Аденомиоз, или внутренний генитальный эндометриоз, представляет собой доброкачественный патологический процесс, характеризующийся появлением в миометрии железистых и стромальных элементов эндометриального происхождения. Классическими симптомами аденомиоза являются аномальные маточные кровотечения (перименструальные мажущие кровянистые выделения и обильные менструальные кровотечения), дисменорея, диспареуния, хроническая тазовая боль, хроническая анемия, а также бесплодие и невынашивание беременности [1]. Распространенность аденомиоза в популяции, по данным различных авторов, варьирует в широчайших пределах – от 10 до 70%, что связано с отсутствием единой классификационной системы, а также с тем, что в прошлом диагноз устанавливался только на основании результатов гистологического исследования после гистерэктомии [2].
Современные представления о патогенезе аденомиоза
В последние годы особый интерес при изучении патогенеза аденомиоза вызывает эпителиально-мезенхимальный переход (ЭМП, Epithelial-Mesenchymal Transition, ЕМТ), который также имеет важное значение в развитии наружного генитального эндометриоза (НГЭ), миомы матки, онкологических процессов, в частности, инвазии и метастазировании рака молочной железы (РМЖ) [3–6]. ЭМП – биологический процесс, в ходе которого эпителиальные клетки теряют свою полярность и межклеточные контакты и приобретают миграционный мезенхимальный фенотип, превращаясь в подвижные мезенхимальные [7, 8]. Изначально ЭМП представляет собой фундаментальную составляющую эмбрионального развития клеток, физиологических процессов созревания стволовых клеток и заживления ран [8]. В процессе ЭМП исчезает апикально-базальная полярность клеток, экспрессия эпителиальных маркеров существенно снижается, а мезенхимальных маркеров – повышается. Кроме того, клетки обретают способность к миграции и инвазии, становятся резистентными к апоптозу, увеличивают секрецию ферментов деградации, лизирующих окружающий внеклеточный матрикс. В процессе ЭМП участвуют многие ферменты, включая матриксные металлопротеиназы (ММР-1, MMP-2, MMP-9), активирующие процесс инвазии при эндометриозе и аденомиозе.
Необходимо отметить, что молекулярная программа ЭМП пластична и может подвергнуться регрессу для возвращения к эпителиальному фенотипу, обратный процесс называется мезенхимально-эпителиальным переходом (МЭП) [9], что открывает широкие перспективы для разработки новых направлений медикаментозной терапии гиперпролиферативных заболеваний.
Среди молекулярных факторов, участвующих в ЭМП, условно можно выделить индукторы, регуляторы и эффекторы [4, 10, 11]. Индукторы представляют собой факторы роста и рецепторы, первоначально сигнализирующие о мезенхимальных изменениях: фактор роста гепатоцитов (HGF) и фибробластов (FGF), трансформирующий ростовой фактор β (TGFβ), тромбоцитарный фактор роста (PDGF), поддерживающие постоянную клеточную пролиферацию и дифференцировку. Факторы роста индуцируют ЭМП с последующей инвазией и миграцией. Регуляторы представлены факторами транскрипции. Эффекторы отвечают за конечную форму клетки, ее способность к инвазии.
Как известно, адгезионные контакты между клетками – это гомодимерные межклеточные соединения, характеризующиеся связью через классические Е- и N-кадгерины. Основным фактором, запускающим ЭМП, является снижение уровня экспрессии эпителиального маркера E-кадгерина [12]. N-кадгерин стимулирует активацию мезенхимальных факторов транскрипции Snail и Slug, вызывая модуляцию рецептора фактора роста фибробластов (FGFR), приводя к увеличению инвазии, пролиферации и метастазирования опухолевых клеток. Snail и Slug являются ключевыми регуляторами ЭМП [7], подавляющими транскрипцию E-кадгерина в последующем. Повышенная экспрессия Snail и Slug в эндометрии при аденомиозе выявляется как в пролиферативной, так и в секреторной фазе менструального цикла [7].
К настоящему времени выявлено, что индукция ЭМП зависит от многочисленных сигнальных путей. Эти каскадные механизмы регулируют воспалительный ответ, фиброз, ангиогенез и пролиферативные процессы при различных заболеваниях и, таким образом, могут являться перспективными фармакодинамическими мишенями в лечении.
Сигнальный путь TGF-β1/Smad3 в патогенезе аденомиоза. В исследовании Zhang Q. и соавт. [3] был изучен процесс активации сигнального пути TGF-β1/Smad3 в эндометриоидных клетках. Установлено, что при аденомиозе и НГЭ тромбоцитарный TGF-β1 активирует данный сигнальный путь, что приводит к запуску ЭМП, метаплазии гладких мышц, трансформации фибробласта в миофибробласт (FMT) и развитию фиброза [3]. Открытие и дальнейшее изучение данного сигнального пути демонстрирует важность тромбоцитарного звена в патогенезе НГЭ [13] и аденомиоза [14]. Обнаружено, что экспрессия генов и белков TGF-β1, фосфорилированных Smad3, в образцах эндометрия женщин с эндометриозом после совместного культивирования с активированными тромбоцитами была значительно повышена по сравнению с показателями в образцах эндометрия женщин группы контроля. Проведенное исследование не исключает возможности того, что миофибробласты могут также происходить из других источников, и предполагает одновременное участие других сигнальных путей или иммунных клеток в ЭМП при эндометриозе.
Сигнальный путь Notch1/Numb/Snail при аденомиозе. Установлено, что сигнальный путь Notch регулирует клеточное развитие, пролиферацию, выживание и дифференцировку клеток в различных органах. Повышенная экспрессия Notch1 вызывает увеличение экспрессии a-SMA и виментина, снижение Е-кадгерина. Перечисленные процессы сопровождаются клеточными морфологическими изменениями и перестройкой цитоскелета, характерными для активации ЭМП, и активно изучаются в патогенезе, развитии и прогрессировании аденомиоза. В процессе ЭМП передача сигналов Notch происходит с участием многочисленных факторов транскрипции и роста, таких как Snail, Slug, TGF-β, FGF и PDGF [15]. Выявлено достоверное повышение экспрессии Notch1 в эндометрии при аденомиозе в течение всего менструального цикла по сравнению с таковой в контрольной группе.
Определено, что в сигнальном пути Notch1/Numb/Snail белок Numb является ингибирующим регулятором передачи сигналов Notch1, который действует, стимулируя убиквитинирование и деградацию внутриклеточного домена Notch1. Его функция заключается в регуляции деления, адгезии и миграции клеток. В исследовании Qi S. и соавт. [7] выявлено снижение экспрессии Numb в эндометрии пациенток с аденомиозом, что свидетельствует о том, что аберрантная негативная регуляция Numb может быть связана с генезом и развитием заболевания.
Значение сигнального пути eIF3 при аденомиозе. Трансляционный контроль играет основную роль в регуляции экспрессии белка и происходит главным образом на этапе инициации, который контролируется множественными эукариотическими факторами инициации трансляции (eIFs) [16]. Недавний транскриптомный анализ эутопического эндометрия у женщин с аденомиозом обнаружил участие сигнальных путей eIF2 (Eukaryotic Initiation Factor 2) и eIF3 (Eukaryotic Initiation Factor 3) в ЭМП. Подавление субъединицы eIF3 (eIF3e) при эндометриозе может привести к увеличению трансляции Snail и Zeb2, что, в свою очередь, запускает механизм ЭМП. Кроме того, известно, что стабильный уровень eIF3e, благодаря усиленному ангиогенезу, способствует заживлению ран. Поскольку эндометриоидные поражения отчасти являются раневыми поверхностями, подвергающимися повторному повреждению и восстановлению, а аденомиоз характеризуется потерей эпителиальных и приобретением мезенхимальных свойств клеток, eIF3e также может играть роль в ЭМП при НГЭ и аденомиозе, что и было продемонстрировано в недавних исследованиях [4, 17]. Выявлено, что eIF3e может участвовать в ЭМП при эндометриозе посредством активации TGF-β1 и способствует пролиферации клеток за счет усиления ангиогенеза в эктопическом эндометрии. При аденомиозе также выявлено значительное снижение иммунореактивности eIF3e по сравнению с эндометрием женщин контрольной группы.
Сигнальный путь эстрадиол(E2)/Slug/VEGF (vascular endothelial growth factor, фактор роста эндотелия сосудов) в развитии аденомиоза. Данный каскадный механизм представляет собой воздействие эстрадиола на транскрипционный фактор Slug, что в последующем приводит к снижению эпителиального фактора Е-кадгерина с помощью механизмов, описанных ранее, с одной стороны, и воздействием на VEGF, являющийся ключевым медиатором ангиогенеза и нейрогенеза, с другой. В работе Huang T.S. и соавт. [18] изучалась роль Е2 в патогенезе аденомиоза. Выявлено, что повышение уровня эстрадиола вызывает запуск сигнального пути E2/Slug/VEGF, что сопровождается увеличением проангиогенной активности в эндотелиальных клетках. В эксперименте на животных подтверждено, что подавление E2 или VEGF приводит к уменьшению степени выраженности аденомиоза. Эти результаты подчеркивают важность эстроген-индуцированного ангиогенеза в развитии аденомиоза и обеспечивают потенциальную стратегию лечения заболевания посредством воздействия на звенья сигнального пути E2/Slug/VEGF [18].
Изучение сигнальных путей и особенностей передачи сигналов необходимо для понимания патогенетических механизмов развития аденомиоза и принципиально важно для подбора эффективной терапии.
Большинство препаратов, применяющихся для лечения аденомиоза, подавляют секрецию эстрадиола и VEGF, а также других факторов роста и являются патогенетически обоснованными.
Методы лечения больных с аденомиозом.
Хирургические методы
В настоящее время в мире не существует единой тактики лечения аденомиоза, а наиболее эффективным методом лечения по-прежнему остается гистерэктомия. Однако радикальный метод лечения не подходит пациенткам, имеющим репродуктивные планы или желающим сохранить матку. Альтернативой гистерэктомии у таких пациенток является операция аденомиомэктомии с восстановлением стенки матки трехлоскутным способом по методике Осада. По опубликованным Osada H. в 2018 г. данным, во всем мире выполнено 2365 операций аденомиомэктомии, из них 2112 (89,8%) операций были выполнены в японских клиниках. После оперативного лечения у 449 (19%) пациенток самостоятельно или с помощью вспомогательных репродуктивных технологий (ВРТ) наступила беременность (из них одна – эктопическая), родами закончились 363 (80,8%) беременности, из них 2 случая мертворождения [19].
Собственный опыт выполнения органосохраняющих хирургических операций при аденомиозе представлен и отечественными авторами. Так, по данным Рухляды Н.Н. и соавт. [20], с 2003 г. были выполнены 203 операции аденомиомэктомии при исходных размерах матки от 9 до 22 недель беременности. Отсутствие кровотечений и анемии наблюдалось у 84%, а значимое уменьшение болевого синдрома – у 74% прооперированных. Беременность наступила у 39/82 женщин, заинтересованных в восстановлении фертильности. Цхай В.Б. и соавт. [21] в 2019 г. представили результаты 29 аденомиомэктомий с реконструкцией стенки матки трехлоскутным методом по методике Осада. У 20 (68,9%) пациенток в анамнезе отмечено аденомиоз-ассоциированное бесплодие, после операции у 3 (15%) пациенток наступила спонтанная беременность, которая закончилась срочными родами путем операции кесарева сечения (КС).
По данным литературы, частота акушерских осложнений после аденомиомэктомии достаточно высока: врастание плаценты наблюдалось у 6,3% пациенток [22], разрыв матки был диагностирован в 3,6% случаев, что гораздо выше частоты разрывов матки после удаления миоматозных узлов (0,26%) [23].
Гормональная терапия
Оптимизация профилей эффективности и безопасности побуждает рассматривать гормональную терапию в качестве золотого стандарта медикаментозного лечения аденомиоза. Несмотря на большое разнообразие применяемых гормональных препаратов, в настоящее время только три группы являются так называемой «специфической терапией» аденомиоза: агонисты гонадотропин-рилизинг-гормона (аГнРГ), антигонадотропины (даназол) и диеногест 2 мг из группы прогестагенов [1]. Также в качестве препарата выбора у пациенток с аденомиозом можно рассматривать левоноргестрелвыделяющую внутриматочную систему (ЛВС). Однако применение первых двух групп препаратов сопровождается выраженными побочными эффектами, что снижает приверженность больных к данному виду лечения. При применении аГнРГ побочные эффекты обусловлены гипоэстрогенемией и включают нейровегетативные и психоэмоциональные симптомы (приливы жара, потливость, нарушения сна, эмоциональная лабильность, депрессия, утомляемость), что является показанием для дополнительного назначения «add-back терапии» (возвратной терапии, или терапии прикрытия). При использовании даназола наблюдаются в основном андроген-обусловленные побочные эффекты (увеличение веса, акне, себорея, гирсутизм, снижение тембра голоса) [1], что привело в настоящее время к отказу от его применения в рутинной практике.
Strowitzki T. и соавт. опубликовали результаты рандомизированного мультицентрового исследования, которое показало, что прием диеногеста в дозе 2 мг в день перорально не только имел эквивалентную эффективность по сравнению с применением аГнРГ для облегчения боли, связанной с эндометриозом, но и обладал преимуществами по профилю безопасности и переносимости [24].
Диеногест является прогестагеном 4-го поколения, производным 19-нортестостерона, который за счет потери этинильного радикала приобрел значимую антиандрогенную эффективность. Терапевтический эффект диеногеста при эндометриозе обусловлен антипролиферативным, антигонадотропным и противовоспалительным действиями, замедлением процессов ангиогенеза, повышением проапоптотической активности, а также снижением ароматазной активности и экспрессии циклооксигеназы-2 [25, 26]. Специфичность действия диеногеста дополняется минимальным влиянием на метаболические параметры [27], что обуславливает хорошую переносимость и высокую биодоступность при приеме внутрь (>90%).
Известно, что на прогрессирование эндометриоза и выраженность болевого синдрома в значительной мере оказывают влияние медиаторы воспаления, в большом количестве синтезирующиеся в эндометриоидных гетеротопиях и вызывающие сенсибилизацию периферических нервов и центральной нервной системы. [28]. В 2014 г. японскими учеными в эксперименте in vivo было продемонстрировано, что диеногест уменьшает уровни TNF-α и интерлейкина (IL)-1β и тем самым подавляет экспрессию фактора роста нервов в эндометриоидной ткани, способствуя уменьшению выраженности болевого синдрома [29].
Таким образом, диеногест является патогенетически обоснованным препаратом выбора для длительного лечения генитального эндометриоза и, в частности, аденомиоза. Osuga Y. и соавт. [30] в 2017 г. опубликовали данные наблюдения за 130 пациентками с аденомиозом, которые получали диеногест 2 мг перорально каждый день в течение 52 недель. Наиболее часто встречающимися побочными эффектами были прорывные маточные кровотечения (96,9%) и приливы (7,7%), однако выраженность побочных эффектов была незначительной и не послужила причиной для отмены препарата. Через год после начала терапии диеногестом 2 мг наблюдались: снижение выраженности болевого синдрома по визуальной аналоговой шкале (ВАШ) (в среднем на 86%), уменьшение размера матки на 30%, а 57% женщин отметили аменорею при длительном приеме препарата [30].
Fawzy M. и соавт. [31] провели проспективное исследование, в котором приняли участие пациентки с аденомиозом, страдающие тазовыми болями и меноррагией. Первая группа больных принимала диеногест (2 мг/день, перорально), вторая группа получала аГнРГ (трипторелина ацетат, 3,75 мг каждые 4 недели, подкожная инъекция) в течение 16 недель. Значительное уменьшение дисменореи и хронической тазовой боли наблюдалось в обеих группах, однако трипторелина ацетат был более эффективен в контроле меноррагии. Таким образом, диеногест может быть альтернативой аГнРГ для лечения дисменореи и тазовых болей у женщин с аденомиозом.
В ретроспективном исследовании пациенток с аденомиозом было продемонстрировано, что диеногест 2 мг может рассматриваться как хорошо переносимый препарат с благоприятным профилем эффективности для долгосрочного приема и как приемлемая альтернатива гистерэктомии. При приеме диеногеста в течение 5 лет было отмечено уменьшение размера матки на 72,6%, при этом значения сывороточного эстрадиола сохранялись в рамках референсных для данного возраста. 70% участниц исследования продолжили принимать диеногест в течение более 80 месяцев или вплоть до наступления менопаузы [32].
Иммуномодулирующий эффект диеногеста подтверждает исследование 2018 г., в котором после гистерэктомии изучалось содержание натуральных киллерных (NK)-клеток (CD57) и макрофагов (CD68) в эндометрии и миометрии женщин с аденомиозом, принимавших диеногест, и в группе контроля без гормональной терапии. На фоне применения диеногеста отмечено достоверное увеличение инфильтрации NK-клеток в железах эутопического эндометрия у пациенток с аденомиозом. По мнению авторов, полученные результаты могут служить объяснением факта повышения частоты наступления беременности после терапии диеногестом у женщин репродуктивного возраста [33].
В ФГБНУ «НИИ АГиР имени Д.О. Отта» также проведен анализ результатов терапии диеногестом у больных аденомиозом.
Цель исследования заключается в оценке и анализе результатов лечения диеногестом 2 мг больных аденомиозом в течение 6–68 месяцев.
Материалы и методы
В проспективное когортное исследование вошли 62 пациентки репродуктивного возраста с установленным диагнозом «аденомиоз», которые получали в качестве терапии диеногест 2 мг ежедневно перорально в течение 6 и более месяцев. Средний возраст пациенток составил 36,3 (3,75) года.
Были оценены жалобы пациенток, наличие болевого синдрома и его выраженность по ВАШ, гинекологический анамнез, размеры матки по данным ультразвукового исследования (УЗИ) органов малого таза в динамике: до лечения и на фоне терапии через 6 месяцев.
В настоящее время возросла значимость визуализационных методов исследования, включающих УЗИ и магнитно-резонансную томографию (МРТ) [34]. Доказана корреляция сонографических признаков аденомиоза с патоморфологическим диагнозом: по данным литературы, в 84,3% случаев, при наличии УЗ-признаков аденомиоза до операции, диагноз подтверждался после гистерэктомии патоморфологическим методом [35]. В настоящем исследовании диагноз внутреннего генитального эндометриоза у всех больных был установлен на основании УЗИ по наиболее частым эхографическим признакам аденомиоза (округлая форма матки за счет увеличения переднезаднего размера, увеличение размеров матки, нечеткий контур М-эха и увеличение переходной зоны, участки повышенной эхогенности в миометрии, асимметрия толщины стенок матки, множественные полосы пониженной эхогенности, ориентированные перпендикулярно плоскости сканирования), нередко у пациенток отмечалась болезненность при трансвагинальном исследовании.
Для вычисления объема матки по данным УЗИ нами была использована следующая формула:
Объем матки (см3) = длина матки (см) ×
толщина матки (см) × ширина матки (см) × 0,52.
Для статистической обработки данных использовали программу Jamovi. Для проверки нормальности распределения применяли критерий Шапиро–Уилка, при p>0,05 распределение считалось нормальным. Нормально распределенные данные описывали как М (SD), а данные с распределением, отличающимся от нормального, как Me (Q1;Q3).
Для сравнения двух парных выборок с ненормальным распределением (объем матки до и после лечения, болевой синдром) был использован критерий знаков Уилкоксона, различия считали статистически значимыми при p<0,05. Для сравнения нормально распределенных выборок (уровень аланинаминотрансферазы (АЛТ), аспартатаминотрансферазы (АСТ), фибриногена в крови до и после лечения) использовали парный t-критерий.
Результаты и обсуждение
У 18/62 (29%) больных диагноз аденомиоза был дополнительно подтвержден результатами МРТ, а у 23/62 (37%) пациенток при проведении гистероскопии выполнена трансцервикальная пункционная биопсия миометрия с последующим гистологическим исследованием. Известно, что на основании результатов гистологических исследований внутренний генитальный эндометриоз традиционно разделяют на следующие формы: диффузную, очаговую и узловую [36], а на втором конгрессе Европейского общества по эндометриозу в Барселоне (SEUD) в 2016 г. было решено добавить также склеротическую форму аденомиоза [37]. В нашем исследовании диффузная форма аденомиоза наблюдалась у подавляющего большинства больных – 54/62 (87,1%) случаев, очаговая и узловая – у 15/62 (24,2%) пациенток.
У большинства пациенток – 33/62 (53,2%) аденомиоз сочетался с НГЭ, что не противоречит данным литературы [38]. Установлена связь между развитием аденомиоза и НГЭ, а также определена роль аденомиоза в возникновении эндометриоз-ассоциированного бесплодия. Так, Kunz G. и соавт. [38] при выполнении МРТ органов малого таза 160 женщинам с НГЭ установили положительную корреляционную связь между толщиной переходной зоны и степенью распространенности НГЭ, а также с возрастом обследуемых женщин. В группе пациенток в возрасте моложе 36 лет сочетание НГЭ, аденомиоза и бесплодия встречалось в 90% случаев, аденомиоз в сочетании с НГЭ – у 79% женщин.
Аденомиоз в сочетании с миомой матки встречался в нашем исследовании у 6/62 (9,7%) пациенток, а в сочетании с миомой и НГЭ – у 17/62 (27,4%) больных. Жалобы на бесплодие в исследуемой группе предъявляли 16/62 (25,8%), из них первичное бесплодие отмечено у 9/16 (56,25%), вторичное – у 7/16 (43,75%) пациенток. Невынашивание беременности в анамнезе встречалось у 15/62 (24,2%) пациенток с аденомиозом, что не противоречит данным литературы. В метаанализе, опубликованном Vercellini P. и соавт. в 2014 г. [39], объединившем в общей сложности 1865 женщин с бесплодием, было отмечено, что у пациенток с аденомиозом вероятность наступления беременности при использовании ВРТ (ЭКО/ИКСИ) снижена на 28% по сравнению с женщинами без аденомиоза.
Частота самопроизвольного выкидыша также была выше в данной группе (31,9%) по сравнению с пациентками без аденомиоза (14,1%) [39]. На основании результатов метаанализа, проведенного Razavi M. и соавт. [40], в который вошли 6 исследований с участием 9742 женщин (322 пациентки с аденомиозом и 9420 женщин контрольной группы), было установлено, что наличие внутреннего генитального эндометриоза ассоциировано с повышением частоты преждевременных родов, низкой массы плода для срока гестации, а также преэклампсии. Таким образом, аденомиоз может оказывать негативное влияние не только на наступление беременности, но и на ее течение и исходы.
В нашем исследовании пациентки получали терапию диеногестом 2 мг в течение 6 месяцев, 15/62 (24,2%) больных продолжили прием диеногеста на более длительный срок, при этом максимальный срок применения препарата составил 68 месяцев у пациентки при сочетании аденомиоза и НГЭ. После приема диеногеста была произведена повторная оценка болевого синдрома, выполнено контрольное УЗИ.
По нашим данным, до начала терапии диеногестом 2 мг дисменорею отмечали у 58/62 (93,5%) пациенток с аденомиозом, медиана оценки боли по ВАШ для альгодисменореи составила 7,87 (6,24;9,13) балла. Диспареуния встречалась у 13/62 (21%), медиана оценки боли по ВАШ – 6,3 (3,9;7,24) балла. Жалобы на тазовую боль, не связанную с менструальным циклом, предъявляли 21/62 (33,87%) пациенток, медиана оценки боли по ВАШ – 5,32 (2,53;6,45) балла. На фоне 6-месячного курса приема диеногеста у 33/58 (53,4%) пациенток интенсивность болевого синдрома снизилась, у 19/58 (31%) пациенток на фоне приема диеногеста 2 мг болевой синдром был полностью устранен.
Жалобы на аномальные маточные кровотечения (АМК) до лечения предъявляли 39/62 (62,9%) больных, после 6 месяцев приема диеногеста у всех пациенток было отмечено снижение интенсивности, частоты и длительности кровянистых выделений.
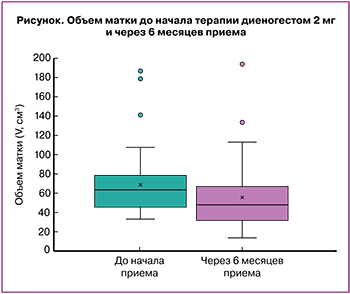 При анализе объема матки в динамике медиана показателя до лечения составила 63,5 (46,2;77,8) см3, после лечения – 47,9 (34,2;66,1) см3, при сравнении выборок с помощью критерия знаков Уилкоксона средняя разница составила 12,3 см3 (95% ДИ 6,81–19,2; р=0,001) (рисунок). На фоне приема диеногеста объем матки уменьшился у 41/58 (70,7 %) пациенток с аденомиозом.
При анализе объема матки в динамике медиана показателя до лечения составила 63,5 (46,2;77,8) см3, после лечения – 47,9 (34,2;66,1) см3, при сравнении выборок с помощью критерия знаков Уилкоксона средняя разница составила 12,3 см3 (95% ДИ 6,81–19,2; р=0,001) (рисунок). На фоне приема диеногеста объем матки уменьшился у 41/58 (70,7 %) пациенток с аденомиозом.
Известно, что миома матки характеризуется повышенной экспрессией прогестероновых рецепторов, в связи с этим диеногест теоретически может обладать разнонаправленным действием на миоматозные узлы и приводить к их росту, поэтому особый интерес представляла их динамика в группе пациенток с миомой матки и аденомиозом. По нашим данным, у пациенток с миомой на фоне приема диеногеста не было отмечено достоверно значимого роста узлов, а у больных с миомэктомией в анамнезе не зафиксировано рецидива заболевания.
Следует отметить, что возраст является одним из наиболее значимых факторов, влияющих на возможность успешной реализации репродуктивной функции, особенно для больных генитальным эндометриозом. В нашем исследовании средний возраст пациенток с аденомиозом и бесплодием составил 29,3 (4,5) лет. После терапии диеногестом у 5/16 (31,3%) женщин, обратившихся по поводу планирования беременности и имеющих в анамнезе бесплодие и невынашивание, наступила беременность: у 3/5 (60%) – в результате ВРТ, а у 2/5 (в 40%) – в естественном цикле. У одной женщины с 10 неудачными попытками экстракорпорального оплодотворения (ЭКО) и привычным выкидышем в анамнезе произошел самопроизвольный выкидыш на раннем сроке беременности. Остальные беременности закончились срочными родами здоровыми детьми.
Наиболее часто встречающимся побочным эффектом при приеме диеногеста являются нарушения менструального цикла. При назначении диеногеста 2 мг нарушения менструального цикла по типу мажущих кровянистых выделений наблюдались у 24/62 (38,7%) больных аденомиозом, эпизоды «прорывных кровотечений» отмечены у 3/62 (4,8%). Аменорея встречалась у 17/62 (27,4%) пациенток, регулярный менструальный цикл – лишь у 8/62 (12,9%), при этом менструации на фоне продолжения терапии диеногестом 2 мг стали более скудными. Вышеперечисленные побочные эффекты не явились причиной для прекращения терапии. Следует отметить, что с увеличением продолжительности терапии отмечалось снижение интенсивности, частоты и длительности кровянистых выделений у всех пациенток. Поводом для досрочной отмены препарата у 3/62 (4,8%) пациенток после 4–5 месяцев приема диеногеста стали следующие побочные эффекты: головная боль, бессонница, масталгия, кисты яичников и боли в эпигастрии. Отмечено, что такие побочные эффекты, как бессонница, головная боль и эмоциональная лабильность, возникали чаще в первые месяцы приема препарата, и со временем их частота и выраженность уменьшалась.
Нами была изучена динамика уровня некоторых лабораторных показателей до начала терапии и через 6 месяцев лечения диеногестом. Для оценки влияния диеногеста на функцию печени были выбраны показатели АЛТ и АСТ в венозной плазме. Их уровень до и после приема диеногеста в дозе 2 мг не имел статистически значимых различий: 15,2 (4,34) и 15,6 (5,91) Eд/л (p=0,921); 18,6 (4,15) и 17,7 (5,19) Eд/л (p=0,387) соответственно и находился в пределах референсных значений. Уровень фибриногена до начала и через 6 месяцев терапии диеногестом 2 мг также статистически значимо не различался (2,96 (0,66) и 3,31 (0,79) г/л; p=0,071).
Заключение
Наш опыт применения диеногеста 2 мг (Визанна) у 62 больных аденомиозом в течение 6–68 месяцев продемонстрировал хорошую переносимость и метаболическую нейтральность при его долговременном применении. При этом снижение интенсивности болевого синдрома было отмечено у 33/58 (53,4%) пациенток, а у 19/58 (31%) больных – полное его устранение. На фоне приема диеногеста объем матки уменьшился у 41/58 (70,7%) пациенток.



