Analysis of the association between the polymorphic variants of estrogen and progesterone receptor genes and genital endometriosis
Aim: To study association between the genetic polymorphism in the estrogen and progesterone receptor genes and genital endometriosis.Altukhova O.B., Radzinsky B.E., Sirotina S.S., Churnosov M.I.
Materials and methods: The study involved 894 women: 105 women with genital endometriosis and 789 women in the control group. The polymorphic loci of the estrogen and progesterone receptor genes were selected for the study: ЕSR1 с.453-397Т>С rs2234693, ЕSR1 c.1029Т>С rs3798577, ЕSR1с.453-351А>G rs9340799, РGR c.38Т>С rs484389, РGR c.1415-11113G>Т rs1042838. CFX96 Touch Real-Time PCR System was used for analysis. To calculate the associations, the odds ratio (OR) and 95% confidence interval (CI) was used.
Results: The associations between molecular genetic markers ЕSR1 с.453-397Т>С rs2234693, ЕSR1 c.1029Т>С rs3798577, ЕSR1с.453-351А>G rs9340799 and development of genital endometriosis were found. A combination of ESR1 А rs9340799, ESR1 Т rs2234693 and ESR1 Т rs3798577alleles (OR=1.86, р=0.003) were found to be a risk factor for the development of genital endometriosis, and a combination of ESR1 rs9340799 G alleles and ESR1 rs3798577 CC genotype (OR=0.33, р=0.003) appeared to prevent the development of the disease. No associations between polymorphic loci of РGR c.38Т>С rs484389 and РGR c.1415-11113G>Т rs1042838 and genital endometriosis were found.
Conclusion: Polymorphic loci of ЕSR1 с.453-397Т>С rs2234693, ЕSR1 c.1029Т>С rs3798577, ЕSR1с.453-351А>G rs9340799 were associated with development of genital endometriosis.
Keywords
Genital endometriosis is a chronic hormone-dependent disease characterized by benign growths of endometrial tissue outside the uterine cavity [1, 2]. According to various authors, genital endometriosis is diagnosed in 15–40% of women of reproductive age, and is the cause of primary and secondary infertility [3].
Clinical and experimental data defined genital endometriosis as an estrogen-dependent disease [4–6]. Estrogens stimulate the survival of endometrial cells, promote inflammation and lesion progression, while progesterone suppresses the expression of estrogen receptors and blocks the effects of estrogen [7, 8]. Estrogen receptors alpha and beta (ESR1 and ESR2), and progesterone receptor (PGR) are key steroid receptors involved in the pathophysiology of genital endometriosis [9, 10]. Some studies have shown a lower level of ESR1 in endometrial tissue, due to the switch from ESR1 to ESR2 predominance [11]. ESR2 RNA expression is 40–140 times higher in stromal cells derived from ovarian endometrioma compared to healthy eutopic endometrial stromal cells [12]. ESR2 expression is also higher in eutopic endometrium in women with endometriosis compared to healthy women. High levels of ESR2 in the endometrium are supposed to increase the risk of endometriosis [13].
Although the pathogenesis of endometriosis is not fully clear, genetic factors play an important role in the development and progression of this disease [14]. Molecular genetics provide an increasing amount of data on genetically determined changes at the level of nucleotide substitutions, which affect the expression of encoded proteins or their functions, which can influence the development of genital endometriosis [15]. Thus, it is important to search for molecular genetic markers of genital endometriosis, which can help to develop new tests for prediction and diagnosis of pathological processes in the early stages of the disease, and to implement new methods of treatment and prevention of genital endometriosis.
The aim of this study was to understand a relationship between polymorphic variants of estrogen and progesterone receptor genes and genital endometriosis.
Materials and methods
The study included 890 women: 102 women with genital endometriosis and 788 women in the control group. All women were natives of the Central Black Earth Region of Russia [16], who had no family relationship with each other. The study group was formed by a complete enumeration at the gynecological department of St. Ioasaph's Belgorod Regional Clinical Hospital. Inclusion criteria: isolated genital endometriosis confirmed echographically, hysteroscopically, and morphologically. Exclusion criteria: previously diagnosed malignant diseases of the uterus, ovaries, endometrium, or pregnancy.
The control group included healthy women without proliferative diseases of the reproductive organs, who was treated in the clinical and diagnostic department of the Perinatal Center of St. Ioasaph's Belgorod Regional Clinical Hospital.
The average age of women with genital endometriosis (41.45±6.34 years) and the control group (41.76±6.89 years) was comparable (Mann–Whitney U-test, p>0.05). This study was approved by the Regional Ethics Committee of the Belgorod State National Research University (Protocol No.7 dated April 18, 2013). All clinical, clinical and instrumental, and genetic studies were performed with informed consent to use biologic material for medical and diagnostic purposes related to the disease.
The material for the study was genomic DNA isolated by phenol-chloroform extraction from peripheral blood leukocytes. The following polymorphic loci of progesterone and estrogen receptors were selected for the analysis: ЕSR1 с453-397Т>С rs2234693, ЕSR1 c.1029Т>С rs3798577, ЕSR1 с.453-351А>G rs9340799, РGR c.38Т>С rs484389, РGR c.1415-11113G>Т rs1042838. To select these polymorphic loci, the data were extracted from the HarloReg database (v.4.1.) (http://compbio.mit.edu/HaploReg), which include the description of their regulatory potential and impact on the level of gene expression [17, 18].
The analysis of the molecular genetic loci of the genes of progesterone and estrogen receptors was carried out by the Real-Time PCR method using oligonucleotide primers and probes (Syntol LLC, Russia) (Table 1).
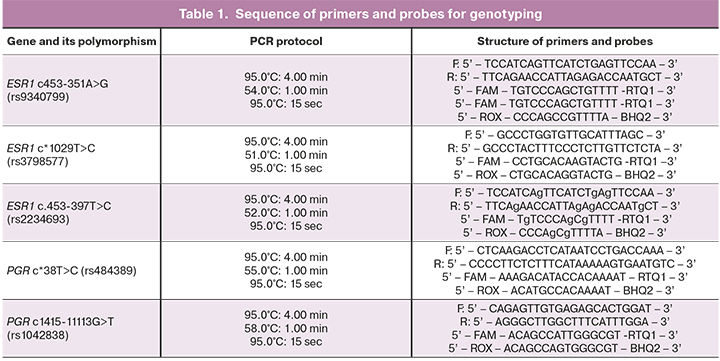
Statistical analysis
The distribution of alleles and genotypes of the polymorphic variants included in the study was assessed in women with genital endometriosis and in the control group, using 2×2 contingency tables and the χ2 test with Yates's correction for continuity. Odds ratio (OR) and its 95% confidence interval (95% CI) were used to identify associations of polymorphic markers with the development of genital endometriosis. The Bonferroni correction (pBonf) was used to avoid the problem of multiple comparisons associated with false-positive results (type I error). The pBonf≤0.05 was considered as statistically significant parameter.
Statistical data processing was performed using STATISTICA for Windows 10.0 software.
Associations of combinations of alleles and genotypes of the analyzed genes with the development of genital endometriosis were assessed using the ARSampler program (https://sourceforge.net/projects/apsampler/) based on the Markov chain Monte Carlo method and Bayesian nonparametric method [19, 20]. Permutation test (pperm) was used to confirm the found associations. Statistically significant level was at pperm<0.05.
Results and discussion
Comparative analysis of the rates of alleles and genotypes of the studied genetic markers in patients with genital endometriosis and in the control group are shown in Tables 2 and 3.
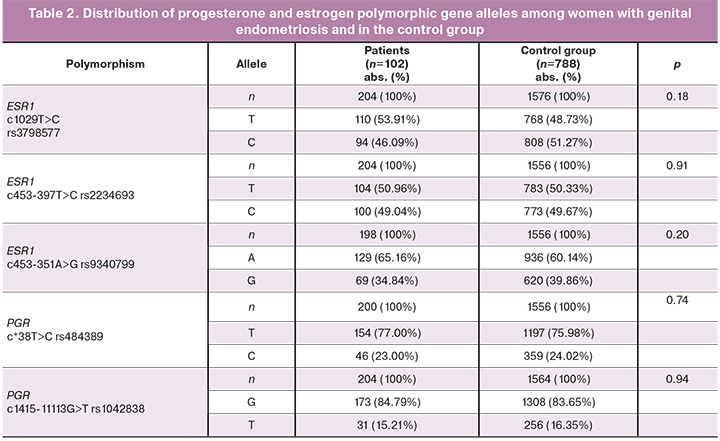
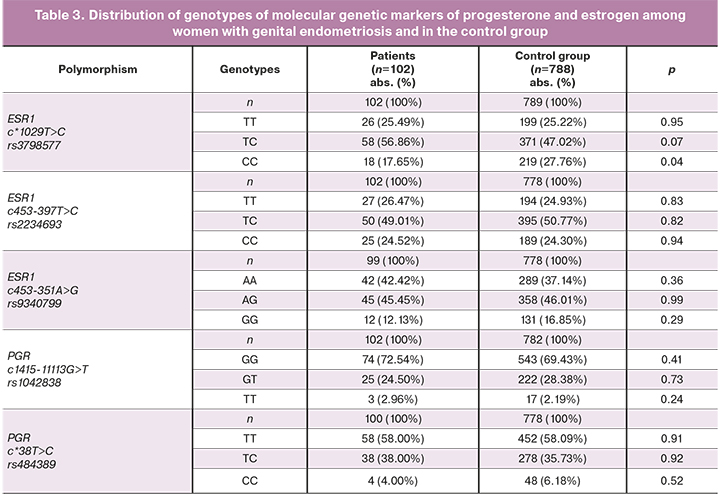
The distribution of genotypes was consistent with the expected data at the Hardy– Weinberg equilibrium for all studied polymorphic loci.
The incidence of CC of ESR1rs3798577 genotype was found to be low in women with genital endometriosis (17.65%) compared to the control group (27.76%, χ2=3.42, p=0.04, OR=0.55, 95% CI 0.3–0.97). However, when Bonferroni correction was used to minimize the risk of type I error associated with false-positive results, the difference between studies patients and control group in the incidence of CC of ESR1 rs3798577 genetic marker appeared not statistically significant (pBonf>0.05). For other studied polymorphic loci of estrogen and progesterone receptors in the analyzed groups, no significant differences were found (p>0.05).
The analysis of combinations of alleles and genotypes of the studied polymorphic variants of progesterone and estrogen receptors revealed reliable differences between women with genital endometriosis and the control group (Table 4). Thus, the combination of the A allele of ESR1 rs9340799 with the T allele of ESR1 rs2234693 and T allele of ESR1 rs3798577 occurs 1.31 times more often in patients with genital endometriosis than in the control group, and this combination is considered as a risk factor for the development of genital endometriosis (OR=1.86, pperm=0.012).
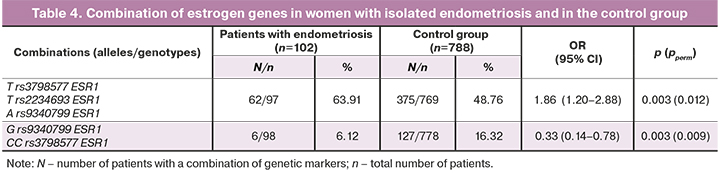
Additionally, the combination of the G allele of ESR1 rs9340799 and the CC genotype of ESR1 rs3798577 occurs 2.5 times less often in women with genital endometriosis than in the control group; this combination protects women from the development of genital endometriosis (OR=0.33, pperm=0.009).
A review of the original studies did not reveal any publications that studied rs3798577 in women with genital endometriosis. The PubMed Central database (https://www.ncbi.nlm.nih.gov/pmc) includes more than 40 publications devoted to the study of the associations of rs3798577 with the development of preeclampsia, miscarriage, development of tumors in the female reproductive system, cardiovascular diseases, etc. For example, Golovchenko O.V. et al. (2020) found that the T allele of ESR1 rs3798577 gene is a risk factor for preeclampsia and fetal growth restriction [21], and Laith N. AL-Eitan et al. (2019) found that the T allele of ESR1 rs3798577 gene is a risk factor for malignant transformation in mammary epithelial cells in Jordanian-Arab women, while the C allele may protect against this disease [22].
Our results are confirmed by the medico-biological effects of estrogens, which influence the regulation of cell proliferation through the activation of intracellular and membrane estrogen receptors [23]. ESR2 expression was found to be associated with the secretory function of the endometrium, while ESR1 is associated with cell proliferation processes [7]. Some studies have found associations of the polymorphic locus of estrogen receptor α gene (ESR1) with diseases of the female reproductive system, including preeclampsia, breast cancer, uterine myoma, and infertility, in different populations [22, 24–26]. Some studies have shown an association of ESR1 polymorphism in women with infertility associated with endometriosis and reproductive health. Ponomorenko I.V. et al. have found the association of ESR1 polymorphism both with BMI and height of adult women, and with endometrial hyperplasia in women of Russian ethnicity [27]. Lamp M. et al. found reliable associations of ESR1 c.453-397T>C (rs2234693) polymorphism with endometriosis [28], and Wang W. et al. showed an association between ESR1 c.453-351A>G (rs9340799) polymorphism with the risk of both endometriosis and infertility with concomitant endometriosis in a female population of China [29].
It is worth noting that currently only associations of individual polymorphic variants of the estrogen receptors are mentioned in the literature, while our study covers significant combinations of molecular genetic markers in the development of genital endometriosis.
Conclusion
The study showed a significant role of molecular genetic markers ESR1 c453-397T>C rs2234693, ESR1 c1029T>C rs3798577, and ESR1 c453-351A>G rs9340799 in the development of genital endometriosis. The combination of G allele of ESR1 rs9340799 and CC genotype of ESR1 rs3798577 (OR=0.33, p=0.003) was found to be protective against the development of genital endometriosis, while the combination of A allele of ESR1 rs9340799, T allele of ESR1 rs2234693 and T allele of ESR1 rs3798577 (OR=1.86, p=0.003) is considered as a risk factor for genital endometriosis. Associations of polymorphic loci RGR c.38T>C rs484389 and RGR c.1415-11113 G>T rs1042838 with the development of genital endometriosis were not found.
References
- Адамян Л.В., Гарданова Ж.Р., Яроцкая Е.Л., Овакимян А.С., Козаченко И.Ф. Особенности болевого синдрома, психоэмоционального состояния и качества жизни женщин с наружным генитальным эндометриозом. Проблемы репродукции. 2016; 22(3): 77-83. [Adamyan L.V., Gardanova Zh.R., Yarotskaya E.L., Ovakimyan A.S., Kozachenko I.F. Features of pain syndrome, psychoemotional state and quality of life of women with external genital endometriosis. Russian journal of human reproduction. 2016; 22(3): 77-83. (in Russian)]. https://dx.doi.org/10.17116/repro201622377-83.
- Балан В.Е., Орлова С.А., Журавель А.С., Овчинникова В.В., Титченко Ю.П., Тихомирова Е.В., Злотникова Ю.П., Торшина З.В., Левкович Е.А., Ананьев В.А., Рижинашвили И.Д., Лазарева И.Н., Белая Ю.М. От истории изучения эндометриоза к современным методам лечения. Российский вестник акушера-гинеколога. 2016; 16(4): 102-6. [Balan V.E., Orlova S.A., Zhuravel A.S., Ovchinnikova V.V., Titchenko Yu.P., Tikhomirova E.V. et al. From the history of the study of endometriosis to modern methods of treatment. Russian bulletin of the obstetrician-gynecologist. 2016;16(4):102-6. (in Russian)]. https://dx.doi.org/10.17116/rosakush2016164102-106.
- Самойлова А.В., Гунин А.Г., Сидоров А.Е., Денисова Т.Г., Чернышов В.В., Смирнова Т.Л. Современные направления изучения этиологии и патогенеза эндометриоза (обзор литературы). Проблемы репродукции. 2020; 26(5): 118-32. [Samoilova A.V., Gunin A.G., Sidorov A.E., Denisova T.G., Chernyshov V.V., Smirnova T.L. Modern trends in the study of the etiology and pathogenesis of endometriosis (literature review). Russian journal of human reproduction. 2020;26(5):118-32. (in Russian)]. https://dx.doi.org/10.17116/repro202026051118.
- Адамян Л.В., Салимова Д.Ф., Кондратович Л.М. Патогенетические аспекты эндометриоз-ассоциированного бесплодия. Проблемы репродукции. 2015; 21(6): 9096. [Adamyan L.V., Salimova D.F., Kondratovich L.M. Pathogenetic aspects of endometriosis-associated infertility. Russian journal of human reproduction. 2015; 21(6): 9096. (in Russian)]. https://dx.doi.org/10.17116/repro201521682-88.
- Jeon D.S., Kim Т.H., Lee H.H., Byun D.W. Endometriosis in a postmenopausal woman on hormonal replacement therapy. J. Мenopausal Мed. 2013; 19(3): 151-3. https://dx.doi.org/10.6118/jmm.2013.19.3.151.
- Артымук Н.В., Тачкова О.А., Данилова Л.Н. Современные возможности медикаментозного контроля эндометриоза. Доктор.Ру. 2015; 11: 39-44. [Artymuk N.V., Tachkova O.A., Danilova L.N. Modern possibilities of drug control of endometriosis. Doctor.ru. 2015; 11:39-44. (in Russian)].
- Golovchenko O., Abramova M., Ponomarenko I., Reshetnikov E., Aristova I., PolonikovA., Dvornyk V., Churnosov M. Functionally significant polymorphisms of ESR1and PGR and risk of intrauterine growth restriction in population of Central Russia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020; 253: 52-7. https://dx.doi.org/10.1016/j.ejogrb.2020.07.045.
- Довжикова И.В., Андриевская И.А. Рецепторы эстрогенов (обзор литературы). Часть 2. Бюллетень физиологии и патологии дыхания. 2019; (73): 125-33. [Dovzhikova I.V., Andrievskaya I.A. Estrogen receptors (literature review). Part 2. Bulletin Physiology and Pathology of Respiration. 2019;(73):125-33. (in Russian)]. https://dx.doi.org/10.36604/1998-5029-2019-73-125-133.
- Monsivais D., Dyson M.T., Yin P., Coon J.S., Navarro A., Feng G. et al. ERβ- and prostaglandin E2-regulated pathways integrate cell proliferation via Ras-like and estrogen-regulated growth inhibitor in endometriosis. Mol. Endocrinol. 2014; 28(8): 1304-15. https://dx.doi.org/10.1210/me.2013-1421.
- Пономаренко И.В., Полоников А.В., Чурносов М.И. Ассоциация полиморфизма rs4986938 гена ESR2 с развитием гиперплазии эндометрия. Акушерство и гинекология. 2019; 4: 66-72. [Ponomarenko I.V., Polonikov A.V., Churnosov M.I. Association of rs4986938 polymorphism of the ESR2 gene with the development of endometrial hyperplasia. Obstetrics and gynecology. 2019; 4: 66-72. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.4.66-72.
- Yilmaz B.D., Bulun S.E. Endometriosis and nuclear receptors. Hum. Reprod. Update. 2019; 25(4): 473-85. https://dx.doi.org/10.1093/humupd/dmz005.
- Yang H., Kang K., Cheng C., Mamillapalli R., Taylor H.S. Integrative analysis reveals regulatory programs in endometriosis. Reprod. Sci. 2015; 22(9): 1060-72. https://dx.doi.org/10.1177/1933719115592709.
- Monsivais D., Dyson M.T., Yin P., Navarro A., Coon JST., Pavone M.E., Bulun S.E. Estrogen receptor beta regulates endometriotic cell survival through serum and glucocorticoid-regulated kinase activation. Fertil. Steril. 2016; 105(5): 1266-73. https://dx.doi.org/10.1016/j.fertnstert.2016.01.012.
- Пономаренко И.В., Полоников А.В., Верзилина И.Н., Чурносов М.И. Молекулярно-генетические детерминанты развития эндометриоза. Вопросы гинекологии, акушерства и перинатологии. 2019; 18(1): 82-6. [Ponomarenko I.V., Polonikov A.V., Verzilina I.N., Churnosov M.I. Molecular genetic determinants of endometriosis development. Questions of gynecology, obstetrics and perinatology. 2019;18(1):82-6. (in Russian)]. https://dx.doi.org/10.20953/1726-1678-2019-1-82-86.
- Matalliotakis M., Zervou M.I., Matalliotaki C., Rahmioglu N., Koumantakis G., Kalogiannidis I. et al. The role of gene polymorphisms in endometriosis. Mol. Med. Rep. 2017; 16(5): 5881-6. https://dx.doi.org/10.3892/mmr.2017.7398.
- Пономаренко И.В., Решетников Е.А., Полоников А.В., Чурносов М.И. Полиморфный локус rs314276 гена LIN28B ассоциирован с возрастом менархе у женщин Центрального Черноземья России. Акушерство и гинекология. 2019; 2: 98-104. [Ponomarenko I.V., Reshetnikov E.A., Polonikov A.V., Churnosov M.I. The polymorphic locus rs314276 of the LIN28B gene is associated with menarche age in women of the Central Chernozem region of Russia. Obstetrics and gynecology. 2019; 2: 98-104. (in Russian)]. https://dx.doi.org/ 10.18565/aig.2019.2.98-104.
- Ward L.D., Kellis M. HaploReg v4: systematic mining of putative causal variants, cell types, regulators and target genes for human complex traits and disease. Nucleic Acids Res. 2016; 44(D1): D877-81. https://dx.doi.org/10.1093/nar/gkv1340.
- Решетников Е.А. Поиск ассоциаций генов-кандидатов, дифференциально экспрессирующихся в плаценте, с риском развития плацентарной недостаточности с синдромом задержки роста плода. Научные результаты биомедицинских исследований. 2020; 6(3): 338-49. [Reshetnikov E.A. Study of associations of candidate genes differentially expressing in the placenta with the development of placental insufficiency with fetal growth restriction. Research Results in Biomedicine. 2020; 6(3): 338-49. (in Russian)]. https://dx.doi.org/10.18413/2658-6533-2020-6-3-0-5.
- Favorov A.V., Andreewski T.V., Sudomoina M.A., Favorova O.O., Parmigiani M.F. A Markov chain Monte Carlo technique for identification of combinations of allelic variants underlying complex diseases in humans. Genetics. 2005; 171(4): 2113-21. https://dx.doi.org/10.1534/genetics.105.0480901.
- Lvovs D., Favorova O.O., Favorov A.V. A polygenic approach to the study of polygenic diseases. Acta Naturae. 2012; 4(3): 59-71.
- Головченко О.В., Абрамова М.Ю., Пономаренко И.В., Чурносов М.И. Полиморфные локусы гена ESR1 ассоциированы с риском развития преэклампсии с задержкой роста плода. Акушерство, гинекология и репродукция. 2020; 14(6): 583-9. [Golovchenko O.V., Abramova M.Yu., Ponomarenko I.V., Churnosov M.I. Polymorphic loci of the ESR1 gene are associated with the risk of developing preeclampsia with fetal growth retardation. Obstetrics, Gynecology and Reproduction. 2020;14(6):583-91. (in Russian)]. https://dx.doi.org/110.17749/2313-7347/ob.gyn.rep.2020.187.
- Al-Eitan L.N., Rababa'h D.M., Alghamdi M.A., Khasawneh R.H. Association between ESR1, ESR2, HER2, UGT1A4, and UGT2B7 polymorphisms and breast Cancer in Jordan: a case-control study. BMC Cancer. 2019; 19(1): 1257. https://dx.doi.org/10.1186/s12885-019-6490-7.
- Wang H.S., Wu H.M., Cheng B.H., Yen Chih-Feng, Chang Pi-Yueh, Chao A. et al. Functional analyses of endometriosis-related polymorphisms in the estrogen synthesis and metabolism-related genes. PLoS One. 2012; 7(11): e47374. https://dx.doi.org/10.1371/journal.pone.0047374.
- Wetendorf М., DeМayo F.J. Тhe progesterone receptor regulates implantation, decidualization, and glandular development via a complex paracrine signaling network . Мol. Cell. Endocrinol. 2012; 357(1-2): 108-18. https://dx.doi.org/10.1016/j.mce.2011.10.028.
- Zhao G., Cai Y., Liu J., Meng T. Association between the estrogen receptor α gene polymorphisms rs2234693 and rs9340799 and severe and mild pre-eclampsia: a meta-analysis. Biosci. Rep. 2019; 39(2): BSR20181548. https://dx.doi.org/10.1042/BSR20181548.
- Xie J., Wang S., He B., Pan Y., Li Y., Zeng Q. et al. Association of estrogen receptor α and interleukin-10 gene polymorphisms with endometriosis in a Chinese population. Fertil. Steril. 2009; 92(1): 54-60. https://dx.doi.org/10.1016/j.fertnstert.2008.04.069.
- Пономаренко И.В., Полоников А.В., Чурносов М.И. Молекулярные механизмы и факторы риска развития эндометриоза. Акушерство и гинекология. 2019; 3: 26-31. [Ponomarenko I.V., Polonikov A.V., Churnosov M.I. Molecular mechanisms and risk factors for endometriosis. Obstetrics and gynecology. 2019;3:26-31. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.3.26-31.
- Lamp M., Peters M., Reinmaa E., Haller-Kikkatalo K., Kaart T., Kadastik U. et al. Polymorphisms in ESR1, ESR2 and HSD17B1 genes are associated with fertility status in endometriosis. Gynecol. Endocrinol. 2011; 27(6): 425-33. https://dx.doi.org/10.3109/09513590.2010.495434.
- Wang W., Li Y., Maitituoheti M., Yang R., Wu Z., Wang T. et al. Association of an oestrogen receptor gene polymorphism in Chinese Han women with endometriosis and endometriosis-related infertility. Reprod. Biomed. Online. 2013; 26(1): 93-8. https://dx.doi.org/10.1016/j.rbmo.2012.09.007.
Received 31.03.2021
Accepted 09.06.2021
About the Authors
Oksana B. Altukhova, MD, Associate Professor, Department of Obstetrics and Gynecology of the Medical Institute, Belgorod State National Research University,+7(4722)30-13-83, kristalinka@yandex.ru, 308015, Russia, Belgorod, Pobedy str., 85.
Viktor E. Radzinsky, MD, Professor, Honored Scientist of the Russian Federation, Academician of the International Academy of Sciences of the Higher School,
Head of the Department of Obstetrics and Gynecology, Faculty of Medicine, Peoples' Friendship University of Russia, +7(495)360-46-69, radzinskiy-ve@rudn.ru,
117198, Russia, Moscow, Miklukho-Maklaya str., 6.
Svetlana S. Sirotina, PhD (Bio), Associate Professor, Department of Biomedical Disciplines of the Medical Institute, Belgorod State National Research University,
+7(4722)30-13-83, sirotina@bsu.edu.ru, https://orcid.org/0000-0002-4163-7863, 308015, Russia, Belgorod, Pobedy str., 85.
Mikhail I. Churnosov, MD, Professor, Head of the Department of Biomedical Disciplines of the Medical Institute, Belgorod State National Research University,
+7(4722)30-13-83, churnosov@bsu.edu.ru, https://orcid.org/0000-0003-1254-6134, 308015, Russia, Belgorod, Pobedy str., 85.
Corresponding author: Svetlana S. Sirotina, sirotina@bsu.edu.ru
Authors’ contributions: Churnosov M.I., Altukhova O.B., Radzinsky V.E. – the concept and design of the study; Altukhova O.B., Churnosov M.I. – material collection and processing; Sirotina S.S. – writing the article; Churnosov M.I., Altukhova O.B. – editing the text of the article.
Conflicts of interest: The authors declare no conflict of interest.
Funding: The study was carried out without any sponsorship.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Altukhova O.B., Radzinsky B.E., Sirotina S.S., Churnosov M.I. Analysis of the association between the polymorphic variants of estrogen and progesterone receptor genes and genital endometriosis.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 9: 93-99 (in Russian)
https://dx.doi.org/10.18565/aig.2021.9.93-99



