Analysis of vitamin D receptor expression in patients with genital endometriosis
Aim. To evaluate expression of vitamin D receptors (VDR) in endometriotic heterotopies and endometrium of patients with genital endometriosis (GE).Yarmolinskaya M.I., Denisova A.S., Tolibova G.Kh., Bespalova O.N., Tral T.G., Zakuraeva K.A., Pyankova V.O.
Materials and methods. 52 women aged 31.8 (4.3) years were included in the study. The main group consisted of 32 patients with surgically and histologically confirmed GE of stages I-IV according to the r-ASRM classification. The control group included 20 women who were examined for infertility and in whom no pelvic disease was found at diagnostic laparoscopy. The expression of VDR in endometriotic lesions and in endometrium of patients with GE as well as in endometrium of the patients from the control group was evaluated. Qualitative and quantitative evaluation of the results of immunohistochemical study was performed using digital microscopy and morphometry. Excel, Statistica 10 and Jamovi programs were used for results processing.
Results. Endometriotic heterotopies are characterized by decreased values of VDR AE in comparison with both proliferative and secretory endometrium of patients with GE, and secretory endometrium from women of the control group. We did not find cyclic changes in VDR expression in endometrium of patients with GE compared to endometrium of the control group.
Conclusion. The results of study elucidate the role of vitamin D in pathogenesis of endometriosis and give us the opportunity to justify the use of cholecalciferol as a promising therapy for this disease.
Keywords
External genital endometriosis (EGE) is a hormone-dependent, progressive, chronic and recurrent disease. EGE is a multifactorial disease, and the important role in its pathogenesis belongs to genetic, immunological and environmental factors. Clinical manifestation of genital endometriosis remains inexplicable and varied; this disease is diagnosed in more than 256 million women worldwide, causes infertility in 35–50% of patients and chronic pain syndrome in 70–80% of women [1, 2]. Genital endometriosis ranks third among all gynecological diseases, and its prevalence tends to increase. As noted above, endometriosis affects 1 in 10 women of reproductive age; however, there were cases when disease affected women in the antenatal period, in childhood, in adolescence, and in peri- and postmenopausal periods [3, 4].
Despite the modern possibilities of hormone-modulating therapy, endometriosis is still characterized by a high recurrence rate. The medications used for the treatment of EGE have a significant number of side effects and are ineffective in some patients. These aspects and the high prevalence of endometriosis urge the need for the development of new methods of drug therapy that could increase the effectiveness of treatment.
Some researchers suggest that vitamin D may play a certain role in the pathogenesis of EGE due to its antiproliferative, anti-inflammatory, antiangiogenic and immunomodulatory effects [5, 6].
Vitamin D is a generic term for several chemical compounds that have similar structure (secosteroids) and features for the organism. However, it is cholecalciferol that is positioned as a “true” vitamin D, while ergocalciferol, dihydrotachysterol, sigma-calciferol and others are considered only as modified derivatives. The stages of calcitriol formation are well known: under the exposure of short-wave ultraviolet radiation on the epidermis 7-dehydrocholesterol is converted to previtamin D3. Then, in the liver and kidneys, respectively, two consecutive hydroxylation reactions take place, converting cholecalciferol into calcitriol – a biologically highly active metabolite with a mechanism of action similar to the steroid hormones [7–9].
A hormone-like role of vitamin D arise a lot of discussions; this feature of vitamin D is evidenced by the ability of calcitriol to bind to specific vitamin D receptors (VDR) located in various tissues and organs, including immune cells, pancreas, skeletal muscles, and the reproductive system [8]. It is believed that the active hormonal form of vitamin D, after binding to the receptors, can provoke such mechanisms of EGE pathogenesis as proliferation, inflammation, neoangiogenesis, and the immune response.
The aim of the study is to evaluate the VDR expression in endometriotic lesions and in the endometrium of patients with EGE compared to the control group.
Materials and methods
The study included 52 patients aged from 23 to 43 years, the mean value was 31.8 (4.3) years.
All patients gave informed consent for participation in the study.
The main group consisted of 32 women with surgically and histologically confirmed EGE. The prevalence rate of the disease was estimated according to the revised classification of the American Society for Reproductive Medicine (r-ASRM): EGE stage I was confirmed in 25% of women, stage II – in 25%, stage III – in 37.5% of patients, stage IV – in 12.5% of patients. The study did not include women with concomitant gynecological pathology (polycystic ovary syndrome, endometrial hyperplasia/polyps and uterine fibroids), with severe somatic diseases, diabetes mellitus, and those taking hormones and/or vitamin D for 6 months before surgery.
Samples for morphological examination were obtained in proliferative and secretory phases of menstrual cycle (days 7 to 11 and days 18 to 22, respectively).
VDR expression in the main group was evaluated in endometriotic lesions and in the endometrium during the secretory (n=22) and the proliferative phases (n=10) of the menstrual cycle.
The control group included 20 women who were examined for infertility and in whom gynecological pathology was not found during diagnostic laparoscopy. In 50% of patients from this group, VDR expression in the endometrium was evaluated in the proliferative phase of the menstrual cycle, and in the other 10 patients – in the secretory phase of the menstrual cycle.
Morphological examination included histological and immunohistochemical (IHC) analysis of endometriotic lesions and endometrium of patients of the main group and the control group. The histological analysis was performed using a standard technique with staining of the material with hematoxylin and eosin [10].
IHC analysis
For the IHC examination, samples were prepared on 3 μm thick paraffin sections. The Abcam Rabbit Specific HRP Plus (ABC) Detection IHC Kit (RTU) [ab93697], (Abcam, UK) was used as a detection system. A standard one-step protocol with antigen retrieval in 0.01 M citrate buffer, pH 6.0, was performed [11]. The IHC examination included a quantitative and qualitative evaluation of Anti Vitamin D Receptor expression (clone ab 3508) – reagents manufactured by Abcam (UK) - with a standard 1: 200 dilution. Morphometry was performed in a program "Morphology 5.2".
After staining, the VDR expression was evaluated in 5 fields of view and calculated by determining the ratio of the area of immunopositive cells to the total area of the preparation [12]:

Statistical analysis
Excel, Statistica 10, and Jamovi programs were used for database compilation and statistical analysis. Quantitative variables with normal distribution were produced by the mean and standard deviation M (SD), and in the case of a distribution other than normal, by the median, 25th and 75th quartiles of Me (Q1; Q3).
All groups in this study were independent, since the sampling was performed once per cycle, i.e., the groups divided according to the phase of the menstrual cycle included data from samples taken from different groups of patients. To analyze non-normally distributed data in independent samples we used: Kruskal–Wallis one-way analysis of variance followed by multiple pairwise comparisons using Dwass–Steel–Critchlow–Fligner method (DSCF).
Differences were considered statistically significant at p<0.05.
Results
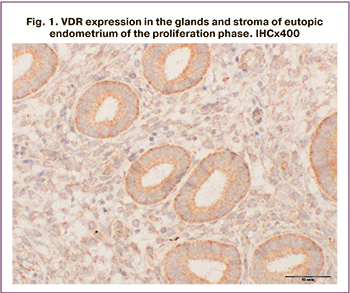
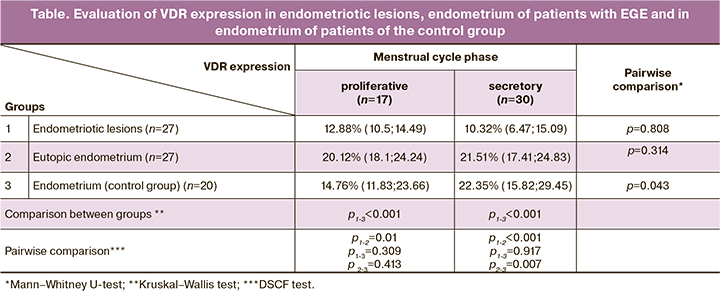
VDR expression was verified in the epithelium of eutopic endometrial glands and in endometriotic lesions (Fig. 1–3), as well as in the endometrium of patients of the control group.
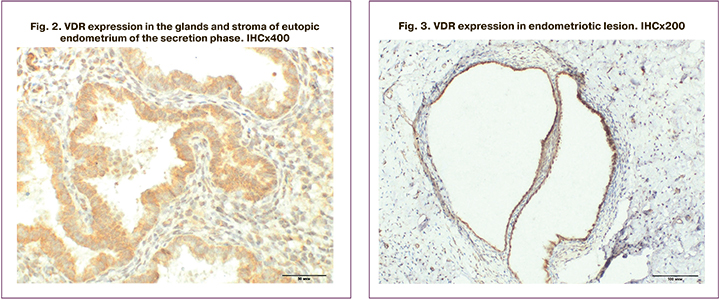
It should be emphasized that histological examination revealed endometrial hyperplasia in 5 patients of the main group, and that this finding was not included in VDR expression analysis. In the remaining patients, the endometrial structure corresponded to the proliferative (n=7) and secretory (n=20) phases of the menstrual cycle. Endometriotic lesions were represented by fragments of fibromuscular tissue with the presence of glands and stroma of the endometriotic type with chronic inflammation and hemorrhages.
Evaluation of VDR expression is presented in Table.
The comparison by the Kruskal–Wallis test revealed statistically significant differences between the groups (p<0.001) in both the proliferative and the secretory phases. The Dwass–Steel–Critchlow–Fligner method (DSCF) was then used to perform multiple pairwise comparison.
VDR expression in endometriotic lesions was significantly lower compared to VDR expression in eutopic endometrium: 1.6 times in the proliferative phase and 2.1 times in the secretory phase of the menstrual cycle (DSCF test, p=0.01; p<0.0001). When comparing VDR expression in endometriotic lesions with that in the endometrium of the control group in the secretory phase of the menstrual cycle, a significant decrease in the expression level was also noted – by 2.2 times (DSCF test, p=0.007). However, in the proliferative phase of the menstrual cycle, no significant differences were found between the VDR expression in the endometriotic lesions of the main group and the endometrium of the control group (DSCF test, p=0.413).
No statistically significant differences in VDR expression in endometriotic lesions in the proliferative and secretory phases of the menstrual cycle were found (Mann–Whitney U-test, p=0.808).
VDR expression in the endometrium in the main group did not statistically differ in the studied phases of the menstrual cycle (Mann–Whitney U-test, p=0.314); while in the control group in the secretory phase of the menstrual cycle a 1.5-fold increase in VDR expression was observed compared to the data obtained in the proliferative phase (Mann–Whitney U-test, p=0.043).
Discussion
The results of IHC study allowed us to conclude that VDR are expressed both in lesions and in the endometrium of patients with EGE, as well as in the endometrium of women of the control group.
Some studies on VDR expression in the endometrium of women have already been published, however VDR expression in endometriotic lesions of women with EGE has not been previously presented.
Zelenko Z. Et al. found that the level of VDR expression is lower in the middle secretory phase, compared to the early secretory phase of the menstrual cycle, both in women with EGE and in the control group [13]. Bergadà L. et al. presented the data of IHC analysis, which showed a decrease in the level of VDR expression in patients with endometrial carcinoma (n=157), compared to healthy endometrium (n=60) [14].
However, Agic A. et al. showed opposite results. The authors evaluated VDR expression in the endometrium and in the ovaries in women with EGE or with endometrial or ovarian cancer and compared it with the expression level in women who underwent surgical treatment for benign ovarian lesions. IHC study of the endometrium showed a more pronounced VDR expression in patients with endometrial cancer than in the comparison group. An increased VDR expression was noted in the endometrium of patients with EGE compared to the patients with benign ovarian lesions, although the changes were not statistically significant. Additionally, in patients with EGE, a significantly higher VDR expression was found in the epithelial component of endometrium compared to stromal component, which was not observed in the comparison group [15].
There are very few studies on VDR expression in patients with endometriosis. The available literature present ambiguous results, which is the reason for continuing research in this direction.
Our study demonstrated that VDR expression was 1.5-fold higher in the secretory phase compared to the expression level in the proliferation phase in women of the control group; the difference was statistically significant (DSCF test, p=0.047). An increase in VDR expression in the secretory phase during the supposed implantation window is probably associated with the progesterone-like effect of vitamin D, which may be important for adequate secretory transformation and successful embryo implantation.
In a prospective cohort study performed by Hormon Q.E. et al. in 2020 was found that patients with vitamin D insufficiency and deficiency had reduced level of total estradiol, free estradiol and progesterone, which indicates that vitamin D directly affects production of steroid hormone and folliculogenesis [16]. In the study of Monastra G. et al. vitamin D is considered as a steroid hormone with progesterone-like activity [17].
In our study, in contrast, no cyclic changes in VDR expression were detected in patients with EGE; this may explain decrease in endometrial receptivity in patients with EGE. However, further research is required.
According to the literature, the frequency of infertility is 3–4 times higher in patients with EGE than in the general population [18]. It was found that calcitriol can activate the transcription of the HOXA10 gene, which plays an important role in the implantation process, and in the interaction of the embryo with endometrium through molecular and cytokine signals that improve blastocyst implantation. Sex hormones (estrogen and progesterone) affect the cyclic expression of HOXA10 in the endometrium, and the functions of this gene include the regulation of endometrial receptivity during the implantation window. The effect of 1,25(OH)2D on the VDR binding to a vitamin D response element (VDRE) in the 5' region of HOXA10 gene promotes the induction of HOXA10 transcription. Vitamin D, as a result of direct activation of HOXA10 transcription, can induce transformation of various tissues, including the endometrium and decidual cells [19].
Thus, changes in vitamin D metabolism in the endometrium and the absence of cyclic changes in VDR expression in patients with EGE can lead to implantation disorders and be one of the causes of endometriosis-associated infertility.
Endometriotic lesions were characterized by a reduced level of VDR expression as compared to the endometrium of women with EGE and the endometrium of women of the control group in the secretory phase of the menstrual cycle. At the same time, in endometriotic lesions, the level of VDR expression was comparable to those in the proliferative endometrium.
For consideration of the use of cholecalciferol as a possible therapy for EGE, the results of the studies on experimental models of surgically induced endometriosis in mice and rats can be used. These studies demonstrated a significant reduction in the area of endometriotic lesion and, in some cases, complete resorption of lesions after therapy with cholecalciferol and its selective agonist – elocalcitol [20, 21, 22]. The authors also noted non-classical effects of vitamin D, including: anti-inflammatory effect by reducing the number of macrophages and the level of the proinflammatory cytokine interleukin-1 (IL-1) in the peritoneal fluid [20]; antiproliferative effect (the development of fibrosis and apoptosis in stromal component of implants) [21], and antiangiogenic effect – reduction of VEGF levels (vascular endothelial growth factor), matrix metalloproteinase-9 (MMP-9), and potential increase in TIMP-2 (tissue inhibitor of metalloproteinases-2) [22].
Our previous study, based on a surgically induced experimental model of endometriosis in rats, made similar findings. Additionally, a positive dose-dependent effect characterized by resorption or regression of endometriotic lesions was found when cholecalciferol was administered orally [23].
Conclusion
VDR expression was found both in the glandular epithelium and endometrial stroma and in endometriotic lesions. However, VDR expression in the lesions was significantly reduced in comparison to VDR expression in the eutopic endometrium of patients with EGE. In the endometrium of the main group, no differences were observed in VDR expression evaluated in different phases of the menstrual cycle; in the control group such difference presented and VDR expression was 1.5 times higher in the secretory phase of the menstrual cycle compared to the proliferative phase.
Results of the study clarify the role of vitamin D in the pathogenesis of EGE and can be a reason for the use of cholecalciferol as a pathogenetic therapy for EGE.
References
- Barnett R., Banks N., Decherney A.H. Endometriosis and fertility preservation. Clin. Obstet. Gynecol. 2017; 60(3): 517-23. https://dx.doi.org/10.1097/GRF.0000000000000311.
- Parasar P., Ozcan P., Terry K.L. Endometriosis: epidemiology, diagnosis and clinical management. Curr. Obstet. Gynecol. Rep. 2017; 6(1): 34-41. https://dx.doi.org/10.1007/s13669-017-0187-1.
- Brosens I., Brosens J., Benagiano G. Neonatal uterine bleeding as antecedent of pelvic endometriosis. Hum. Reprod. 2013; 28(11): 2893-7. https://dx.doi.org/10.1093/humrep/det359.
- Ярмолинская М.И., Айламазян Э.К. Генитальный эндометриоз. Различные грани проблемы. СПб.: Эко-Вектор; 2017. 615 с. [Yarmolinskaya M.I., Ajlamazyan E.K. Genital endometriosis. Different facets of the problem. SPb.: Eko-Vektor; 2017. 615 p. (in Russian)].
- Muscogiuri G., Altieri B., de Angelis C., Palomba S., Pivonello R., Colao A. et al. Shedding new light on female fertility: The role of vitamin D. Rev. Endocr. Metab. Disord. 2017; 18(3): 273-83. https://dx.doi.org/10.1007/s11154-017-9407-2.
- Денисова А.С., Ярмолинская М.И. Роль витамина D в патогенезе генитального эндометриоза. Журнал акушерства и женских болезней. 2017; 66(6): 81-8. [Denisova A.S., Yarmolinskaya M.I. The role of vitamin D in the pathogenesis of genital endometriosis. Zhurnal akusherstva i zhenskih boleznej / Journal of obstetrics and women's diseases. 2017; 66(6): 81-8. (in Russian)]. https://doi.org/10.17816/JOWD66681-88.
- Cermisoni G.C., Alteri A., Corti L., Rabellotti E., Papaleo E., Viganò P., Sanchez A.M. Vitamin D and endometrium: a systematic review of a neglected area of research. Int. J. Mol. Sci. 2018; 19(8): 2320. https://dx.doi.org/10.3390/ijms19082320.
- Nandi A., Sinha N., Ong E., Sonmez H., Poretsky L. Is there a role for vitamin D in human reproduction? Horm. Mol. Biol. Clin. Investig. 2016; 25(1): 15-28. https://dx.doi.org/10.1515/hmbci-2015-0051.
- Мальцев С.В., Мансурова Г.Ш. Метаболизм витамина D и пути реализации его основных функций. Практическая медицина. 2014; 9: 12-8. [Mal'tsev S.V., Mansurova G.Sh. Vitamin D metabolism and ways to implement its main functions. Prakticheskaya medicina/ Practical medicine. 2014; 9: 12-8. (in Russian)].
- Суслова Е.В., Ярмолинская М.И., Ткаченко Н.Н., Клейменова Т.С., Нетреба Е.А. Значение пролактина, дофамина и их рецепторного профиля в развитии генитального эндометриоза. Проблемы репродукции. 2019; 25(6): 86-94. [Suslova E.V., Yarmolinskaya M.I., Tkachenko N.N., Klejmenova T.S., Netreba E.A. The role of prolactin, dopamine and their receptor profile in the development of genital endometriosis. Problemy reprodukcii/Russian Journal of Human Reproduction. 2019; 25(6): 86-94. (in Russian)]. https://doi.org/10.17116/repro20192506186.
- Ярмолинская М.И., Тхазаплижева С.Ш., Молотков А.С., Ткаченко Н.Н., Бородина В.Л., Андреева Н.Ю., Клейменова Т.С., Лысенко В.В. Мелатонин и наружный генитальный эндометриоз: роль в патогенезе и возможности применения в терапии заболевания. Журнал акушерства и женских болезней. 2019; 68(3): 51-60. [Yarmolinskaya M.I., Thazaplizheva S.Sh., Molotkov A.S., Tkachenko N.N., Borodina V.L., Andreeva N.Yu. et al. Melatonin and external genital endometriosis: role in the pathogenesis and possible use in the treatment of the disease. Zhurnal akusherstva i zhenskih boleznej/ Journal of obstetrics and women's diseases. 2019; 68(3): 51-60 (in Russian)]. https://dx.doi.org/10.17816/JOWD68351-60.
- Толибова Г.Х., Траль Т.Г., Клещёв М.А., Кветной И.М., Айламазян Э.К. Эндометриальная дисфункция: алгоритм гистологического и иммуногистохимического исследования. Журнал акушерства и женских болезней. 2015; 64(4): 69-77. [Tolibova G.H., Tral' T.G., Kleshchyov M.A., Kvetnoj I.M., Ajlamazyan E.K. Endometrial dysfunction: an algorithm for histological and immunohistochemical examination. Zhurnal akusherstva i zhenskih boleznej/ Journal of obstetrics and women's diseases. 2015; 64(4): 69-77 (in Russian)].
- Zelenko Z., Aghajanova L., Irwin J.C., Giudice L.C. Nuclear receptor, coregulator signaling, and chromatin remodeling pathways suggest involvement of the epigenome in the steroid hormone response of endometrium and abnormalities in endometriosis. Reprod. Sci. 2012; 19(2): 152-62. https://dx.doi.org/10.1177/1933719111415546.
- Bergadà L., Pallares J., Arcidiacono M.V., Cardus A., Santacana M., Valls J. et al. Role of local bioactivation of vitamin D by CYP27A1 and CYP2R1 in the control of cell growth in normal endometrium and endometrial carcinoma. Lab. Invest. 2014; 94(6): 608-22. https://dx.doi.org/10.1038/labinvest.2014.57.
- Agic A., Xu H., Altgassen C., Noack F., Wolfler M.M., Diedrich K. et al. Relative expression of 1,25-dihydroxyvitamin D3 receptor, vitamin D 1 alpha-hydroxylase, vitamin D 24-hydroxylase, and vitamin D 25-hydroxylase in endometriosis and gynecologic cancers. Reprod. Sci. 2007; 14(5): 486-97. https://dx.doi.org/10.1177/1933719107304565.
- Harmon Q.E., Kissell K., Jukic A.M.Z., Kim K., Sjaarda L., Perkins N.J. et al. Vitamin D and reproductive hormones across the menstrual cycle. Hum. Reprod. 2020; 35(2): 413-23. https://dx.doi.org/10.1093/humrep/dez283.
- Monastra G., De Grazia S., De Luca L., Vittorio S., Unfer V. Vitamin D: a steroid hormone with progesterone-like activity. Eur. Rev. Med. Pharmacol. Sci. 2018; 22(8): 2502-12.
- Адамян Л.В., Сонова М.М., Тихонова Е.С., Зимина Э.В., Антонова С.О. Медицинские и социальные аспекты генитального эндометриоза. Проблемы репродукции. 2011; 17(6): 78-81. [Adamyan L.V., Sonova M.M., Tihonova E.S., Zimina E.V., Antonova S.O. Medical and social aspects of genital endometriosis. Problemy reprodukcii/ Russian Journal of Human Reproduction. 2011; 17(6): 78-81. (in Russian)].
- Бахарева И.В. Витамин D и эндометриоз: в поиске новых возможностей. Российский вестник акушера-гинеколога. 2018; 18(4): 35-43. [Bakhareva I.V. Vitamin D and endometriosis: in search of new opportunities. Rossijskij vestnik akushera-ginekologa/ Russian Bulletin of Obstetrican-Gynecologist. 2018; 18(4): 35-43. (in Russian)]. https://dx.doi.org/10.17116/rosakush201818435.
- Mariani M., Vigano P., Gentilini D., Camisa B., Caporizzo E., Di Lucia P. et al. The selective vitamin D receptor agonist, elocalcitol, reduces endometriosis development in a mouse model by inhibiting peritoneal inflammation. Hum. Reprod. 2012; 27(7): 2010-19. https://dx.doi.org/10.1093/humrep/des150.
- Abbas M.A., Taha M.O., Disi A.M., Shomaf M. Regression of endometrial implants treated with vitamin D3 in a rat model of endometriosis. Eur. J. Pharmacol. 2013; 715(1-3): 72-5. https://dx.doi.org/10.1016/j.ejphar.2013.06.016.
- Yildirim B., Guler T., Akbulut M., Oztekin O., Sariiz G. 1–Alpha, 25–Dihydroxyvitamin D3 regresses endometriotic implants in rats by inhibiting neovascularization and altering regulation of matrix metalloproteinase. Postgrad. Med. 2014; 126(1): 104-10. https://dx.doi.org/10.3810/pgm.2014.01.2730.
- Ярмолинская М.И., Денисова А.С., Андреева Н.Ю. Эффективность применения витамина D3 (колекальциферола) в терапии хирургически индуцированного эндометриоза у крыс. Российский вестник акушера-гинеколога. 2019; 19(3): 37-42. [Yarmolinskaya M.I., Denisova A.S., Andreeva N.YU. Efficacy of vitamin D3 (colecalciferol) in the treatment of surgically induced endometriosis in rats. Rossijskij vestnik akushera-ginekologa/ Russian Bulletin of Obstetrican-Gynecologist. 2019; 19(3): 37-42. (in Russian)]. https:/dx./doi.org/10.17116/rosakuch20191903137.
Received 09.11.2020
Accepted 04.12.2020
About the Authors
Maria I. Yarmolinskaya, Professor of the Russian Academy of Sciences, Dr. Med. Sci., Professor, Head of the Department of Gynecology and Endocrinology, Head of the center "Diagnostics and treatment of endometriosis", D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology; Professor of the Departmentof Obstetrics and Gynecology, I.I. Mechnikov North-Western State Medical University, Ministry of Health of Russia. Tel.: +7(812)334-35-85.
E-mail: m.yarmolinskaya@gmail.com. ORCID: 0000-0002-6551-4147. 199034, Russia, Saint Petersburg, Mendeleevskaya Liniya, 3.
Alexandra S. Denisova, post-graduate student, junior researcher of the Department of Gynecology and Endocrinology, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology. Tel.: +7(911)179-15-88. E-mail: al.ser.denisova@gmail.com. ORCID: 0000-0003-3607-2420. 199034, Russia, Saint Petersburg, Mendeleevskaya Liniya, 3.
Gulrukhsor Kh. Tolibova, Dr. Med. Sci., Head of the Laboratory of Immunohistochemistry, Head of the Laboratory of Morphology, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology. Tel.: +7(812)328-98-60. E-mail: gulyatolibova@yandex.ru.
ORCID: 0000-0002-6216-6220. 199034, Russia, Saint Petersburg, Mendeleevskaya Liniya, 3.
Olesya N. Bespalova, Dr. Med. Sci., Deputy Director for Research, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology. Tel.: +7(812)328-98-89. E-mail: shiggerra@mail.ru. ORCID: 0000-0002-6542-5953. 199034, Russia, Saint Petersburg, Mendeleevskaya Liniya, 3.
Tatyana G. Tral, PhD, senior researcher of the Laboratory of Morphology, Head of the Department of Pathologic Anatomy, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology; assistant of the Department of Pathologic Anatomy with the course of forensic medicine, Saint Petersburg State Pediatric Medical University, Ministry of Health of Russia. Tel.: +7(812)328-98-60. E-mail: ttg.tral@yandex.ru. ORCID: 0000-0001-8948-4811. 199034, Russia, Saint Petersburg, Mendeleevskaya Liniya, 3.
Karina A. Zakuraeva, clinical resident, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology. Tel.: +7(960)374-12-22. E-mail: KareenZ@yandex.ru. ORCID: 0000-0002-8128-306X. 199034, Russia, Saint Petersburg, Mendeleevskaya Liniya, 3.
Valeria O. Pyankova, post-graduate student, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology. Tel.: +7(922)666-10-22.
E-mail: pyankova.valeriia@rambler.ru. ORCID: 0000-0002-9101-1981. 199034, Russia, Saint Petersburg, Mendeleevskaya Liniya, 3.
For citation: Yarmolinskaya M.I., Denisova A.S., Tolibova G.Kh., Bespalova O.N., Tral T.G., Zakuraeva K.A., Pyankova V.O. Analysis of vitamin D receptor expression in patients with genital endometriosis.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2021; 3: 117-123 (in Russian)
https://dx.doi.org/10.18565/aig.2021.3.117-123