Characteristics of folliculogenesis, steroidogenesis, and oogenesis during ovarian stimulation in the luteal phase of the menstrual cycle
Aim. To investigate the effectiveness of ovarian stimulation in different phases of the menstrual cycle to preserve reproductive material in cancer patients.Korneeva I.E., Martirosyan Ya.O., Koval’chuk A.I., Nazarenko T.A., Biryukova A.M., Veyukova M.A., Ivanets T.Yu.
Materials and methods. Patients requiring gonadotoxic treatment for cancer (n=140) underwent ovarian stimulation according to the standard protocol with the gonadotrophin-releasing hormone (GnRH) antagonist in the follicular phase (n=68) and random-start protocol in the luteal phase without GnRH antagonists (n=72). The comparative analysis included starting and total gonadotropin doses, stimulation duration, and outcomes (mature oocyte and blastocyst counts, fertilization rate).
Results. All patients included in the study were comparable in age and serum levels of anti-Müllerian hormone (AMH). The mean age of women in groups I and II was 33.3 (3.7) and 33.3 (5.47) years, respectively. AMH level was (3.0 (2.1) and 2.7 (0.4) ng/ml, respectively. There was no statistically significant difference in the starting, daily, and total gonadotropin doses between the two groups. The findings demonstrated that ovarian stimulation with gonadotropins in the luteal phase is accompanied by a high progesterone level, which does not require a GnRH antagonist administration while inducing follicular growth. This produces comparable results with stimulation in the follicular phase in terms of the number of obtained and mature oocytes and the fertilization rate.
Conclusion. The present study’s findings suggest equal effectiveness of stimulation protocols irrespective of menstrual cycle phase, which allows tailoring treatment both for cancer patients and in the routine practice of ART. The protective effect of progesterone against spontaneous LH-surge allows stimulation in the luteal phase without additional administration of a GnRH antagonist.
Keywords
Ovarian stimulation is an essential step in the success of in vitro fertilization (IVF). Gonadotropin administration during the early follicular phase of the cycle results in supraphysiological hormone concentrations during the periovulatory period. Recent studies have shown that recruited antral follicles are constantly present in the ovaries throughout the menstrual cycle. Several cohorts of 2–5-mm antral follicles are found on different days of the cycle. Among fertile women, waves of follicle development detected by ultrasound examination were described by Baerwald A.R. in 2003 [1]. The first wave occurred in the follicular phase and a second wave occurred in the luteal phase. Some women had a third wave in the middle follicular phase, and their menstrual cycle is somewhat longer than in women with two waves [2].
The concept of polycyclic follicle development during the menstrual cycle has led to the emergence of new approaches to ovarian stimulation
The possibility of starting gonadotropins for ovarian stimulation in both the follicular and luteal phases allowed the use of ART protocols, including random-start protocols and double stimulation protocols [3]. Currently, protocols with the initiation of stimulation on any day of the cycle are used for fertility preservation for female cancer patients who do not have enough time to start treatment in the early follicular phase of the cycle [4, 5].
Along with new knowledge about ovarian physiology, the methods of oocyte/embryo vitrification were improved, which made it possible to abandon the obligatory transfer of embryos in the treatment cycle, cryopreserve all obtained embryos and subsequently thaw and transfer them in a cryopreserved cycle [5].
The comparative efficacy of standard luteal phase ovarian stimulation protocols versus luteal phase stimulation continues to be debated. Despite the increase in the duration of stimulation and the total dose of gonadotropins, the number and quality of oocytes not only do not differ between the phases of the menstrual cycle but, according to some data, are better in the luteal phase, compared with standard approaches [6–8]. However, this statement requires verification and objective evidence.
The present study aimed to investigate the effectiveness of ovarian stimulation in different phases of the menstrual cycle to preserve reproductive material in cancer patients.
Materials and methods
The study included 140 female cancer patients seeking retrieval and cryopreservation of oocytes and/or embryos before cancer treatment. Among them 44 had breast cancer, 37 hematological cancers, 30 cervical cancer, and other malignancies (n=29). The patients were managed at the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
All participants signed an informed consent form before entering the study, approved by the Research Ethics Committee.
The criteria for inclusion in the studies were age from 18 to 42 years, AMH ≥ 0.75 ng/ml, planned gonadotoxic therapy (chemotherapy, radiation therapy).
The exclusion criteria were extremely reduced ovarian reserve, oocyte retrieval failure, recurrent oncological diseases, distant metastases, a history of gonadotoxic therapy, and contraindications to ovarian stimulation.
Depending on the day of treatment, the patients were divided into two groups. Group I included 68 women who underwent ovarian stimulation according to the standard protocol with the gonadotrophin-releasing hormone (GnRH) antagonist in the follicular phase (days 2–3 of the menstrual cycle). Patients in group II (n=72) were stimulated during the luteal phase. The criterion for the initiation of ovarian stimulation was the presence of a corpus luteum. These women received ovarian stimulation during the luteal phase of the cycle. We used recombinant FSH (rFSH), human menopausal gonadotropin (hMH), or their combination. GnRH antagonists were not used in the group II patients stimulated in the luteal phase.
During stimulation, ultrasound examination was used to count the number of antral, growing, and preovulatory follicles. The parameters of steroidogenesis were assessed on the day of starting ovarian stimulation, day 6 of ovarian stimulation , and the day of final oocyte maturation trigger injection. The transvaginal ovarian puncture was performed 36 hours after administering 10,000 IU of human chorionic gonadotropin (hCG).
Primary Study Outcomes
The number of retrieved oocytes, mature oocyte counts, daily and total gonadotropin doses, and the duration of stimulation was evaluated. After five days of culture, embryological outcomes were assessed, and cryopreservation of embryos was carried out.
Statistical analysis
Statistical analysis was performed using Microsoft Excel 2010 and SPSS V22.0. The distribution of continuous variables was tested for normality using the Kolmogorov-Smirnov or the Shapiro – Wilk test, depending on the sample size. Quantitative variables showing normal distribution were expressed as means (M) and standard deviation (SD) ); otherwise, the median (Me) and the quartiles Q1 and Q3 were reported. Categorical variables were summarized using counts and percentages (%). Normally distributed continuous variables were compared with Student’s t-test for unpaired samples. Variables not meeting normality assumptions were compared with a nonparametric Mann–Whitney test. Differences between the groups were considered statistically significant at p<0.05.
Results
Patients of both groups were comparable in age [group I 33.3 (3.7) and group II 33.3 (5.5) years], p=0.855, body mass index [23.2 (2.2 ) kg/m2 and 21.2 (1.6)] kg/m2 and AMH levels [3.0 (2.1) ng/ml and 2.7 (0.4) ng/ml].
However, women in group II at the time of starting stimulation had significantly fewer ultrasound-detected antral follicles [5.8 (1.1) versus 9.2 (1.2)] (p=0.04). This difference is due to the presence of a corpus luteum in one of the ovaries, making it difficult to count follicles accurately.
The characteristics of the stimulation protocols depending on the phase of the menstrual cycle are presented in Table 1.
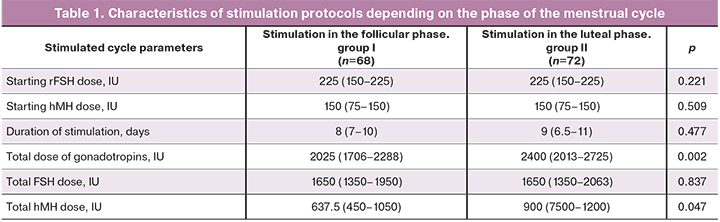
The data in Table 1 show that despite a longer duration of stimulation and higher total dose of gonadotropins in group II, there were no statistically significant differences in the duration of gonadotropic stimulation, starting and total doses of gonadotropins.
All patients of the study groups had preserved ovarian reserve at the time of starting ovarian stimulation. The mean AMH level in women in groups I and II were 1.5 (1.1–3.2) and 1.8 (1.2–3.5) ng/ml, FSH 9, 1 (5.7–14.2) IU/L, and 7.3 (4.8–11.4) IU/L, respectively.
Significant statistical differences between the groups were found in steroid hormone profiles. Thus, the mean concentrations of estradiol and progesterone at the time of starting ovarian stimulation in group II were 332 (222–570) pmol/l and 14.0 (2.7–36.3) nmol/ l, respectively, which was significantly higher (p<0.001), than in group I [estradiol 186 (155–284) pmol/l and progesterone 1.2 (0.6–1.6) nmol/l].
On day 6 of ovarian stimulation and the day of the ovulation trigger administration, serum concentrations of estradiol did not differ between the groups and amounted to 2765 (1200–5336) pmol/l in group I and 1827 (936–2988) pmol/l in group II, and also 7800 (4139–12943) pmol/l and 5544 (3047–8126) pmol/L, respectively.
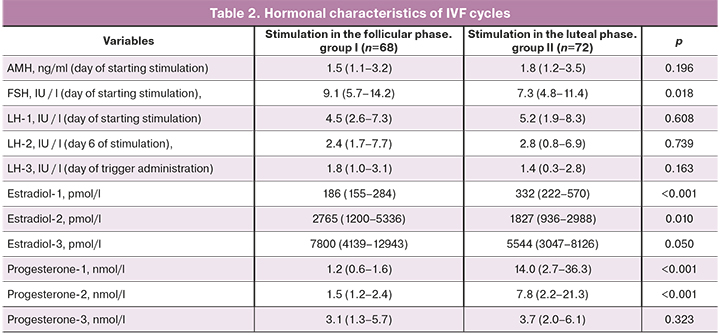
In group II, levels of progesterone on day 6 of ovarian stimulation [7.8 (2.2–21.3) nmol/l] were significantly higher than in group I [1, 5 (1.2–2.4) nmol/l] (p <0.001). They decreased on the day of ovulation trigger administration to 3.7 (2.0–6.1) nmol/L, which was comparable with the values in group I patients [3.1 (1, 3–5.7) nmol/l].
The LH level in the patients of the study groups remained within the normal range throughout the entire period of ovarian stimulation and did not differ significantly.
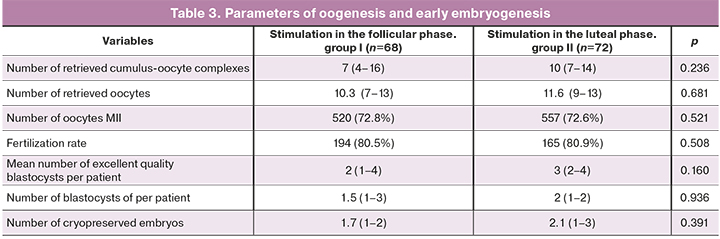
Our study findings did not show an adverse effect of a high concentration of progesterone on the quality of oocytes, which was confirmed by a similar number of retrieved and mature oocytes, both during stimulation in the follicular and in the luteal phase. Mean plasma concentrations of progesterone at the start of ovarian stimulation in groups II, and I were 14.0 (2.7–36.3) nmol/L and 1.2 (0.6–1.6) nmol/L, (p<0.001), the number of retrieved oocytes was 10.3 (7–13) and 11.6 (9–13), p=0.681, the number of cryopreserved embryos on day 5 of cultivation was 1.7 (1–2) and 2.1 (1–3), respectively, p=0.391.
Discussion
Current evidence suggests that during ovarian stimulation in the early follicular phase, GnRH antagonists should be used to prevent premature LH surge [9]. However, the feasibility of this approach for stimulation in the luteal phase of the cycle continues to be discussed. According to the present study's findings, at the start of ovarian stimulation in the luteal phase, there was no spontaneous release of LH, despite the concentration of estradiol, comparable to that on day 6 of stimulation in the follicular phase.
Similar data was published by Wei L.-H. et al. They suggested that high progesterone concentrations block the release of GnRH, which in turn leads to a decrease in LH secretion [8].
This study demonstrates a decrease in the concentration of progesterone by the day of ovulation trigger administration. In group II, the progesterone concentration significantly decreased from 14.0 (2.7–36.3) nmol/L to 3.7 (2.0–6.1) nmol/L and reached values comparable to those in group I [3.1 (2.0–6.1) nmol/l]. Thus, by the day of ovulation trigger administration in women of group II, the level of progesterone did not differ from that in standard protocols of ovarian stimulation, which may indirectly indicate a reduction in the corpus luteum. In group II, we did not use GnRH antagonists to prevent a premature LH surge and gestagens for stopping the menstrual-like reaction. In this regard, the hypothesis of the effect of high progesterone levels on preventing a premature LH surge can be debated. The literature has discussed a hypothesis that the absence of a premature LH surge in the luteal phase of the menstrual cycle may be associated not with progesterone but with other mediators secreted by the corpus luteum (inhibins, follistatin, etc.) [10].
Estradiol levels in women of the study groups had comparable dynamics of concentration increase, which corresponded to the ovarian response to gonadotropin administration, regardless of the stimulation protocol. These findings are consistent with the study by Wei L.-H. [8].
Analysis of the embryological stage of the IVF program did not reveal a statistically significant increase in the number of obtained oocytes, mature oocytes, normally fertilized oocytes, and embryos obtained after stimulation in different phases of the menstrual cycle. This observation confirms the previously published data on the lack of influence of the phase of ovarian stimulation on the embryological stage of the IVF program.
At the same time, Kuang Y. et al. in 2014 published data suggesting that ovarian stimulation in the luteal phase resulted in significantly more retrieved oocytes than with stimulation in the follicular phase [(3.5 (3.2) and 1.7 (1.0), respectively]. However, it should be noted that the total gonadotropin dose used by the authors for ovarian stimulation in the luteal phase was significantly higher than in the follicular phase [3266.4 (248.9) and 1802.5 (712.7) IU, respectively] [11]. In our study, the starting and total doses of gonadotropins and the number of days of ovarian stimulation did not differ significantly.
Based on our study results, we believe that the outcome of stimulation does not depend on the phase of the menstrual cycle when it was started, but on the characteristics of the patient's reproductive system, including age, ovarian reserve, i.e., on those indicators that are generally accepted in the practice of ART.
Conclusion
The dynamics of sex steroid and gonadotropic hormone concentrations during ovarian stimulation in the follicular and luteal phases of the menstrual cycle is characterized by comparable serum levels of estradiol and LH on day 6 of stimulation in all study participants and a significant increase in the level of progesterone in patients of group II.
Indicators of oogenesis and early embryogenesis during stimulation in the follicular and luteal phases do not differ significantly, despite the high serum level of progesterone in phase 2 of the cycle.
Despite the decrease in progesterone level on day 6 of ovarian stimulation in the luteal phase, indicating a reduction in the corpus luteum functional activity, there was no premature LH surge, which suggests the participation of other mediators in this process.
The results of this study demonstrate comparable efficacy of ovarian stimulation in the follicular and luteal phases of the cycle.
There is no need for additional administration of GnRH antagonists at the beginning of ovarian stimulation in the early luteal phase.
References
- Baerwald A.R., Adams G.P., Pierson R.A. A new model for ovarian follicular development during the human menstrual cycle. Fertil. Steril. 2003; 80(1): 116-22. https://dx.doi.org/10.1016/s0015-0282(03)00544-2.
- Baerwald A.R., Adams G.P., Pierson R.A. Characterization of ovarian follicular wave dynamics in women. Biol. Reprod. 2003; 69(3): 1023-31. https://dx.doi.org/10.1095/biolreprod.103.017772.
- Kuang Y., Chen Q., Hong Q., Lyu Q., Ai A., Fu Y., Shoham Z. Double stimulations during the follicular and luteal phases of poor responders in IVF/ICSI programmes (Shanghai protocol). Reprod. Biomed. Online. 2014; 29(6): 684-91. https://dx.doi.org/10.1016/j.rbmo.2014.08.009.
- Cakmak H., Katz A., Cedars M.I., Rosen M.P. Effective method for emergency fertility preservation: random-start controlled ovarian stimulation. Fertil. Steril. 2013; 100(6): 1673-80. https://dx.doi.org/10.1016/j.fertnstert.2013.07.1992.
- von Wolff M., Thaler C.J., Frambach T., Zeeb C., Lawrenz B., Popovici R.M., Strowitzki T. Ovarian stimulation to cryopreserve fertilized oocytes in cancer patients can be started in the luteal phase. Fertil. Steril. 2009; 92(4): 1360-5. https://dx.doi.org/10.1016/j.fertnstert.2008.08.011.
- Kim J.H., Kim S.K., Lee H.J., Lee J.R., Jee B.C., Suh C.S., Kim S.H. Efficacy of random-start controlled ovarian stimulation in cancer patients. J. Korean Med. Sci. 2015; 30(3): 290-5. https://dx.doi.org/10.3346/jkms.2015.30.3.290.
- Muteshi C., Child T., Ohuma E., Fatum M. Ovarian response and follow-up outcomes in women diagnosed with cancer having fertility preservation: Comparison of random start and early follicular phase stimulation – cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018; 230: 10-4. https://dx.doi.org/10.1016/j.ejogrb.2018.09.007.
- Wei L.H., Ma W.H., Tang N., Wei J.H. Luteal-phase ovarian stimulation is a feasible method for poor ovarian responders undergoing in vitro fertilization/intracytoplasmic sperm injection-embryo transfer treatment compared to a GnRH antagonist protocol: A retrospective study. Taiwan. J. Obstet. Gynecol. 2016; 55(1): 50-4. http://dx.doi.org/10.1016/j.tjog.2015.07.001.
- Borm G., Mannaerts B. Treatment with the gonadotrophin-releasing hormone antagonist ganirelix in women undergoing ovarian stimulation with recombinant follicle stimulating hormone is effective, safe and convenient: results of a controlled, randomized, multicentre trial. Hum. Reprod. 2000; 15(7): 1490-8. https://dx.doi.org/10.1093/humrep/15.7.1490.
- Messinis I.E. Ovarian feedback, mechanism of action and possible clinical implications. Hum. Reprod. Update. 2006; 12(5): 557-71. https://dx.doi.org/10.1093/humupd/dml020.
- Kuang Y., Chen Q., Fu Y., Wang Y., Hong Q., Lyu Q., Ai A., Shoham Z. Medroxyprogesterone acetate is an effective oral alternative for preventing premature luteinizing hormone surges in women undergoing controlled ovarian hyperstimulation for in vitro fertilization. Fertil. Steril. 2015; 104(1):62-70. e3. https://dx.doi.org/10.1016/j.fertnstert.2015.03.022.
Received 26.03.2021
Accepted 12.04.2021
About the Authors
Irina E. Korneeva, Dr. Med. Sci., Professor, Senior Researcher at the F. Paulsen Research and Educational Center for ART with the Clinical Department, V.I. KulakovNMRC for OG&P, Ministry of Health of Russia. E-mail: irina.korneeva@inbox.ru. 4 Oparin str., Moscow, Russian Federation, 117997.
Yana O. Martirosyan, Junior Researcher at the F. Paulsen Research and Educational Center for ART with the Clinical Department, V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia. Tel.: +7(925)124-99-99. E-mail: ya_martirosyan@oparina4.ru. 4 Oparin str., Moscow, Russian Federation, 117997.
Alla I. Kovalchuk, Ph.D. Student at the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Tel.: +7(999)999-40-47. E-mail: kovalchukalla27@mail.ru.
4 Oparin str., Moscow, Russian Federation, 117997.
Tatiana A. Nazarenko, Dr. Med. Sci., Professor, Head of the Institute of Reproductive Medicine, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
E-mail: t_nazarenko@oparina4.ru. 4 Oparin str., Moscow, Russian Federation, 117997.
Almina M. Birukova, Ph.D (Med. Sci.), Clinical Supervisor at the F. Paulsen Research and Educational Center for ART with the Clinical Department, V.I. Kulakov
NMRC for OG&P, Ministry of Health of Russia. E-mail: a_birukova@oparina4.ru. 4 Oparin str., Moscow, Russian Federation, 117997.
Mariya A. Veyukova, Ph.D (Bio. Sci.), Head of the Embryological Laboratory, F. Paulsen Research and Educational Center for ART with the Clinical Department, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. E-mail: m_veukova@oparina4.ru. 4 Oparin str., Moscow, Russian Federation, 117997.
Tatiana Yu. Ivanets, Dr. Med. Sci., Head of the Clinical Diagnostic Laboratory, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. E-mail: t_ivanets@oparina4.ru.
4 Oparin str., Moscow, Russian Federation, 117997.
For citation: Korneeva I.E., Martirosyan Ya.O., Koval’chuk A.I., Nazarenko T.A., Biryukova A.M., Veyukova M.A., Ivanets T.Yu. Characteristics of folliculogenesis, steroidogenesis, and oogenesis during ovarian stimulation in the luteal phase of the menstrual cycle.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 7: 107-112 (in Russian)
https://dx.doi.org/10.18565/aig.2021.7.107-112



