Hormonal profile of oocyte donors during ovarian stimulation at different phases of the menstrual cycle
Objective: To compare the hormonal profile of folliculogenesis in oocyte donors during ovarian stimulation in the follicular and luteal phases of the menstrual cycle. Materials and methods: This study included 30 oocyte donors who underwent ovarian stimulation in both the follicular and luteal phases of the menstrual cycle. Sixty donor stimulation cycles were analyzed. Hormone levels (LH, estradiol, progesterone) were measured on the day of stimulation, day 6 of stimulation, day of ovulation trigger administration, and day of transvaginal ovarian puncture (TOP). The number of retrieved oocytes and the MII of oocytes were also evaluated. Results: Statistically significant differences were observed between the groups in the levels of LH, estradiol, and progesterone on the day of the superovulation stimulation. There were no significant differences in estradiol levels on day 6 of stimulation, on the day of ovulation trigger administration, or on the day of TOP (p>0.05). Progesterone levels were significantly higher during the luteal phase ovarian stimulation on the day of ovarian stimulation (p<0.001) and on day 6 of stimulation (p<0.001). However, on the day of ovulation trigger administration, the progesterone levels in the study groups were similar, and on the day of TOP, progesterone levels were also comparable, regardless of the stimulation phase. There were no statistically significant differences in the number of retrieved oocytes and mature oocytes during the stimulation of donors in the different phases of the cycle (p>0.1). Conclusion: Ovarian stimulation in the luteal phase of the menstrual cycle in oocyte donors is significantly different from ovarian stimulation in the follicular phase of the menstrual cycle in terms of the hormonal profile. However, the characteristics identified do not adversely affect the ovarian response and the production of quality oocytes. Authors' contributions: Lapina V.S., Martazanova B.A., Durinyan E.R., Amyan T.S., Korolkova A.I., Gavisova A.A. – the conception and design of the study, review of relevant literature, data collection, statistical analysis, manuscript drafting, final approval for submission. Conflicts of interest: The authors have no conflicts of interest to declare. Funding: There was no funding for this study. Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P. Patient Consent for Publication: All patients provided informed consent for the publication of their data. Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator. For citation: Lapina V.S., Martazanova B.A., Durinyan E.R., Amyan T.S., Korolkova A.I., Gavisova A.A. Hormonal profile of oocyte donors during ovarian stimulation at different phases of the menstrual cycle. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (8): 96-102 (in Russian) https://dx.doi.org/10.18565/aig.2023.152Lapina V.S., Martazanova B.A., Durinyan E.R., Amyan T.S., Korolkova A.I., Gavisova A.A.
Keywords
Traditionally, ovarian stimulation in in vitro fertilization (IVF) programs has always commenced on the first day of a woman's menstrual cycle. According to the prevailing concept of folliculogenesis for several decades, recruitment of a single wave of antral follicles occurs only at the end of the luteal phase of the preceding menstrual cycle. Following regression of the corpus luteum and a decrease in the production of estradiol and inhibin, there is an increase in the level of circulating follicle-stimulating hormone (FSH), which, in turn, is responsible for stimulating (or preventing atresia) a cohort of 2–5 mm antral follicles in the ovaries [1, 2].
Although studies indicate that there is no apparent change in the number of antral follicles throughout the menstrual cycle, the dominant view is that follicles recruited during other phases of the menstrual cycle, influenced by the corpus luteum, are predominantly atretic, contain fewer granulosa cells, and secrete less estradiol than follicles in the early follicular phase.
Some studies confirm that antral follicles of 2–5 mm appear continuously throughout the menstrual cycle, whereas other studies suggest cyclical waves or cohorts of follicular development during the menstrual cycle. In a large study, the appearance of two or three waves of 4–14 follicles ≥4–5 mm has been observed during the menstrual cycle in healthy women [2, 3]. Currently, 68% of women are considered to have two waves of follicle recruitment during the intra-ovulatory interval and 32% have three waves [2].
Therefore, evidence demonstrates that ovarian stimulation initiated at the end of the follicular/early or mid-luteal phases of the menstrual cycle can be successful [4].
However, not all researchers reported comparable results when conducting stimulation in different phases of the menstrual cycle; there have been cases of protocol cancellation owing to a lack of ovarian response [5, 6]. To date, stimulation protocols, the choice of gonadotropins, methods for preventing a spontaneous peak of luteinizing hormone (LH), and luteinization of follicles when initiating ovarian stimulation in the late follicular and early/middle luteal phases have not been fully defined. Additionally, the role of the preovulatory follicle and the processes of follicular atresia and corpus luteum in the formation of the follicular pool and the quality of oocytes remain unclear. The role of hormonal markers of ovarian reserve in the choice of stimulation strategy, gonadotropins, etc., for ovarian stimulation initiated at different phases of the menstrual cycle is also unknown.
This study aimed to compare the hormonal profile of folliculogenesis in oocyte donors during ovarian stimulation during the follicular and luteal phases of the menstrual cycle.
Materials and methods
A prospective randomized study was conducted at V.I. Kulakov NMRC for OG&P of the Ministry of Health of Russia. The study included 30 oocyte donors, each of whom underwent ovarian stimulation twice (at the first stage in the follicular phase, then again after 3 months in the luteal phase), which suggested stratifying them into two groups depending on the phase of ovarian stimulation:
- Group I (n=30) included oocyte donors who received ovarian stimulation during the follicular phase of their menstrual cycle.
- Group II (n=30) included the same oocyte donors, but ovarian stimulation was carried out in the luteal phase of the menstrual cycle.
Thus, 60 cycles of ovarian stimulation of donors were analyzed.
Inclusion criteria were age from 18 to 35 years, basal FSH concentration<10 mIU/ml, the presence of more than 10 antral follicles on day 2 of the menstrual cycle, body mass index 18–25 kg/m2, and informed consent to participate in the study.
For patients in group I, the program was carried out using a protocol with gonadotropin-releasing hormone antagonists (GnRH antagonists). Starting on day 2 or 3 of the menstrual cycle, ovarian stimulation was performed with recombinant FSH and/or human menopausal gonadotropin, the doses of which were determined individually. Once the follicles reached ≥14 mm in diameter, as determined by pelvic ultrasound, GnRH antagonists were administered daily until the end of ovarian stimulation to prevent a premature LH peak.
To determine the day of ovulation trigger administration, the donors underwent pelvic ultrasonography to measure the diameter of the antral follicles. When at least three of these reached ≥18 mm, gonadotropin-releasing hormone agonists (GnRH agonists) were used as triggers. A transvaginal ovarian puncture (TOP) was performed 36 h after ovulation trigger administration. Peripheral blood sampling was performed on the day of the start of ovarian stimulation, on the day of GnRH antagonist administration, and on the day of administration of the ovulation trigger to determine the concentrations of progesterone, estradiol, and LH.
Three months after ovarian stimulation in the follicular phase of the menstrual cycle, the oocyte donors entered the program again. The criteria for the initiation of ovarian stimulation in the luteal phase were the presence of ≥5 antral follicles with a diameter of 3–8 mm. When the leading follicles reached a diameter of ≥14 mm and until the day of TOP, 10 mg of norethisterone (Norcolut) was administered to prevent a menstrual-like reaction. When pelvic ultrasound showed ≥3 follicles ≥18 mm in diameter in the ovaries, patients were administered GnRH agonists as an ovulation trigger. TOP was performed 36 h after ovulation-trigger administration.
Similarly, a study of the hormonal profile in the blood was carried out on day 1 of the administration of drugs for ovarian stimulation, the day of the administration of norethisterone, the day of ovulation trigger administration, and the TOP of the ovaries. The serum concentrations of LH, estradiol, and progesterone were measured using the IMMULITE 2000 analyzer with Progesterone, LH, Estradiol test systems (Siemens, USA).
The study was reviewed and approved by the Research Ethics Committee of V.I. Kulakov NMRC for OG&P.
Statistical analysis
Statistical analysis was performed using the Statistical Analysis Package for Microsoft Office Excel 2007 and SPSS V22.0. Statistical analysis was performed using the generally accepted methods of statistical variation. Quantitative variables showing normal distribution are expressed as mean (M) and standard deviation (SD) and presented as M (SD). Categorical variables are presented as counts and percentages.
The distribution of continuous variables was tested for normality using the Kolmogorov–Smirnov test and graphical data analysis. Methods of parametric statistics (Student's t-test) were used to compare numerical data with a normal distribution. A nonparametric Mann–Whitney test was used to compare numerical data with a non-normal distribution. The Mann–Whitney test was considered significant at p<0.05.
Sample size
The sample size was not calculated due to the lack of similar studies on oocyte donors at the time of data collection.
The primary endpoint was the number of mature oocytes obtained from an oocyte donor. Secondary endpoints: LH, estradiol, and progesterone concentrations.
Results
Clinical and anamnestic data of donors
The age of oocyte donors was 27.7 (4.2) years, and the mean body mass index was 21.1 (3.1) kg/m2. Most oocyte donors had a history of childhood infections. None of the donors had somatic comorbidities. Three donors had a history of laparoscopic appendectomy.
The characteristics of the donors’ menstrual cycles are presented in Table 1. All the donors had regular menstrual cycles. The mean age at menarche was 12.5 (0.5) years.
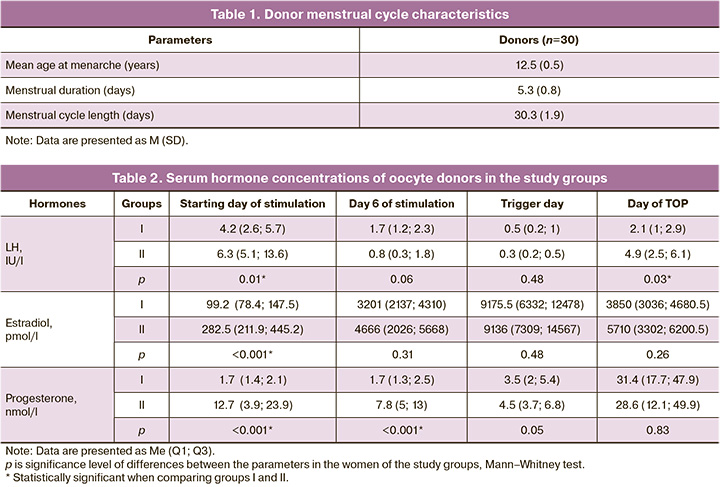
No statistically significant difference was found when comparing ovarian stimulation parameters at different menstrual cycle phases: the starting dose of gonadotropins was 271.2 (37.2) and 283.7 (32.2) IU in groups I and II (p>0.05); the total dose of gonadotropins was 2556.0 (399.8) and 2898.8 (511.5) IU in groups I and II (p>0.05). The duration of stimulation in group I, 0.1 (0.8) days, was similar to that in group II, 10.8 (1.4) days. There was also no significant difference (p>0.05) in basal FSH concentration between groups I, 4.6 (3.2) IU/ml, and II, 3.8 (2.9) IU/ml (p>0.05).
Table 2 presents the serum concentrations of LH, estradiol, and progesterone in women undergoing ovarian stimulation during the different phases of the menstrual cycle.
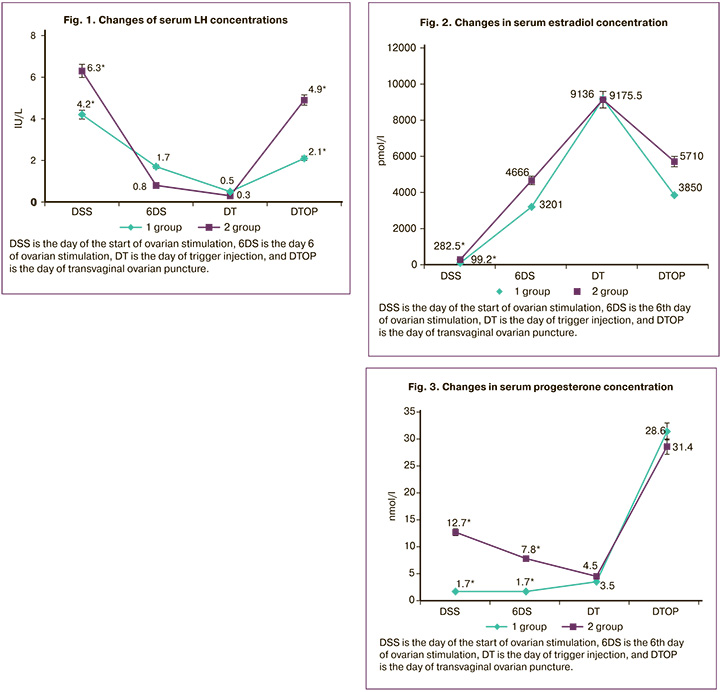
On day 1 of ovarian stimulation, statistically significant differences were found in the concentrations of LH (Fig. 1), estradiol (Fig. 2), and progesterone (Fig. 3), which was due to the onset of stimulation in the luteal phase of the cycle during the ultrasound diagnosis of ovulation (p<0.05). Analysis of the same values at subsequent points (on day 6 of stimulation, the day of the trigger, and the day of TOP) revealed a gradual, but statistically insignificant (p>0.05) increase in serum estradiol levels between groups, accompanied by the growth of follicles (Fig. 2). Progesterone concentrations (Fig. 3) were significantly higher on the day of stimulation (p<0.001) and on day 6 (p<0.001) in group II (luteal phase), which can be explained by the continued hormonal activity of the corpus luteum. However, the concentrations of progesterone in the study groups level off on the day of ovulation trigger, probably due to the extinction of the activity of the corpus luteum. On the day of TOP, progesterone levels were also comparable regardless of the phase of the menstrual cycle in which ovarian stimulation was performed.
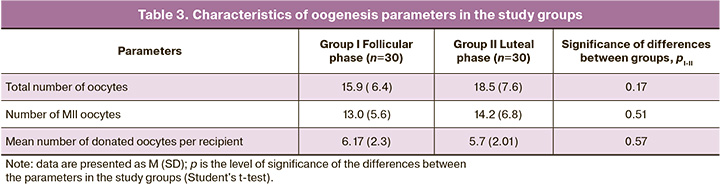
When comparing the oogenesis parameters in both groups, no statistically significant differences were found in the number of obtained, mature, and donated oocytes (Table 3).
Discussion
To study the effect of ovarian stimulation in different phases of the menstrual cycle on the parameters of oogenesis, we chose the donor-recipient program since donors are the ideal model for studying the effect of the onset of superovulation stimulation on the quality of oocytes and embryos, which are represented by a group of healthy, potentially or proven fertile women who voluntarily undergo an ovarian stimulation program.
In our study, when studying the dynamics of changes in the concentrations of sex hormones during the program in oocyte donors in different phases of the menstrual cycle, a statistically significant difference was found in the concentration of LH on the first day of administration of ovarian stimulation drugs in the luteal phase of the menstrual cycle, which reflects the ovulatory peak of LH and the start of the program immediately after visualization of the presence of the corpus luteum according to pelvic ultrasound. In group I, the LH level on the day of stimulation was 4.2 (2.6;5.7) IU/l, in group II it was 6.3 (5.1;13.6) IU/l (p<0.05). Further analysis of the LH level dynamics did not reveal any statistically significant differences. An important feature of the ovarian stimulation protocol carried out in the luteal phase of the menstrual cycle was the absence of a parasitic LH peak at a comparable level of estradiol on day 6 of gonadotropic drug administration (in group I, the level of estradiol on day 6 of stimulation was 3201 (2137; 4310) pmol /l, in group II – 4666 (2026; 5668) pmol/l (p>0.05).The luteal phase of the cycle is characterized by a high concentration of progesterone on the day of ovarian stimulation initiation. In group I, its level was 1.7 (1.4 ;2.1) nmol/l, in group II – 12.7 (3.9;23.9) nmol/l (p<0.001). On day 6 of ovarian stimulation in group I, the progesterone level was 1.7 (1.3;2.5) nmol/l, in group II – 7.8 (5;13) nmol/l (p<0.001). However, by the day of ovulation trigger administration, the concentration of progesterone had decreased, reaching values comparable to those in group I (group I – 3.5 (2; 5.4) nmol/l, group II – 4.5 (3.7; 6.8) nmol/l (p>0.05)). To prevent a menstrual-like reaction in the luteal phase of the cycle, women received 10 mg/day of norethisterone, the concentration of which in the serum was not determined by the test systems we used. Similar data were also reported by Wei L.-H. et al. [7], where the study group included patients with reduced ovarian reserve who underwent double ovarian stimulation within the same menstrual cycle.
In our study, there was no negative effect of high progesterone concentrations on the quality of the obtained oocytes, which is confirmed by the number of obtained and mature oocytes during stimulation, both in the follicular and luteal phases (the number of mature oocytes (MII) was 13.0 (5.6) and 14.2 (6.8) in groups I and II, respectively). These data can probably be explained by the absence of progesterone receptors in oocyte-cumulus complexes [8].
Based on the above data, we concluded that ovarian stimulation in the luteal phase of the menstrual cycle makes it possible provides a hormonal profile and parameters of folliculogenesis comparable to those during stimulation in the follicular phase of the menstrual cycle.
High concentrations of progesterone and LH in the blood serum, observed during ovarian stimulation in the luteal phase of the menstrual cycle, did not adversely affect the parameters of oogenesis in donors, which was confirmed in our study by the absence of a statistically significant difference between the number of received and mature oocytes in the two groups.
The feasibility of starting ovarian stimulation in any phase of the menstrual cycle is consistent with the multiwave theory of folliculogenesis [9]. Previous studies did not find differences in the number and competence of oocytes obtained during the IVF program in the luteal phase compared with conventional stimulation [10–14], and some authors have demonstrated an increase in the number of oocytes obtained during stimulation in the luteal phase [13, 14].
Conclusion
Despite the significant differences in hormonal profile of oocyte donors during ovarian stimulation in the luteal phase of the cycle compared with that in the follicular phase of the menstrual cycle, the identified characteristics do not adversely affect ovarian response, follicular and oogenesis parameters and ultimately the retrieval of high-quality donor oocytes.
References
- Gougeon A. Dynamics of follicular growth in the human: a model from preliminary results. Hum. Reprod. 1986; 1(2): 81-7. https://dx.doi.org/10.1093/oxfordjournals.humrep.a136365.
- Baerwald A.R., Adams G.P., Pierson R.A. Ovarian antral folliculogenesis during the human menstrual cycle: a review. Hum. Reprod. Update. 2012; 18(1): 73-91. https://dx.doi.org/10.1093/humupd/dmr039.
- Kirillova A., Martazanova B., Mishieva N., Semenova M. Follicular waves in ontogenesis and female fertility. Biosystems. 2021; 210: 104558.https://dx.doi.org/10.1016/j.biosystems.2021.104558.
- Mishieva N., Martazanova B., Bogatyreva Kh., Korolkova A., Kirillova A.,Veyukova M. et al. Cumulus cell gene expression in luteal-phase-derived oocytes after double stimulation in one menstrual cycle. Reprod. Biomed. Online. 2020; 41(3): 518-26. https://dx.doi.org/10.1016/j.rbmo.2020.05.002.
- Bedoschi G.M., de Albuquerque F.O., Ferriani R.A., Navarro P.A. Ovarian stimulation during the luteal phase for fertility preservation of cancer patients: case reports and review of the literature. J. Assist. Reprod. Genet. 2010; 27(8): 491-4. https://dx.doi.org/10.1007/s10815-010-9429-0.
- Wang N., Wang Y., Chen Q., Dong J., Tian H., Fu Y. et al. Luteal-phase ovarian stimulation vs conventional ovarian stimulation in patients with normal ovarian reserve treated for IVF: a large retrospective cohort study. Clin. Endocrinol. (Oxf). 2016; 84(5): 720-8. https://dx.doi.org/10.1111/cen.12983.
- Wei L.-H., Ma W.-H., Tang N., Wei J.-H. Luteal-phase ovarian stimulation is a feasible method for poor ovarian responders undergoing in vitro fertilization/intracytoplasmic sperm injection-embryo transfer treatment compared to a GnRH antagonist protocol: a retrospective study. Taiwan. J. Obstet. Gynecol. 2016; 55(1): 50-4. https://dx.doi.org/10.1016/j.tjog.2015.07.001.
- Iliodromiti S., Kelsey T.W., Wu O., Anderson R.A., Nelson S.M. The predictive accuracy of anti-Müllerian hormone for live birth after assisted conception: a systematic review and meta-analysis of the literature. Hum. Reprod. Update. 2014; 20(4): 560-70. https://dx.doi.org/10.1093/humupd/dmu003.
- de Mello Bianchi P.H., Serafini P., Monteiro da Rocha A., Hassun P.A., Alves da Motta E.L., Baruselli P.S., Baracat E.C. Review: follicular waves in the human ovary: a new physiological paradigm for novel ovarian stimulation protocols. Reprod. Sci. 2010; 17(12): 1067-76. https://dx.doi.org/10.1177/1933719110366483.
- Kuang Y., Hong Q., Chen Q., Lyu Q., Ai A., Fu Y., Shoham Z. Luteal-phase ovarian stimulation is feasible for producing competent oocytes in women undergoing in vitro fertilization/intracytoplasmic sperm injection treatment, with optimal pregnancy outcomes in frozen-thawed embryo transfer cycles. Fertil. Steril. 2014; 101(1): 105-11. https://dx.doi.org/10.1016/j.fertnstert.2013.09.007.
- Jochum F., Sananès N., Teletin M., Lichtblau I., Rongières C., Pirrello O. Luteal phase stimulation, the future of fertility preservation? Retrospective cohort study of luteal phase versus follicular phase stimulation. J. Gynecol. Obstet. Hum. Reprod. 2019; 48(2): 91-4. https://dx.doi.org/10.1016/j.jogoh.2018.11.003.
- Martínez F., Clua E., Devesa M., Rodríguez I., Arroyo G., González C.et al. Comparison of starting ovarian stimulation on day 2 versus day 15of the menstrual cycle in the same oocyte donor and pregnancy rates among the corresponding recipients of vitrified oocytes. Fertil. Steril. 2014;102(5): 1307-11. https://dx.doi.org/10.1016/j.fertnstert.2014.07.741.
- Kalra S.K., Ratcliffe S., Gracia C.R., Martino L., Coutifaris C., Barnhart K.T. Randomi zed controlled pilot trial of luteal phase recombinant FSH stimulation in poor responders. Reprod. Biomed. Online. 2008; 17(6): 745-50.https://dx.doi.org/10.1016/s1472-6483(10)60400-2.
- Богатырева Х.А., Мишиева Н.Г., Мартазанова Б.А., Лапина В.С., Абубакиров А.Н. Эффективность протоколов стимуляции функции яичников в различные фазы менструального цикла у пациенток со сниженным овариальным резервом. Акушерство и гинекология. 2017; 11: 78-83. [Bogatyreva Kh.A., Mishieva N.G., Martazanova B.A., Lapina V.S., Abubakirov A.N. Efficiency of ovarian stimulation protocols in different phases of menstrual cycle in patients with diminished ovarian reserve. Obstetrics and Gynecology. 2017; (11): 78-83. (in Russian)]. https://dx.doi.org/10.18565/aig.2017.11.78-83.
Received 15.06.2023
Accepted 07.08.2023
About the Authors
Vera S. Lapina, obstetrician-gynecologist at the 1st Gynecology Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(909)920-23-05, v_lapina@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.Bella A. Martazanova, Ph.D., Senior Researcher at the 1st Gynecology Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(967)123-88-24, dr.bella.ivf@gmail.com, 117997, Russia, Moscow, Ac. Oparina str., 4.
Evelina R. Durinyan, Ph.D., Clinical Care Supervisor at the 1st Gynecology Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(916)612-99-30, evelina_durinyan@mail.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Tatiana S. Amyan, Ph.D., Junior Researcher at the 1st Gynecology Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(926)163-28-33, t_amyan@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Anna I. Korolkova, Ph.D., Researcher at the 1st Gynecology Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(915)322-08-79, korolkovaai@icloud.com, 117997, Russia, Moscow, Ac. Oparina str., 4.
Alla A. Gavisova, Ph.D., Head of the 1st Gynecology Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(916)829-05-90, a_gavisova@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Corresponding author: Vera S. Lapina, v_lapina@oparina4.ru



