Синдром поликистозных яичников (СПКЯ) рассматривают как эндокринно-метаболический симптомокомплекс, частота которого в популяции среди женщин репродуктивного возраста достигает 20%. По оценкам National Institute of Health (NIH), в 2012 г. только в США число женщин репродуктивного возраста с СПКЯ достигло 5 млн [1]. Многообразие клинических проявлений СПКЯ создает ряд трудностей в диагностике синдрома. По данным исследования Gibson-Helm M. (2016), каждая третья женщина с СПКЯ тратит более двух лет на постановку корректного диагноза, посетив более трех специалистов [2]. Вероятно, это связано с тем, что традиционно используемые Роттердамские критерии нельзя считать абсолютно надежными ввиду недостаточной чувствительности и специфичности каждого признака, взятого в отдельности. Наиболее перспективным с позиций как оптимизации диагностики, так и изучения патогенетических основ синдрома представляется проведение молекулярно-генетических исследований. Генетические маркеры СПКЯ могли бы использоваться в повседневной практике и как самостоятельные критерии для выявления женщин группы риска по развитию синдрома, и в качестве метода постановки корректного диагноза у пациенток со стертой симптоматикой. В более ранних исследованиях изучалась ассоциация СПКЯ с полиморфизмом генов андрогенового рецептора, гена инсулинового рецептора и генов, регулирующих его активность [3, 4]. Проведение полногеномных исследований в группе из 16 тысяч женщин китайской популяции позволило установить ассоциацию СПКЯ с полиморфизмом 11 генов [5, 6]. Это гены, ответственные за действие гонадотропинов и биосинтез андрогенов (LHCGR, FSHR, DENND1A), функцию сигнальных путей инсулина (INSR), а также гены, ассоциированные с развитием сахарного диабета (HMG2, THADA и область, содержащая гены RAB5B/SUOX, ERBB3), регулирующие рост органов и клеточную пролиферацию (YAP1, SUMO1P1, ТОХ3). Воспроизведение результатов этих исследований американскими и европейскими учеными позволило установить ассоциацию СПКЯ с полиморфизмом 6 генов (LHCGR, FSHR, DENND1A, THADA, RAB5B/SUOX, YAP1) [7–9]. Российскими учеными исследован полиморфизм генов FMR1, CYP11A1, CYP17, CYP19 [10, 11], однако не представлены данные об ассоциации локусов, ранее выявленных в ходе полногеномных исследований. В связи с чем целью проведенного исследования явилось изучение взаимосвязи СПКЯ с полиморфизмом генов, ассоциированных с метаболической дисфункцией, нарушением биосинтеза андрогенов и фолликулогенеза.
Материалы и методы
В исследование были включены 298 женщин, из них 163 с СПКЯ в возрасте от 18 до 35 лет (средний возраст – 25,2 (4,6) года, средний индекс массы тела (ИМТ) – 23,9 (5,3) кг/м2) и 135 женщин группы контроля (средний возраст – 49,8 (5,8) года, средний ИМТ – 27,4 (5,8) кг/м2). Критериями включения в основную группу явились: наличие СПКЯ, установленного в соответствии с Роттердамскими критериями (2003), отсутствие сопутствующей эндокринной и некомпенсированной экстрагенитальной патологии, а также гормональной терапии в течение 3 месяцев до включения в исследование. Группу контроля составили женщины старше 45 лет, c реализованной репродуктивной функцией без нарушения менструального цикла, преждевременной недостаточности яичников, СПКЯ и андрогенизации в анамнезе.
Всем пациенткам с СПКЯ на 5–7-й день менструального цикла проведено ультразвуковое трансвагинальное исследование органов малого таза с частотой 7,5 Мгц на аппарате 2000 Toshiba SSA-240 (Япония). На 2–3-й день менструального цикла в сыворотке крови оценивались уровни лютеинизирующего (ЛГ), фолликулостимулирующего (ФСГ) гормонов, общего и свободного тестостерона (Тобщ и Тсв), андростендиона (А4), глобулина, связывающего половые стероиды (ПССГ), пролактина, тиреотропного гормона (ТТГ) (иммунохемилюминесцентным методом на автоматическом анализаторе Immulite 2000 (Siemens, USA)), уровень антимюллерова гормона (АМГ) (методом ELISA с использованием тест-систем AMH Gen II ELISA (Beckman Coulter, USA)). На основании результатов клинико-лабораторного исследования определяли фенотип СПКЯ – классический фенотип А (гиперандрогения (ГА) + поликистозные яичники (ПКЯ) + олигоменорея (ОМ)), ановуляторный фенотип В (ГА + ОМ), овуляторный фенотип С (ГА + ПКЯ) и неандрогенный фенотип Д (ПКЯ + ОМ). Проведен 2-часовой оральный глюкозотолерантный тест с 75 г глюкозы с определением уровня инсулина на фоне углеводной нагрузки. В рамках исследования липидного спектра крови определяли уровни холестерина, триглицеридов, липопротеидов низкой и высокой плотности, производили расчет коэффициента атерогенности.
Анализ полиморфизма генов производился в лаборатории молекулярно-генетических методов ФГБУ «НМИЦ АГП им. академика В.И. Кулакова» МЗ РФ. Генотипирование по 45 локусам проводилось с применением полимеразной цепной реакции с анализом кривых плавления или таргетного секвенирования на приборах IonS5, чипы 520 и 530 (таблица).
Статистический анализ
Статистический анализ проводился с помощью программы SPSS (IBM Statistical Package for the Social Sciences, 21-я версия). С помощью теста Колмогорова–Смирнова установлено нормальное распределение данных. Количественные показатели представлены как среднее арифметическое и стандартное отклонение (М (SD)). Сравнение производилось с помощью t-критерия Стьюдента. Сравнение качественных характеристик производилось с помощью критерия χ2 Пирсона. Количественная оценка риска проводилась на основании расчета отношения шансов (ОШ) с доверительным интервалом 95%. ОШ выбрано для интерпретации рисков применительно к аллелям и генотипам как симметричная величина, наиболее приемлемая для генетических исследований. Cтатистически значимыми считались результаты при достижении уровня ошибки p<0,05.
Результаты и обсуждение
Проведение клинико-лабораторного обследования показало, что средний уровень ЛГ составил 11,3 (6,8) МЕ/мл, ФСГ – 5,8 (1,6) МЕ/мл, АМГ – 11,8 (7,8) пг/мл, ПССГ – 50,0 (23,6) нмоль/л, Тобщ – 2,8 (1,3) нмоль/л, Тсв – 6,4 (5,3) нмоль/л, А4 – 4,3 (6,1) нмоль/л. Биохимическая ГА (фенотипы А+В+С) диагностирована у 73,0% пациенток (n=119), неандрогенный фенотип D – у 27,0% (n=44) пациенток. Частота нарушенной толерантности к глюкозе составила 20,8%, инсулинорезистентности – 28,2%, дислипидемии – 33,7%. Анализ распределения частот генотипов и аллелей среди пациенток с СПКЯ женщин группы контроля показал ассоциацию СПКЯ с 6 полиморфными локусами: SLCO1B1 [rs4149056], YAP1 [rs1894116], RAB5B/SUOX [rs705702], THADA [rs12468394], OCT1 [rs6282031], DENND1A [rs10818854] (рис. 1). Исходя из этого, значимая взаимосвязь с СПКЯ установлена только для 4 (YAP1, RAB5B/SUOX, THADA, DENND1A) из 11 генов, ранее заявленных как гены-кандидаты СПКЯ по результатам полногеномных китайских исследований. Авторы предшествующих зарубежных исследований предполагают участие генов SLCO1B1, Rub5B/SUOX, THADA, OCT1 в формировании метаболической дисфункции при СПКЯ, а генов YAP1 и DENND1A – в генезе репродуктивных нарушений и ГА [12, 13].
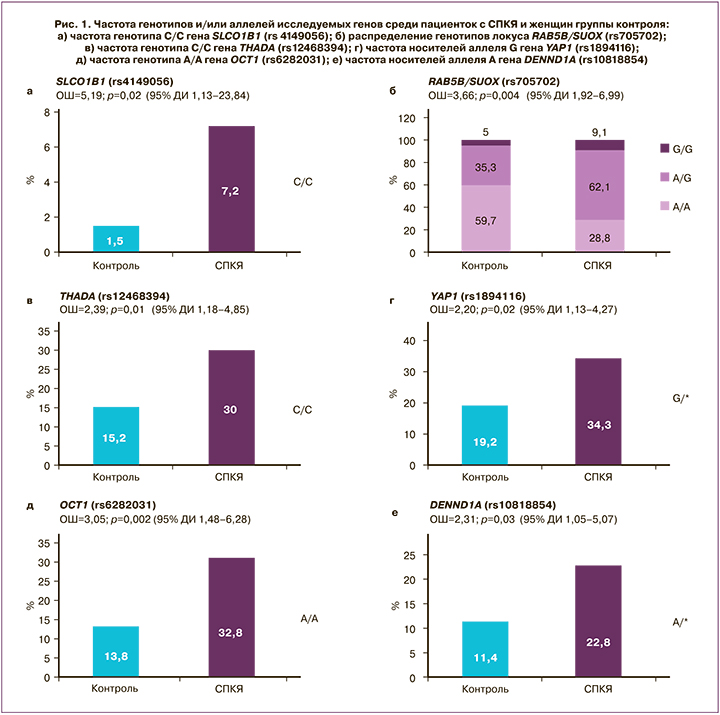
Ассоциация СПКЯ с полиморфизмом гена DENND1A, кодирующего белок DENN/MADD домен 1А, широко экспрессирующийся в тканях и вовлеченный в эндосомальный мембранный трафик, впервые была обнаружена в ходе полногеномных исследований на китайской популяции [6]. Согласно результатам представленного исследования, носительство аллеля А гена DENND1A rs10818854 увеличивает риск развития СПКЯ в 2,3 раза по сравнению с носителями генотипа G/G; предполагается аутосомно-доминантная модель наследования (р=0,03; ОШ=2,31; 95% ДИ 1,05–5,07) (рис. 1е). В исследовании Ye Tian et al. (Китай, 2020) указывается на взаимосвязь полиморфизма DENND1A (rs2479106, rs10818854) с СПКЯ [14]. Результаты настоящего исследования согласуются с работами европейских авторов, в которых данная взаимосвязь обнаружена только для rs10818854 [7]. Предполагают, что DENND1A может затрагивать различные механизмы формирования СПКЯ, как ассоциированные, так и не ассоциированные с инсулинорезистентностью [12, 13], которой отводится важная роль в патогенезе эндометриоидной аденокарциномы [15]. Функциональные исследования показали, что при СПКЯ в клетках теки яичников отмечается увеличение экспрессии DENND1A.V2, что приводит к формированию поликистозной морфологии и к повышенному синтезу андрогенов [16].
Локус RAB5B/SUOX (rs705702) расположен на хромосоме 12q13.2 между генами RAB5B и SUOX. Ген RAB5B является Rab-ГТФ-азой, которая, по имеющимся данным, вовлечена в эндоцитоз и рециркуляцию рецепторов и является молекулой, взаимодействующей с доменом DENND, а также с PI3K, PKB и MAPK/ERK [17]. Впервые данные о взаимосвязи rs705702 с СПКЯ были представлены в работе китайских исследователей Shi et al. [5.], а позднее аналогичные результаты были получены группой европейских ученых [18]. Согласно результатам проведенного нами исследования, носители генотипов А/G и G/G локуса RAB5B/SUOX (rs705702) имеют в 3,6 раза больший шанс развития СПКЯ по сравнению с женщинами, гомозиготными по аллелю А (генотип А/А, рис. 1б). Предполагается аутосомно-доминантная модель наследования (р<0,001; ОШ=3,66; 95% ДИ 1,92–6,99). Поиск взаимосвязей полиморфизма данного гена с клинико-лабораторными показателями пациенток с СПКЯ установил, что носители аллеля G гена RAB5B/SUOX (rs705702), по сравнению с носителями генотипа А/А, имели более высокие значения тощаковой глюкозы (5,65 (0,07) ммоль/л и 5,00 (0,09) ммоль/л соответственно; р<0,05). Взаимосвязь полиморфизма гена RAB5B с метаболической дисфункцией продемонстрирована в работе Welter D. (2014), согласно результатам которой, RAB5B известен как локус риска сахарного диабета 1 типа и ожирения у детей. Другим геном-кандидатом СПКЯ, ассоциированным с увеличенным риском метаболической дисфункции, считается ген THADA (thyroid adenoma associated), первоначально связанный с аденомой щитовидной железы и с сахарным диабетом 2 типа [19]. Известно, что ген THADA принимает участие в регуляции работы β-клеток поджелудочной железы. Однако не все исследования подтвердили взаимосвязь полиморфизма гена ТНАDA с сахарным диабетом 2 типа или инсулинорезистентностью после внесения поправки по ИМТ [20, 21]. Взаимосвязь гена THADA и СПКЯ впервые была продемонстрирована в ходе полногеномных исследований на популяции китайских женщин [6] и позднее воспроизведена на других когортах [7, 8] в ходе семейных исследований [22] и в межэтническом метаанализе [18]. Согласно результатам нашего исследования, носительство генотипа С/С по сравнению с генотипом А/* увеличивает риск развития СПКЯ в 2,4 раза (р=0,01; ОШ=2,39; 95% ДИ 1,18–4,85) (рис. 1в). Предполагается аутосомно-рецессивная модель наследования.
Ген YAP1, который рассматривают как транскрипционный ко-активатор сигнального Hippo-пути, принимает участие в регуляции роста органов, в том числе яичников. Согласно результатам проведенного исследования, носительство аллеля G гена YAP1 (rs1894116) увеличивает риск развития СПКЯ в 2,2 раза по сравнению с генотипом А/*. Установлена аутосомно-доминантная модель наследования (р=0,02; ОШ=2,20; 95% ДИ 1,13–4,27) (рис. 1г). Носители генотипа G/G гена YAP1 (rs1894116), по сравнению с женщинами с генотипом А/*, обладали высоким уровнем Тобщ (4,5 (3,7) нмоль/л и 1,9 (1,3) нмоль/л соответственно, р=0,02) и индекса свободных андрогенов (37,5 (15,2) и 4,7 (2,9) соответственно, р=0,04). Ген YAP1 кодирует Yes-ассоциированный протеин, ключевой транскрипционный коактиватор Hippo-пути, регулирующий рост тканей и органов, а также процессы апоптоза [23, 24]. YAP1 принимает участие во множестве сигнальных путей, которые регулируют морфологию органов, в том числе, вероятно, увеличение размеров яичников, ключевой признак СПКЯ. Здоровые женщины существенно отличаются по частоте полиморфизма гена YAP1 (rs11225161, rs11225138 и rs11225166), и данный ген можно рассматривать как новый потенциальный ген-кандидат развития СПКЯ [25]. В настоящее время исследования по изучению функции данного гена и его влияния на развитие СПКЯ немногочисленны. Есть данные о том, что YAP1 реализует свое действие через регуляцию процессов фосфорилирования. Более того, предполагается, что в генезе СПКЯ играет роль степень метилирования гена YAP1, которая оказалась значительно более низкой по сравнению с женщинами группы контроля; при этом наблюдалось значительное повышение уровня mRNA YAP1 и экспрессии белка. Показано, что тестостерон может приводить к гипометилированию гена YAP1, был продемонстрирован дозозависимый эффект [26]. В настоящем исследовании полиморфизм гена YAP1 (rs1894116) ассоциирован с двукратным увеличением риска СПКЯ и повышенными концентрациями Тобщ, что согласуется с результатами представленного выше исследования. Белковый продукт гена YAP1, Yes-1-тирозинкиназа, является членом семейства нерецепторных Src тирозинкиназ, которые широко экспрессируются во всех органах и тканях и регулируют функцию ряда мембранных белков; в том числе субстратом Yes-1-тирозинкиназы является транспортер органических катионов ОСТ (organic cation transporter ).
В настоящем исследовании установлена взаимосвязь генотипа А/А гена ОСТ1 rs6282031 с СПКЯ, повышающего риск развития синдрома в 3 раза, по сравнению с женщинами с генотипом G/* (р<0,001; ОШ=3,05; 95% ДИ 1,48–6,28) (рис. 1д). Ген ОСТ1 является высокополиморфным, что приводит к большому количеству аминокислотных замен и нарушает транспорт катионов через мембрану гепатоцитов. Данные о вкладе полиморфизма гена в формирование СПКЯ достаточно противоречивы. Некоторые авторы считают, что ген ОСТ1 не принимает участия в генезе ПКЯ, ОМ или ГА, однако может способствовать развитию инсулинорезистентности и сахарного диабета 2 типа [27]. Генотип А/А гена OCT1 (rs6282031), по результатам проведенного исследования, ассоциировался с более высоким уровнем глюкозы (7,90 (2,11) ммоль/л и 7,45 (1,81) ммоль/л соответственно, p<0,05) и инсулина на фоне глюкозотолерантного теста (103,4 (46,4) мкЕд/мл и 66,6 (38,5) мкЕд/мл соответственно, p<0,05), тогда как все носители G-аллеля имели нормальный уровень инсулина на фоне нагрузки глюкозой.
Ген SLCO1B1, транспортер органических анионов (solute carrier organic anion transporter), кодирует полипептид, транспортирующий органические анионы 1B1 (ОАТР1В1) в гепатоцитах. Результаты настоящего исследования показали, что обладательницы гомозиготного генотипа С/С гена SLCO1B1 [rs4149056] имеют больший риск развития СПКЯ (р=0,02; ОШ=5,19; 95% ДИ 1,13–23,84) по сравнению с носителями генотипов Т/* (рис. 1а), однако для точной оценки ОШ необходимо проведение исследования на большей выборке. Группа ученых из Германии представила результаты, согласно которым ген SLCO1B1 участвует в регуляции накопления субстратов для синтеза дегидроэпиандростеронсульфата [28]. Также представлены данные о влиянии изученного нами полиморфизма на конъюгацию эстрогенов и, вероятно, на синтез ПССГ. Ранее выявлена связь аллеля C гена SLCO1B1 (rs414956, 521 T>C) с более высоким ИМТ и повышенным уровнем гликированного гемоглобина. В нашем исследовании установлена ассоциация генотипа С/С гена SLCO1B1 (rs4149056) с более высоким уровнем глюкозы на фоне проведения глюкозотолерантного теста (7,80 (2,11) ммоль/л и 6,70 (1,94) ммоль/л соответственно, р=0,03), более высоким индексом НОМА (9,8 и 6,3 соответственно, р=0,04) по сравнению с пациентками с генотипом Т/*.
 Гетерогенность СПКЯ предполагает различные генетические основы формирования отдельных его признаков. Пациентки с андрогенными фенотипами СПКЯ обладают повышенным риском развития сахарного диабета 2 типа и сердечно-сосудистых заболеваний как по сравнению с женщинами без нарушений репродуктивной системы, так и по сравнению с пациентками с неандрогенным фенотипом, что делает обоснованным раннее выявление пациенток с ГА. Однако оценить андрогенный статус пациенток не всегда представляется возможным в силу проведенной ранее гормональной терапии, конверсии фенотипа с возрастом. Выявление генетических основ ГА при СПКЯ может не только повысить точность диагностики при наличии «стертой» формы, но и способствовать изучению патогенетических механизмов формирования различных фенотипов синдрома.
Гетерогенность СПКЯ предполагает различные генетические основы формирования отдельных его признаков. Пациентки с андрогенными фенотипами СПКЯ обладают повышенным риском развития сахарного диабета 2 типа и сердечно-сосудистых заболеваний как по сравнению с женщинами без нарушений репродуктивной системы, так и по сравнению с пациентками с неандрогенным фенотипом, что делает обоснованным раннее выявление пациенток с ГА. Однако оценить андрогенный статус пациенток не всегда представляется возможным в силу проведенной ранее гормональной терапии, конверсии фенотипа с возрастом. Выявление генетических основ ГА при СПКЯ может не только повысить точность диагностики при наличии «стертой» формы, но и способствовать изучению патогенетических механизмов формирования различных фенотипов синдрома.
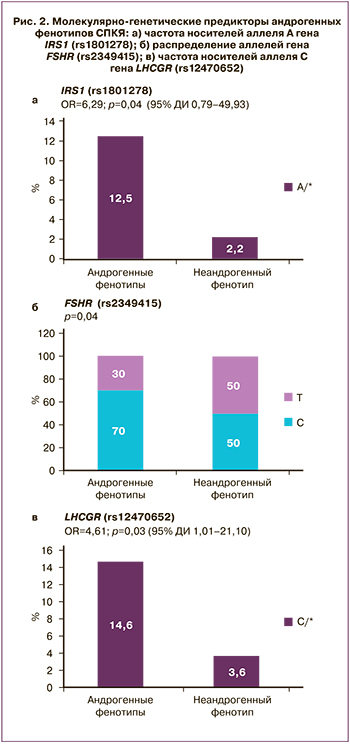
В настоящем исследовании было проведено изучение распределения частоты аллелей и генотипов 45 локусов между пациентками с биохимической ГА (фенотипы А+В+С) и пациентками без ГА (фенотип D). Установлена ассоциация 3 полиморфных локусов с ГА: гены IRS1 (rs1801278), FSHR (rs 2349415), LHCGR (rs12470652) (рис. 2). Среди пациенток с андрогенными фенотипами по сравнению с неандрогенным фенотипом D значительно чаще встречались пациентки с генотипом А/* гена IRS1 rs1801278 по сравнению с генотипом G/G (р=0,04; ОШ=6,29; 95% ДИ 0,79–49,93), пациентки с генотипом С/* гена LCHGR (rs12470652) по сравнению с генотипом Т/Т (р=0,03; ОШ=4,61; 95% ДИ 1,01–21,10), а также носители аллеля С по сравнению с аллелем Т гена FSHR (rs2349415) (р=0,04), однако для точной оценки ОШ и модели наследования целесообразно исследование указанных закономерностей на большей выборке.
Известно, что нарушение функции инсулинового рецептора приводит к развитию выраженной инсулинорезистентности. Активация инcулинового рецептора является реакцией аутофосфорилирования. После связывания инсулина с α-субъединицей рецептора происходит аутофосфорилирование β-субъединиц и в последующем — субстратов инсулинового рецептора, которые обеспечивают трансляцию сигнала в ядро клетки. Эти активированные субстраты запускают каскад последующих процессов. Ассоциация СПКЯ с генами IRS1 и IRS2 была показана в нескольких исследованиях. В некоторых из них при СПКЯ была выявлена более высокая частота мутации Gly972Arg IRS1 [29], в других данная взаимосвязь не обнаружена [30]. В настоящем исследовании среди пациенток с андрогенными фенотипами СПКЯ по сравнению с женщинами с неандрогенным фенотипом D значительно чаще встречалось носительство аллеля А гена IRS1 (rs1801278) по сравнению с аллелем G (р=0,04). Нарушение секреции ЛГ рассматривают как один из важных факторов увеличения синтеза андрогенов и развития СПКЯ, который опосредован снижением конверсии андрогенов в эстрогены. Другим геном-кандидатом развития андрогенного фенотипа СПКЯ, согласно результатам проведенного исследования, является ген FSHR (rs2349415). Ген FSHR расположен на коротком плече 2-й хромосомы и состоит из 14 экзонов, кодирует G-белок-связанный рецептор, взаимодействующий с ФСГ и необходимый для развития и функции гонад. Взаимосвязь мутации гена FSHR (rs2349415) с развитием СПКЯ была показана в ходе китайских полногеномных исследований [6].
Ассоциация гиперандрогении при СПКЯ с носительством полиморфных локусов IRS1 (rs1801278), FSHR (rs 2349415), LHCGR (rs12470652) указывает на генетически детерминированный вклад нарушений функции гипоталамо-гипофизарно-яичниковой оси и метаболической дисфункции в формирование синдрома. В настоящее время продолжаются исследования по изучению молекулярно-генетических основ различных фенотипов синдрома. Показано, что при полиморфизме генов THADA, INSR, TOX3 и DENND1A могут формироваться метаболически неблагополучные формы СПКЯ за счет инсулинорезистентности и сопутствующих метаболических нарушений [14]. Не исключено, что в перспективе распределение пациенток с СПКЯ по фенотипическим подгруппам будет проводиться не только на основании уже известных клинико-лабораторных показателей, но и с учетом генотипа пациентки.
Заключение
Проведенное исследование позволило выявить 6 генов, потенциально повышающих риск развития СПКЯ, что подтверждает мультигенную природу заболевания. Для некоторых локусов (THADA, RAB5B, SUOX) уже известна их ассоциация с нарушениями углеводного обмена или нарушениями фолликулогенеза (DENND1A, YAP1), вклад других в генез СПКЯ еще предстоит выяснить. Для 3 полиморфных локусов установлена ассоциация с формированием андрогенного фенотипа. Это может указывать на их роль в нарушении стероидогенеза как ключевого фактора формирования ГА при СПКЯ. На основе полученных результатов возможны разработка шкалы индивидуального риска развития СПКЯ и проведение популяционного скрининга, наиболее актуального среди девочек-подростков и женщин со стертыми формами синдрома.



