 9(1): 5-21. [Golovchenko I.O. Genetic determinants of sex hormone levels in endometriosis patients. Research Results in Biomedicine. 2023; 9(1): 5-21 (in Russian)]. https://dx.doi.org/10.18413/2658-6533-2023-9-1-0-1.
- Favorov A.V., Andreewski T.V., Sudomoina M.A., Favorova O.O., Parmigiani M.F. A Markov chain Monte Carlo technique for identification of combinations of allelic variants underlying complex diseases in humans. Genetics. 2005; 171(4): 2113-21. https://dx.doi.org/10.1534/genetics.105.048090.
- Che R., Jack J.R., Motsinger-Reif A.A., Brown C.C. An adaptive permutation approach for genome-wide association study: evaluation and recommendations for use. BioData. Min. 2014; 7: 9. https://dx.doi.org/10.1186/1756-0381-7-9.
- Джемлиханова Л.Х., Смирнова М.Ю., Ниаури Д.А., Кветной И.М. Экспрессия рецепторов половых стероидных гормонов и фактора роста в миометрии при миоме матки и аденомиозе. Вестник СПбГУ. 2019; (4): 222-30. [Dzhemlikhanova L.Kh., Smirnova M.Yu., Niauri D.A., Kvetnoj I.M. Expression of sex steroid hormone and growth factor receptors in the myometrium in uterine fibroids and adenomyosis. Vestnik SPbGU. 2019; (4): 222-30. (in Russian)].
- Тюрина А.А., Ящук А.Г., Нафтулович Р.А., Хуснутдинов Ш.М. Пролиферативная активность и экспрессия рецепторов половых стероидных гормонов в ткани миомы матки вне и во время беременности. Практическая Медицина. 2016; 1(93): 101-5. [Tyurina A.A., Yashchuk A.G., Naftulovich R.A., Husnutdinov Sh.M. Proliferative activity and expression of sex steroid hormone receptors in uterine fibroid tissue outside and during pregnancy. Practical Medicine. 2016; 1(93): 101-5. (in Russian)].
- Мустафин Р.Н., Хуснутдинова Э.К. Ретроэлементы как мишени таргетной терапии опухолей (обзор). Научные результаты биомедицинских исследований. 2024; 10(1): 5-22. [Mustafin R.N., Khusnutdinova E.K. Retroelements in targeted antitumor therapy (review). Research Results in Biomedicine. 2024; 10(1): 5-22. (in Russian)]. https://dx.doi.org/10.18413/2658-6533-2024-10-1-0-1.
- Ponomarenko M.S., Reshetnikov E.A., Churnosova M.M., Reshetnikova Yu.N., Churnosov V.I., Ponomarenko I.V. Comorbidity and syntropy of benign proliferative diseases of the female reproductive system: non-genetic, genetic, and epigenetic factors (review). Research Results in Biomedicine. 2023; 9(4): 544-56. https://dx.doi.org/10.18413/2658-6533-2023-9-4-0-9.
- Алтухова О.Б., Радзинский В.Е., Сиротина С.С., Чурносов М.И. Анализ ассоциации полиморфных вариантов генов рецепторов эстрогенов и прогестерона с развитием генитального эндометриоза. Акушерство и гинекология. 2021; 9: 93-9. [Altukhova O.B., Radzinsky B.E., Sirotina S.S., Churnosov M.I. Analysis of the association between the polymorphic variants of estrogen and progesterone receptor genes and genital endometriosis. Obstetrics and Gynecology. 2021; (9): 93-9 (in Russian)]. https://dx.doi.org/10.18565/aig.2021.9.93-99.
- Ящук А.Г., Мусин И.И., Гумерова И.А. Современные аспекты в изучении этиологии миомы матки. Российский вестник акушера-гинеколога. 2019; 19(3): 49-56. [Yashchuk A.G., Musin I.I., Gumerova I.A. Current aspects of the study of uterine myoma etiology. Russian Bulletin of Obstetrician-Gynecologist. 2019; 19(3): 49-56. (in Russian)]. https://dx.doi.org/10.17116/rosakush20191903149.
- Печковский Е.В. Аллель РROGINS гена рецептора прогестерона РGR ассоциирован с риском рака молочной железы в Западно-Сибирском регионе России. Онкология. 2012; 13: 207-17. [Pechkovskij E.V. The RROGINS allele of the progesterone receptor gene PGR is associated with the risk of breast cancer in the West Siberian region of Russia. Oncology. 2012; (13): 207-17. (in Russian)].
- Golovchenko O., Abramova M., Ponomarenko I., Reshetnikov E., Aristova I., Polonikov A. et al. Functionally significant polymorphisms of ESR1and PGR and risk of intrauterine growth restriction in population of Central Russia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020; 253: 52-7. https://dx.doi.org/10.1016/j.ejogrb.2020.07.045.
- Головченко О.В. Молекулярно-генетические детерминанты преэклампсии. Научные результаты биомедицинских исследований. 2019; 5(4): 139-49. [Golovchenko O.V. Molecular genetic determinants of pre-eclampsia. Research Results in Biomedicine. 2019; 5(4): 139-49. (in Russian)]. https://dx.doi.org/10.18413/2658-6533-2019-5-4-0-11.
Received 25.11.2024
Accepted 11.02.2025
About the Authors
Oxana B. Altukhova, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology, Medical Institute, Belgorod State National Research University,308015, Russia, Belgorod, Pobedy str., 85, +7(4722)30-13-83, https://orcid.org/0000-0003-4674-8797
Viktor E. Radzinsky, Dr. Med. Sci., Professor, Honored Scientist of the Russian Federation, Academician of the International Academy of Sciences of the Higher School,
Head of the Department of Obstetrics and Gynecology, Faculty of Medicine, Peoples’ Friendship University of Russia, 117198, Moscow, Miklukho-Maklaya str., 6,
+7(495)360-46-69.
Svetlana S. Sirotina, PhD (Bio), Associate Professor at the Department of Biomedical Disciplines, Belgorod State National Research University, 308015, Russia, Belgorod, Pobedy str., 85, +7(4722)30-13-83, sirotina@bsuedu.ru, https://orcid.org/0000-0002-4163-7863
Mikhail I. Churnosov, Dr. Med. Sci., Professor, Head of the Department of Biomedical Disciplines of the Medical Institute, Belgorod State National Research University, 308015, Russia, Belgorod, Pobedy str., 85, +7(4722)30-13-83, https://orcid.org/0000-0003-1254-6134
Valentina S. Orlova, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology of the Medical Institute, Belgorod State National Research University,
308015, Russia, Belgorod, Pobedy str., 85, +7(4722)30-13-83, https://orcid.org/0000-0003-3882-9191
Irina V. Batlutskaya, Dr. Bio. Sci., Associate Professor, Head of the Department of Biotechnology and Microbiology, Belgorod State National Research University,
308015, Russia, Belgorod, Pobedy str., 85, +7(4722)30-13-83, https://orcid.org/0000-0003-0068-6586
Olga A. Efremova, Dr. Med. Sci., Associate Professor, Head of the Department of Faculty Therapy of the Medical Institute, Belgorod State National Research University, 308015, Russia, Belgorod, Pobedy str., 85, +7(4722)30-13-83, https://orcid.org/0000-0003-4967-2556
Oksana N. Belousova, Dr. Med. Sci., Professor at the Department of Hospital Therapy of the Medical Institute, Belgorod State National Research University,
308015, Russia, Belgorod, Pobedy str., 85, +7(4722)30-13-83, https://orcid.org/0000-0001-6862-0829
Natalia A. Rudyh, PhD (Bio), Associate Professor at the Department of Biomedical Disciplines, Belgorod State National Research University,
308015, Russia, Belgorod, Pobedy str., 85, +7(4722)30-13-83, https://orcid.org/0000-0003-0953-3463



