Здоровье женщин является частью здоровья населения, которое Устав Всемирной организации здравоохранения (ВОЗ) определяет как «состояние полного физического, психического и социального благополучия, а не просто отсутствие болезней или недугов» [1].
Здоровье женщины включает репродуктивное здоровье (здоровье матери и ребенка), здоровье половых органов и молочной железы, а также эндокринное (гормональное) здоровье, включая контроль менструального цикла, рождаемости и менопаузы.
Вопросы, связанные со здоровьем женщин, являются особо значимыми в медицинском, социальном и индивидуально-психологическом плане.
Фармацевтическая компания ООО «Фармасинтез-Тюмень», Россия, разработала воспроизведенные гормональные препараты линейки «ДляЖенс»: «ДляЖенс метри», «ДляЖенс про», «ДляЖенс климо». Указанные препараты предназначены для применения у женщин с различными заболеваниями репродуктивных органов и бесплодием, а также для проведения менопаузальной гормонотерапии (МГТ) и профилактики постменопаузального остеопороза.
Лекарственный препарат «ДляЖенс метри» (диеногест), таблетки, 2 мг, является воспроизведенным препаратом по отношению к оригинальному (референтному) препарату «Визанна», таблетки, 2 мг («Байер Фарма АГ», Германия). Оригинальный препарат «Визанна» разрешен к медицинскому применению в Российской Федерации с 2011 г.
Диеногест предназначен для лечения эндометриоза, который является болезнью века, в связи с ростом заболеваемости и тенденцией к прогрессированию и рецидивирующему течению.
Эндометриоз встречается у 6–10% женщин репродуктивного возраста. При хронических тазовых болях и бесплодии наблюдается у 40–70 и 30–50% соответственно [2–5].
При доброкачественном характере заболевания может наблюдаться его агрессивное течение с локальной инвазией, широким распространением и диссеминацией очагов. Минимально распространенный эндометриоз нередко сопровождается выраженной тазовой болью, а эндометриоидные кисты больших размеров – бессимптомным течением [6].
Поиск эффективных методов фармакотерапии различных форм эндометриоза является актуальной задачей [7–9].
Эндометриоз традиционно считают персистирующим, рецидивирующим заболеванием, требующим длительного лечения, поэтому возникают вопросы, связанные с безопасностью и переносимостью лекарственных препаратов, предназначенных для его лечения. К терапии первой линии эндометриоза относятся комбинированные пероральные контрацептивы и прогестагены [10, 11].
Диеногест – синтетический прогестин с выраженным прогестагенным и умеренным антигонадотропным эффектами, но без андрогенной, глюкокортикоидной и минералокортикоидной активности [12–14]. Доклинические и клинические исследования показали, что диеногест подавляет овуляцию при умеренном снижении синтеза эстрадиола в яичниках, концентрация последнего находится в пределах терапевтического окна действия эстрогенов [13, 15]. Это не приводит к повышению пролиферации в очагах эндометриоза, но позволяет избежать развития симптомов дефицита эстрогенов. Особенность химической структуры диеногеста – отсутствие этинильного радикала, что обусловливает его метаболическую нейтральность. Это преимущество позволяет применять диеногест длительно. Высокая эффективность терапии диеногестом, немногочисленные нежелательные реакции, возможность длительного применения способствуют обоснованному выбору диеногеста в качестве препарата выбора для лечения эндометриоза.
Следует отметить тот факт, что многие женщины с эндометриозом имеют овуляторный менструальный цикл и нуждаются в контрацепции. В настоящее время имеются данные, свидетельствующие о том, что диеногест (2 мг/сут) обеспечивает надежный антиовуляторный эффект за счет апоптоза гранулезных клеток доминантного фолликула [15–17]. Ингибирование овуляции осуществляется также за счет слабого центрального эффекта (подавления секреции фолликулостимулирующего гормона (ФСГ) и лютеинизирующего гормона (ЛГ) и умеренного снижения концентрации эстрогенов в сыворотке крови), что является преимуществом диеногеста по сравнению с различными формами агониста гонадотропин-рилизинг-гормона [13, 15]. Во многих работах получены убедительные данные о быстром восстановлении фертильности (в среднем 30 дней) и об успешном наступлении беременности у женщин, получавших достаточно длительное время (сроком до 1 года) диеногест (2 мг/сут) для лечения эндометриоза [18–20].
Лекарственный препарат «ДляЖенс про» (прогестерон), капсулы 100 мг, 200 мг, является воспроизведенным препаратом по отношению к оригинальному (референтному) препарату «Утрожестан», капсулы 100 мг, 200 мг («Безен Хелскеа СА», Бельгия). Оригинальный препарат «Утрожестан» разрешен к медицинскому применению в Российской Федерации с 1999 г.
Прогестерон является не только важнейшим регулятором нормальной репродуктивной функции у человека в матке, яичниках, молочных железах и мозге, но и играет роль в функционировании нерепродуктивных органов кардиоваскулярной, костной, центральной нервной, иммунной систем и в метаболических процессах (обмен воды, электролитов, липидов, углеводов, белков, а также компонентов гемостаза и фибринолиза) [21].
В настоящее время отечественный фармацевтический рынок представлен рядом препаратов прогестерона в различных лекарственных формах, предназначенных для перорального, интравагинального, подкожного, внутримышечного и трансдермального введения.
Препараты прогестерона применяются для лечения прогестерондефицитных состояний у женщин: при угрожающем аборте или для предупреждения привычного аборта; при бесплодии вследствие лютеиновой недостаточности, предменструальном синдроме, нарушениях менструального цикла вследствие нарушения овуляции или ановуляции, фиброзно-кистозной мастопатии; для МГТ в пери- и постменопаузе (в сочетании с эстрогенсодержащими препаратами), МГТ в случае дефицита прогестерона при функционирующих (отсутствующих) яичниках (донорство яйцеклеток); для предупреждения преждевременных родов у женщин из группы риска, поддержки лютеиновой фазы во время подготовки к экстракорпоральному оплодотворению и поддержки лютеиновой фазы в спонтанном или индуцированном менструальном цикле; при преждевременной менопаузе [22–25].
Лекарственный препарат «ДляЖенс климо» (левоноргестрел+эстрадиол), таблетки, покрытые оболочкой, 2 мг и 0,15 мг+2 мг, является воспроизведенным препаратом по отношению к оригинальному (референтному) препарату «Климонорм», набор таблеток, покрытых пленочной оболочкой, 2 мг и 0,15 мг+2,0 мг («Йенафарм ГмбХ и Ко.КГ», Германия). Оригинальный препарат «Климонорм» разрешен к медицинскому применению в Российской Федерации с 1993 г.
Комбинация левоноргестрел+эстрадиол стала в Российской Федерации одним из первых препаратов, широко применяемых для МГТ.
Левоноргестрел+эстрадиол предназначен для проведения МГТ при симптомах дефицита эстрогенов у женщин с интактной (сохраненной) маткой в постменопаузальном периоде (не ранее чем через 6 месяцев после последней менструации в случае естественной менопаузы или сразу же после менопаузы в результате хирургического вмешательства, например, после двусторонней овариэктомии или лучевой терапии), а также для профилактики постменопаузального остеопороза у женщин с высоким риском переломов при непереносимости или наличии противопоказаний к применению других лекарственных средств для профилактики остеопороза [26, 27].
Постепенное снижение и прекращение синтеза половых стероидов в яичниках, происходящее в возрасте 43–53 лет, способствует возникновению психоэмоциональных, вазомоторных, урогенитальных нарушений, предрасполагает к развитию атеросклероза и остеопороза. Поэтому очевидно значение МГТ для устранения или уменьшения тяжести возникающих патологических процессов, сохранения трудоспособности и качества жизни женщины. В этот период целесообразно применение комбинированных эстроген-гестагенных препаратов [27, 28].
На обмен жиров половые гормоны оказывают разнонаправленное действие: эстрогены улучшают соотношение липопротеидов высокой и низкой плотности, обладают антиоксидативным эффектом, антиатеросклеротическим потенциалом, но повышают содержание в крови триглицеридов; андрогены негативно действуют на соотношение липопротеидов и снижают синтез триглицеридов в печени. Для женщин с избытком массы тела особенно важно отсутствие эстрогенозависимого повышения концентрации триглицеридов, что часто наблюдается при применении препаратов, в состав которых входят гестагены. К позитивным свойствам комбинации левоноргестрел+эстрадиол следует отнести статистически значимое снижение концентрации триглицеридов, обусловленное остаточной андрогенной активностью левоноргестрела. Кроме того, незначительное уменьшение концентрации инсулина натощак, вероятно, связанное со столь же незначительным снижением массы тела, приводит к достоверной редукции индекса инсулинорезистентности HOMA-IR, что свидетельствует об улучшении чувствительности тканей к инсулину [29].
Профиль метаболических эффектов левоноргестрела+эстрадиол позволяет рекомендовать его к широкому применению у женщин с нормальным жировым и углеводным обменом без обязательного контроля биохимических параметров жирового и углеводного обмена [29].
Внедрение в клиническую практику воспроизведенных препаратов, обладающих системной биодоступностью (за исключением водных растворов), предусматривает предварительное проведение исследования биоэквивалентности. Биоэквивалентность лекарственных препаратов предполагает их терапевтическую эквивалентность. Исследование биоэквивалентности является основным видом медико-биологического контроля качества воспроизведенных препаратов, содержащих такое же количество действующего вещества, как и соответствующий оригинальный лекарственный препарат, и регулируется нормативными документами [30–32].
С целью оценки биоэквивалентности новых воспроизведенных лекарственных препаратов «ДляЖенс метри», «ДляЖенс про», «ДляЖенс климо» компанией ООО «Фармасинтез-Тюмень», Россия, проведены исследования их биоэквивалентности в сравнении с соответствующими оригинальными (референтными) препаратами.
Материалы и методы
Исследования биоэквивалентности лекарственных препаратов «ДляЖенс метри» (диеногест), «ДляЖенс про» (прогестерон), «ДляЖенс климо» (левоноргестрел+эстрадиол) были проведены в Российской Федерации в соответствии с принципами, изложенными в Хельсинкской декларации Всемирной медицинской ассоциации «Рекомендации для врачей, занимающихся биомедицинскими исследованиями с участием людей» (Бразилия, Форталеза, 2013 г.); принципами Надлежащей клинической практики [33–35]; международными правилами проведения клинических исследований (ICH GCP); требованиями к проведению исследований биоэквивалентности российского законодательства и ЕАЭС [30–32], а также в соответствии с утвержденными в установленном порядке протоколами исследований биоэквивалентности.
Каждое из исследований биоэквивалентности было проведено в одном клиническом центре в Российской Федерации: НУЗ «Дорожная клиническая больница на ст. Ярославль ОАО «РЖД» (Ярославль). В исследовании биоэквивалентности препаратов диеногеста аналитическая часть выполнена в биоаналитической лаборатории ФГБУ ВЦЭРМ им. А.М. Никифорова МЧС России (Санкт-Петербург); в исследованиях биоэквивалентности препаратов прогестерона и левоноргестрела+этинилэстрадиол – в Испытательной лаборатории ООО «СИВИлаб» (Иркутск). Статистичсекая обработка результатов исследования проведена контрактно-исследовательской организацией ООО «КлинФармДевелопмент» (Ярославль).
В исследование биоэквивалентности препаратов диеногеста были рандомизированы 24 добровольца [36]; в исследование биоэквивалентности препаратов прогестерона – 54 добровольца [37]; в исследование биоэквивалентности препаратов левоноргестрела+эстрадиол – 40 добровольцев [38].
В исследования включались женщины, добровольно изъявившие желание участвовать в исследовании, прошедшие физикальное и лабораторно-инструментальное обследование и соответствующие следующим критериям: верифицированный диагноз «здоров» по данным стандартных клинических, лабораторных и инструментальных методов обследования; индекс массы тела (ИМТ) в пределах 18,5–30 кг/м2 по формуле Кетле; подписанное письменное информированное согласие; согласие на все ограничения, налагаемые в ходе исследования; согласие участвовать во всех требуемых процедурах исследования.
Дополнительными критериями включения в исследования биоэквивалентности препаратов диеногеста и прогестерона являлись: возраст 18–45 лет, женский пол, европеоидная раса, согласие придерживаться адекватных негормональных методов контрацепции (презерватив или диафрагма+спермицид) на протяжении всего исследования (от момента подписания формы информированного согласия и в течение 14 дней после завершения исследования).
Дополнительными критериями включения в исследование биоэквивалентности препаратов диеногеста явились: нормальный менструальный цикл продолжительностью 28±2 дня на протяжении не менее 6 месяцев до включения в исследование; отсутствие грудного вскармливания; вторая фаза менструального цикла; отрицательный тест на беременность; отрицательный тест Папаниколау.
Дополнительными критериями включения в исследование биоэквивалентности препаратов прогестерона являлись: регулярный менструальный цикл продолжительностью 28±2 дня.
Дополнительными критериями включения в исследование биоэквивалентности препаратов левоноргестрела+эстрадиол являлись: возраст 40–60 лет для здоровых некурящих женщин с интактной маткой, в период естественной постменопаузы; отсутствие менструаций в течение как минимум 12 месяцев; значение концентрации 17β-эстрадиола, ФСГ в сыворотке крови в диапазоне, соответствующем постменопаузе. Выбор женщин в постменопаузе в качестве субъектов исследования исключал воздействие гормонов, выделяемых функциональными яичниками [22].
В исследование не включались: женщины с отягощенным аллергологическим анамнезом; известной гиперчувствительностью к действующим веществам исследуемых препаратов; лекарственной непереносимостью; с любыми острыми и хроническими заболеваниями; психическими расстройствами, в том числе в анамнезе; приступами судорог, эпилепсии и любыми другими неврологическими нарушениями в анамнезе; гинекологическими заболеваниями; кровянистыми выделениями из половых путей; перенесшие гинекологические операции, хирургические вмешательства на желудочно-кишечном тракте за исключением аппендэктомии, острые инфекционные заболевания менее чем за 4 недели до начала исследования. В исследования биоэквивалентности не включались женщины, соблюдающие особую диету или образ жизни (работа в ночное время, экстремальные физические нагрузки); при приеме более чем 10 ед. алкоголя в неделю; употреблении цитрусов (в т.ч. грейпфрут и грейпфрутовый сок) и клюквы (в т.ч. соки, морсы и др.) за 14 дней до начала исследования; при наличии анамнестических сведений об алкоголизме, наркомании, злоупотреблении лекарственными препаратами; принимавшие гестагены менее чем за 6 месяцев до включения в исследование, любые препараты, имеющие системное всасывание, за 14 дней до начала исследования, безрецептурные препараты, включая травы и пищевые добавки, за 7 дней до приема первой дозы. Прием лекарственных препаратов, оказывающих выраженное влияние на гемодинамику, функцию печени также являлся критерием невключения в исследование. В случае использования гормональных контрацептивов они должны быть отменены не менее чем за 2 месяца до начала исследования.
В исследования биоэквивалентности не включались добровольцы, принимавшие участие в других клинических исследованиях лекарственных средств менее чем за 3 месяца до начала исследования.
Дополнительными критериями невключения в исследование биоэквивалентности препаратов прогестерона являлись: тромбоз глубоких вен, тромбофлебит, тромбоэмболические нарушения (тромбоэмболия легочной артерии, инфаркт миокарда [27], инсульт), внутричерепное кровоизлияние или наличие данных состояний/заболеваний в анамнезе.
Целью проведенных исследований биоэквивалентности были изучение сравнительной фармакокинетики и оценка биоэквивалентности препаратов «ДляЖенс метри» (диеногест), таблетки, 2 мг (ООО «Фармасинтез-Тюмень», Россия) и «Визанна», таблетки, 2 мг («Байер Фарма АГ», Германия); препаратов «ДляЖенс про» (прогестерон), капсулы, 200 мг (ООО «Фармасинтез-Тюмень», Россия) и «Утрожестан», капсулы, 200 мг («Безен Хелскеа СА», Бельгия); препаратов «ДляЖенс климо» (левоноргестрел+эстрадиол), таблетки, покрытые оболочкой, 0,15 мг+2 мг (ООО «Фармасинтез-Тюмень», Россия) и «Климонорм», таблетки, покрытые пленочной оболочкой, 0,15 мг+2 мг («Йенафарм ГмбХ и Ко.КГ», Германия) у здоровых добровольцев женского пола при однократном приеме их натощак.
Задачи исследований биоэквивалентности включали оценку фармакокинетических параметров, биодоступности, биоэквивалентности, безопасности и переносимости исследуемых и референтных препаратов у здоровых добровольцев женского пола при однократном приеме их натощак.
Выбор препаратов «Визанна», «Утрожестан», «Климонорм» в качестве референтных препаратов для препаратов «ДляЖенс метри», «ДляЖенс про», «ДляЖенс климо» соответственно в исследованиях биоэквивалентности обоснован следующим: исследуемые препараты (T) и препараты сравнения (R) имеют одинаковые действующие вещества; являются оригинальными препаратами; производятся в одинаковой лекарственной форме; содержат равную дозу действующего вещества; вспомогательные вещества, входящие в состав сравниваемых препаратов, хорошо изучены и не должны оказывать влияния на фармакокинетику данных препаратов [39].
Исследования биоэквивалентности препаратов диеногеста [36] и левоноргестрела+эстрадиол [38] проводились как открытые сравнительные рандомизированные перекрестные одноцентровые исследования биоэквивалентности с двумя этапами, с однократным приемом одинаковой дозы каждого из сравниваемых препаратов в условиях стационара и на амбулаторных визитах.
Клиническая фаза исследования состояла из этапа скрининга (до 7 дней), двух равнозначных этапов исследования, когда осуществлялся прием исследуемых препаратов и отбор образцов крови, и «отмывочного» периода между этапами исследования (в исследовании биоэквивалентности препаратов диеногеста – 28 суток, в исследовании препаратов левоноргестрела+эстрадиол – 14 суток от момента первого приема одного из исследуемых препаратов).
Длительность каждого этапа в исследовании биоэквивалентности препаратов диеногеста составила около 2,5 суток (приблизительно 60 ч), в исследовании препаратов левоноргестрела+эстрадиол – около 3,5 суток (приблизительно 84 ч).
Этап скрининга включал набор добровольцев и проведение их обследования с целью определения критериев включения/невключения [22].
Каждый доброволец был госпитализирован в клинический центр для проведения первого этапа исследования не позднее чем за 12 ч до момента приема одного из исследуемых препаратов. Госпитализация на первом этапе исследования длилась 36 ч, после чего, в случае отсутствия показаний для продления госпитализации, каждый доброволец в исследовании биоэквивалентности препаратов диеногеста был отпущен домой до амбулаторных визитов через 36 ч и 48 ч [36], в исследовании препаратов левоноргестрела+эстрадиол – через 48 ч и 72 ч [38].
В начале первого этапа добровольцы, соответствующие критериям включения/невключения, были рандомизированы в две группы в соотношении 1:1 к одной из последовательностей приема препаратов: RT или TR. Добровольцы, включенные в группу 1, на первом этапе исследования получали исследуемый препарат (Т), а добровольцы группы 2 – препарат сравнения (R).
На втором этапе исследования назначение препарата было обратным: добровольцы группы 1 получали препарат сравнения (R), а добровольцы группы 2 – исследуемый препарат (Т). Таким образом, группа 1 получала лекарственный препарат в последовательности TR, а группа 2 получала лекарственный препарат в последовательности RT, т.е. общая схема исследования была «TR/RT».
Схема рандомизации была сгенерирована перед началом исследования при помощи методом генерации случайных чисел. Рандомизационный номер присваивался добровольцам в порядке их прибытия в клинический центр.
Ослепление в настоящих исследованиях не проводилось. Однако врачи-аналитики, проводящие фармакокинетический анализ, не знали план рандомизации до получения результатов.
На каждом этапе в исследовании биоэквивалентности препаратов диеногеста каждый доброволец принимал внутрь натощак 2 мг диеногеста (1 таблетка), в исследовании препаратов левоноргестрела+эстрадиол каждый доброволец принимал внутрь натощак 2 мг+0,15 мг левоноргестрела+эстрадиол (1 таблетка).
Указанные дозировки препаратов были выбраны в связи с тем, что соотетствующие референтные препараты зарегистрированы и применяются в данных дозировках; они является терапевтическими дозами препаратов диеногеста и препаратов левоноргестрела+эстрадиол. Способ приема препаратов соответствует инструкциям по медицинскому применению референтных препаратов [13, 26].
Поскольку прием пищи не оказывает значимого влияния на абсорбцию действующих веществ [11, 40], исследования биоэквивалентности были проведены при приеме препаратов натощак, так как это условие соответствует наибольшей чувствительности для выявлений различий между сравниваемыми лекарственными препаратами [30–32].
За 1 день до начала второго этапа исследования добровольцы прибывали на амбулаторный визит в клинический центр для сдачи промежуточных анализов крови и мочи (для оценки критериев включения/невключения). После этого добровольцы приглашались для госпитализации на второй этап. Процедуры второго этапа исследования были идентичны первому этапу.
На каждом этапе исследования биоэквивалентности препаратов диеногеста образцы крови для фармакокинетического анализа отбирались в 19 временных точках: 0 мин (до приема препарата); 15 мин; 30 мин; 45 мин; 1 ч; 1 ч 15 мин; 1 ч 30 мин; 1 ч 45 мин; 2 ч; 2 ч 30 мин; 3 ч; 4 ч; 6 ч; 8 ч; 12 ч; 18 ч; 24 ч; 36 ч; 48 ч после приема лекарственного препарата.
На каждом этапе исследования биоэквивалентности препаратов левоноргестрела+эстрадиол образцы крови для фармакокинетического анализа отбирались в следующих временных точках:
- для измерения концентраций левоноргестрела: за 30 мин до приема препарата; 30 мин; 45 мин; 1 ч; 1 ч 15 мин; 1 ч 30 мин; 1 ч 45 мин; 2 ч; 2 ч 15 мин; 2 ч 30 мин; 2 ч 45 мин; 3 ч; 3 ч 30 мин; 4 ч; 6 ч; 9 ч; 12 ч; 24 ч; 48 ч; 72 ч (всего 20 проб);
- для измерения концентраций эстрадиола: -1 ч 30 мин; -1 ч, -30 мин (до приема препарата); 30 мин; 1 ч; 2 ч; 3 ч; 4 ч; 5 ч; 6 ч; 7 ч, 8 ч; 9 ч; 10 ч; 12 ч; 18 ч; 24 ч; 48 ч; 72 ч (всего 19 проб).
После проведения всех процедур второго этапа исследования каждому добровольцу был проведен завершающий осмотр, после которого, в случае отсутствия нежелательных явлений (НЯ) и показаний для продления госпитализации, исследование для добровольцев считалось завершенным.
Общая продолжительность исследования биоэквивалентности препаратов диеногеста для каждого добровольца составила 34–37 дней, исследования биоэквивалентности препаратов левоноргестрела+эстрадиол – 24 дня.
Дизайн исследования биоэквивалентности препаратов прогестерона [37] – открытое сравнительное рандомизированное повторное четырехпериодное исследование биоэквивалентности, с однократным приемом одинаковой дозы каждого из сравниваемых препаратов в каждом периоде исследования.
Исследование проводилось в условиях стационара и на амбулаторных визитах.
Исследование состояло из периода скрининга (14 суток), 4 равнозначных периодов исследования, когда осуществлялись прием исследуемых препаратов и отбор образцов крови и трех «отмывочных» интервалов (7 суток) между периодами исследования. Длительность каждого периода отбора проб около 2,5 суток (до точки через 48 ч после приема препарата).
За 1 день до начала третьего периода добровольцы прибывали на оценочный визит.
Завершающий визит проводился после отбора крови в точке через 48 ч в четвертом периоде.
С первого по четвертый периоды исследования добровольцы прибывали в клинику за 12 ч до приема препаратов и госпитализировались до точки отбора крови через 24 ч после приема препарата. Отборы крови в точке через 48 ч производились амбулаторно [37].
В начале первого этапа добровольцы, соответствующие всем критериям включения/невключения, были включены в активную фазу исследования.
Добровольцы каждой группы получали внутрь однократно исследуемый препарат и препарат сравнения прогестерона в дозе 200 мг по открытой перекрестной схеме. Способ приема соответствует инструкции по медицинскому применению референтного препарата [25].
В каждом периоде исследования биоэквивалентности препаратов прогестерона образцы крови для фармакокинетического анализа отбирались в 20 временных точках: -1 ч; -30 мин; 0 мин (до приема препарата); 20 мин; 40 мин; 1 ч; 1 ч 20 мин; 1 ч 40 мин; 2 ч; 2 ч 20 мин; 2 ч 40 минут; 3 ч; 3 ч 30 мин; 4 ч; 5 ч; 6 ч; 8 ч; 12 ч; 24 ч; 48 ч.
Общая продолжительность исследования для каждого добровольца составляла 36 суток.
Фармакокинетические параметры и статистический анализ данных
Для каждого добровольца рассчитывались следующие фармакокинетические параметры, необходимые для оценки биоэквивалентности сравниваемых лекарственных препаратов: AUC0–t – площадь под кривой «плазменная концентрация-время» с момента приема до последней определяемой концентрации во временной точке t; AUC0–∞ – площадь под кривой «плазменная концентрация-время» с момента приема препарата до бесконечности; AUCresid, AUCresid% – остаточная площадь (в абсолютных и относительных единицах); Cmax – максимальная плазменная концентрация; TСmax – время достижения Cmax; T½ – период полувыведения из плазмы; f – относительная биодоступность исследуемого препарата по отношению к препарату сравнения AUC0-∞(T)/AUC0-∞(R); f’ – относительная биодоступность исследуемого препарата по отношению к препарату сравнения AUC0-t(T)/AUC0-t(R); f’’ – относительная степень всасывания, определяемая отношением Cmax(T)/Cmax(R); Cmax/AUC – относительная скорость всасывания; MRT – среднее время удерживания препарата в организме; kel – константа скорости терминальной элиминации [41].
Статистическая обработка и оформление результатов проводились с помощью пакетов программного обеспечения StatSoft STATISTICA v. 13, Microsoft Excel 2007 и статистического пакета R v. 3.6.0, модуль Bear v. 2.8.4.
Для проверки гипотез о статистической значимости вклада различных факторов (различия между препаратами, различия между добровольцами, последовательность приема препаратов, периоды исследования) в наблюдаемую вариабельность применялся дисперсионный анализ. Расчет фармакокинетических параметров и статистический анализ полученных данных выполнены в предположении о лог-нормальном распределении параметров AUC и Cmax. Полученная с помощью дисперсионного анализа оценка остаточной вариации использовалась при расчете доверительного интервала для отношения средних геометрических значений соответствующего параметра [41].
Вывод о биоэквивалентности сравниваемых препаратов был сделан на основе оценки 90% доверительных интервалов для отношений средних геометрических значений параметров AUC0-t и Сmax исследуемых и референтных препаратов. После проведения логарифмического преобразования эти показатели анализировались с помощью дисперсионного анализа (ANOVA; параметрический метод) [41]. Полученные результаты остаточной вариации использовалась при расчете доверительного интервала для отношения средних значений соответствующего параметра с обратным преобразованием из логарифмических в исходные единицы.
Препараты считались биоэквивалентными, если границы оцененного 90% доверительного интервала для отношений средних геометрических значений параметров AUC0-t и Cmax исследуемых и референтных препаратов диеногеста, прогестерона, левоноргестрела+эстрадиол находились в пределах 80,00–125,00%.
Аналитический анализ данных
Анализ концентрации действующих веществ препаратов в плазме крови проводили биоаналитическим методом с использованием высокочувствительной и селективной высокоэффективной жидкостной хроматографии с тандемным масс-спектрометрическим детектированием (ВЭЖХ-МС/МС) [22].
Нижний предел количественного определения диеногеста составлял 0,5 нг/мл.
Нижний предел количественного определения левоноргестрела составлял 1 пг/мл. Нижний предел для эстрадиола – 1 пг/мл.
Нижний предел количественного определения прогестерона составлял 2 пг/мл. Поскольку прогестерон является эндогенным соединением,
исследование биоэквивалентности препаратов прогестерона предполагает оценку среднего значения концентрации эндогенного прогестерона [40]. Для этого были отобраны три пробы крови до приема препаратов прогестерона у каждого добровольца на каждом этапе во временных точках -1 ч, -0,5 ч и 0 ч.
Результаты
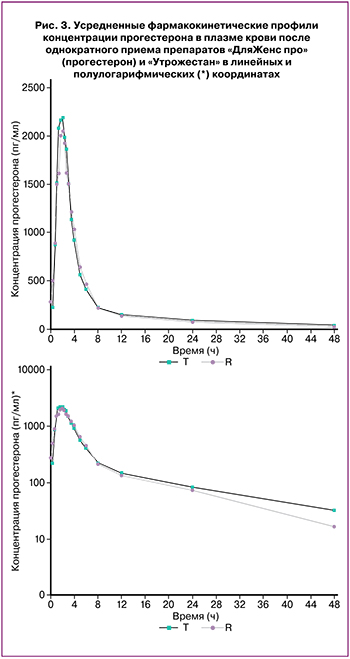
В ходе трех исследований биоэквивалентности определение концентраций диеногеста, прогестерона и левоноргестрела+эстрадиол было проведено в плазме крови 24, 52 и 39 здоровых добровольцев женского пола соответственно. На рисунках 1–3 представлены усредненные фармакокинетические профили концентрации диеногеста, прогестерона, левоноргестрела+эстрадиол в плазме крови добровольцев после однократного приема исследуемых и референтных препаратов в линейных и полулогарифмических координатах.

Построение фармакокинетических профилей концентрации прогестерона проводилось с учетом среднего значения концентраций эндогенного прогестерона, рассчитанного у каждого добровольца на каждом этапе.
На основании полученных данных можно сделать вывод, что исследуемые и референтные препараты диеногеста, прогестерона, левоноргестрела+эстрадиол характеризуются высокой степенью сходства показателей фармакокинетики. Индивидуальные и усредненные профили фармакокинетических кривых исследуемых и референтных препаратов имеют совпадающие формы. Препараты характеризуются близкими значениями относительной биодоступности и Cmax.
Границы оцененных 90% доверительных интервалов для отношений средних геометрических значений AUC0-t и Cmax исследуемых и референтных препаратов диеногеста, прогестерона, левоноргестрела+эстрадиол соответствуют допустимым пределам 80,00–125,00%.
Фармакокинетические параметры, характеризующие биодоступность диеногеста, были следующие: AUC0-∞ – 102,94%, AUC0-t – 102,91%, Cmax – 98,98%. Для левоноргестрела AUC0-72 – 101,65%, Cmax – 102,39%. Для эстрадиола AUC0-72 – 105,78%, Cmax – 99,66%. В случае прогестерона: AUC0-∞ – 99,22%, AUC0-t – 99,22%, Cmax – 97,41%.
Рассчитанные значения 90% доверительных интервалов для отношений средних геометрических значений фармакокинетических параметров диеногеста, левоноргестрела+эстрадиол, прогестерона представлены в таблицах 1–3.

Таким образом, лекарственные препараты «ДляЖенс метри» (диеногест), таблетки, 2 мг (ООО «Фармасинтез-Тюмень», Россия) и «Визанна», таблетки, 2 мг («Байер Фарма АГ», Германия); «ДляЖенс про» (прогестерон), капсулы, 200 мг (ООО «Фармасинтез-Тюмень», Россия)
и «Утрожестан», капсулы, 200 мг («Безен Хелскеа СА», Бельгия), «ДляЖенс климо» (левоноргестрел+эстрадиол), таблетки, покрытые оболочкой 0,15 мг+2,0 мг (ООО «Фармасинтез-Тюмень», Россия) и «Климонорм», таблетки, покрытые пленочной оболочкой, 0,15 мг+2,0 мг («Йенафарм ГмбХ и Ко.КГ», Германия), являются биоэквивалентными.
Оценка безопасности
Данные по безопасности анализировались исходя из следующих показателей:
- клинический осмотр;
- измерение жизненных показателей: АД, ЧСС, ЧДД и температуры тела;
- лабораторные обследования по окончании второго/четвертого этапа/периода исследования;
- при необходимости – лабораторные и инструментальные обследования;
- мониторинг НЯ/серьезных НЯ (СНЯ).
В исследовании биоэквивалентности препаратов диеногеста переносимость препаратов была хорошей. НЯ не выявлены.
В исследовании биоэквивалентности препаратов прогестерона переносимость препаратов была удовлетворительной. Было зарегистрировано 22 НЯ у 15 добровольцев. В отмывочный период зарегистрировано 1 НЯ – ОРВИ. Нежелательные явления наблюдались как после приема исследуемого препарата (13 НЯ), так и после приема препарата сравнения (8 НЯ).
Нежелательными явлениями были: головная боль (10 случаев), боль внизу живота (2 случая), тахикардия (4 случая); в единичных случаях – головокружение, жидкий стул, миграция водителя ритма из желудочков в предсердия, миграция синусового водителя ритма в предсердия, спаечная кишечная непроходимость болевая форма, ОРВИ.
Связь НЯ с приемом препаратов прогестерона расценили как «вероятную», «возможную» и «сомнительную». Было зарегистрировано 1 СНЯ – спаечная кишечная непроходимость, болевая форма, которое завершилось неполным разрешением. В случаях миграции водителя ритма из желудочков в предсердия, миграции синусового водителя ритма в предсердия исход неизвестен. Во всех остальных случаях НЯ разрешились самостоятельно. 1 НЯ (СНЯ) имело умеренную степень тяжести. Все остальные НЯ имели легкую степень тяжести.
В исследовании биоэквивалентности препаратов левоноргестрела+эстрадиол переносимость препаратов была хорошей. Было зарегистрировано 1 НЯ – судорожный синдром. Данное НЯ развилось после приема исследуемого препарата во втором периоде. Оно имело среднюю степень тяжести, для его купирования потребовалось назначение дополнительной терапии. Исходом НЯ являлось разрешение возникшего состояния. СНЯ выявлено не было.
Во всех исследованиях биоэквивалентности не было выявлено отклонений в лабораторных показателях крови и мочи добровольцев во время исследования, а также случаев возникновения беременности.
Таким образом, по результатам исследований биоэквивалентности профили безопасности сравниваемых препаратов были сопоставимы.
Заключение
Границы оцененных 90% доверительных интервалов для отношений средних геометрических значений фармакокинетических параметров AUC0-t и Cmax исследуемых и референтных препаратов диеногеста, прогестерона, левоноргестрела+эстрадиол находятся в пределах 80,00–125,00%. Эти данные подтверждают, что лекарственные препараты «ДляЖенс метри» (диеногест), таблетки 2 мг (ООО «Фармасинтез-Тюмень», Россия) и «Визанна», таблетки 2 мг («Байер Фарма АГ», Германия); «ДляЖенс про» (прогестерон), капсулы 200 мг (ООО «Фармасинтез-Тюмень», Россия) и «Утрожестан», капсулы 200 мг («Безен Хелскеа СА», Бельгия); «ДляЖенс климо» (левоноргестрел+эстрадиол), таблетки, покрытые оболочкой 0,15 мг+2,0 мг (ООО «Фармасинтез-Тюмень», Россия) и «Климонорм», драже 0,15 мг+2,0 мг («Йенафарм ГмбХ и Ко.КГ», Германия), биоэквивалентны.
Исследуемые препараты обладают сопоставимыми профилями безопасности в сравнении с оригинальными препаратами.



