Fertility outcomes in women after therapy for non-Hodgkin lymphoma
Aim: To assess menstrual and reproductive function in women, who underwent treatment non-Hodgkin (NHL) lymphoma.Dmitrieva I.E., Nazarenko T.A., Burduli A.G., Polushkina E.S., Khokhlova S.V.
Materials and methods: The study included 39 female patients diagnosed with NHL. All patients still had remission by the end of the study. To collect retroaspective data, a telephone survey of the women under the study was conducted, or the data were collected from their medical records.
Results: Before conception, the patients underwent 3–4 or even more than 11 chemotherapy cycles. In one patient, menses never resumed after 14 cycles of chemotherapy. However, a single observation was not enough for making statistically significant conclusion. Most treatment regimens included highly gonadotoxic substances (Cyclophosphan in 94.87% of the cases, Doxorubicin and platinum-based drugs in 89.74%). R-CHOP was the most common regimen, which was used among the studied cohort.
Regular menstrual cycle resumed after treatment in 82.05% of patients. Of them, 46.88% of women received combined oral contraceptive pills (COCP), and 9.38% received depot GnRH agonist (aGnRH) for ovarian protection. After treatment with COCP, 16.66% of patients had permanent amenorrhea. None of the patients, who were treated with depot aGnRH, had persistent amenorrhea. The median period of menstrual cycle restoration was 2.41 months. In 62.07% of women, who received COCP, menstruation restored immediately or 1 month after treatment. Restoration period was longer (1–2 months) in patients, who received depot aGnRH. In 34/48% of women, the episodes of ovarian dysfunction (oligomenorrhea) was observed.
Spontaneous pregnancy rate was 82.76% among those who had menses. Pregnancy occurred in a period from 24 to 54 months after the completion of chemotherapy treatment. Infertility rate increased from 10.26 to 23.07%. Two patients got pregnant after undergoing ART treatment.
82.76% of women with restored menses spontaneously conceived 24 to 54 months after polychemotherapy. The rate of infertility among the treated women increased from 10.26% to 23.07%. Two patients became pregnant after IVF.
Conclusion: The critical factors for restoration of menstrual function after treatment for non-Hodgkin lymphoma were; the age of patients, high ovarian reserve, chemotherapy duration and regimens. Recovery of menstrual cycle does not always result in restoration of fertility. Medical ovarian protection does not completely protect from gonadotoxic impact of polychemotherapy. Due to this, it is important that the patients with oncologic diseases should undergo examination by a gynecologist not only after being diagnosed with the oncologic disease, but also after treatment. Only cooperation between oncologists and gynecologists may contribute to correct prognosis, reduction of reproductive losses women’s understanding of their reproductive prospective.
Keywords
Non-Hodgkin lymphoma (NHL) is a heterogeneous group of malignant neoplasms that derive from 2 types of lymphocytes (B-cells and T-cells) at various stages of differentiation [1]. Most often the tumor develops and/or is diagnosed in lymphoid tissues (lymph nodes, spleen, bone marrow). The most common extranodal tumors are localized in the skin, gastrointestinal tract, and central nervous system [2].
Hodgkin lymphoma is a B-cell lymphoma with a pronounced reactive polymorphocellular environment (Hodgkin cells, Berezovsky-Sternberg cells, lacunar cells, mummified cells, and LP cells) characterized by a single immunophenotype (CD-30, CD-15, PAX-5). Most of the tumor cells have Epstein-Barr virus (EBV). All other types of lymphoproliferative disorders (up to 90%) are grouped into the general group of non-Hodgkin lymphomas (NHL). In addition to EBV, other infectious agents, as well as trauma, genetic and autoimmune disorders are considered to be possible etiological factors for this disease; immunosuppression is the most proven cause of this pathological process.
The group of hemoblastosis is the most common among cancers that develop in patients under the age of 30. According to data from the P. Hertsen Moscow Oncology Research Institute in 2019, among patients aged 20 to 35 years, NHL is diagnosed in 11.64% of cases and lymphogranulematosis in 26.92% of cases [3]. The rate of NHL is increasing: the incidence over the past 10 years increased by 13.32%.
The pattern of the disease depends on the forms of NHL, ranging from the indolent course (slow-growing and not aggressive) in case of follicular lymphoma to more aggressive forms of diffuse large B-cell lymphoma and Burkitt lymphoma. NHL, unlike Hodgkin lymphoma, is mostly asymptomatic and therefore diagnosed late, when the disease is already severe and requires more aggressive treatment.
Chemotherapy is the main treatment for NHL. Aggressive lymphomas usually require immediate treatment. The mean age of death for women with lymphoproliferative diseases in Russia in 2019 was 67.6 years, and statistics indicate that treatment became more effective (primarily due to improvements in treatment protocols, and the introduction of targeted therapy). According to the American Cancer Society, the five-year survival rate for NHL ranges from 65 to 84%, depending on the severity of the malignant process [4]. Effective treatment in female patients increases survival rate; this induces a question of the impact of high-dose chemotherapy on the ovarian reserve in young patients and a possibility of reproductive function preservation after treatment.
Joint efforts of oncologists and obstetricians-gynecologists to treat oncological diseases, particularly NHL show the necessity to inform young patients about the risks of decreased ovarian reserve prior to treatment. Medical treatment to minimize the gonadotoxic impact of polychemotherapy (PCT) (combined oral contraceptives (COCs), gonadotropin-releasing hormone agonists (aGnRH)), as well as preliminary ovarian stimulation to vitrify obtained oocytes should be available prior to the start of cancer treatment [5]. This is the only way to ensure full planning of pregnancy after remission. Recently, an increase in the number of consultations of such patients by obstetricians-gynecologists has been noted [6]. Counselling on reproductive issues increases the awareness of women and allows them to make an informed decision about fertility preservation [7].
The most important and unsolved issue today is the prediction of reproductive function of patients with NHL after chemotherapy. The solution of this issue will enable to create the personalized programs for each patient and ensure their reproduction.
Therefore, the aim of the present study was to analyze the state of menstrual cycle and reproductive function in women after NHL treatment.
Materials and methods
The study included 39 women diagnosed with NHL. The study was approved by the Ethics Committee of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of the Ministry of Health of the Russian Federation.
The age of women at the time of NHL diagnosis was 24 (20; 27) years. Five patients were younger than 18 years, two of them had not reached menarche. All patients were in remission at the time of the study. The retrospective data from most of patents was collected by phone and from their medical records (stage and form of NHL, assessment of menstrual status before and after PCT, use of COCs or aGnRH during PCT, PCT regimen, obstetric and gynecological history, somatic disease history).
Table 1 presents an analysis of somatic disorders before the diagnosis of NHL.
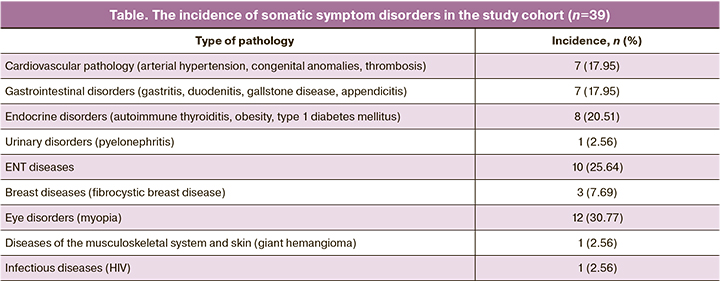
The spectrum and incidence of somatic disorders were similar to those in general population.
The incidence of hormone-specific diseases, such as polycystic ovary syndrome (PCOS) (15.4%), uterine fibroids (30.8%), uterine polyps (12.8%), deserves special attention. The incidence of other gynecological diseases, such as adenomyosis (5.1%), chronic salpingo-ophoritis (5.1%), ovarian cysts (5.1%), chronic endometritis (7.7%), sexually transmitted infections (15.4%), was also similar to the general population. The studied patients have previously underwent the following surgeries: coagulation of cervical ectopy (41%) and oophorectomy (7.7%).
The majority of the studied women were young and had a good ovarian reserve. Before treatment, the average length of their menstrual cycle was 28 days, and the duration of menstrual bleeding was 5 days. In addition to the mentioned patients with diagnosed PCOS, menstrual cycle disorders such as oligomenorrhea and multifollicular ovaries were noted in 7 women (17.95%) before PCT treatment.
Before the onset of the disease, 74.36% of the patients were nulligravidae. 17.95% of patients became pregnant before treatment, while each of them had given birth at least once; out of 13 pregnant women, only 2 had abortions, and 2 more women had missed miscarriage. 10.26% of those who applied for treatment already had a diagnosis of infertility.
Results
A retrospective assessment of NHL severity at the time of diagnosis revealed that stage I of the disease was diagnosed in 2 patients (8.7%), stage II – in 9 patients (39.1%), stage III – in 4 patients (17.4%), stage IV – in 8 patients (34.8%). All women underwent PCT from 3 to 14 cycles; one women was diagnosed with NHL in pregnancy, and therefore PCT was started after delivery. PCT together with radiotherapy was used in 16 of 39 women (41,03%), with surgical treatment (for histologic verification of the diagnosis) – in 9 of 39 women (23,08%). Radiation therapy for the area bordering the pelvic organs (right inguinal lymph nodes) was performed in 1 patient diagnosed with follicular lymphoma.
We tried to analyze the dependence between the number of PCT cycles and the state of reproductive function of the woman after the end of treatment. No clear pattern was found, although the small number of cases did not allow us to make any definite conclusions. Women who became pregnant as the result of treatment underwent from 3-4 to more than 11 cycles (Figure 1). In one woman who underwent 14 cycles of PCT, menstrual function did not resume; however, a single case is insufficient to make a reliable conclusion.
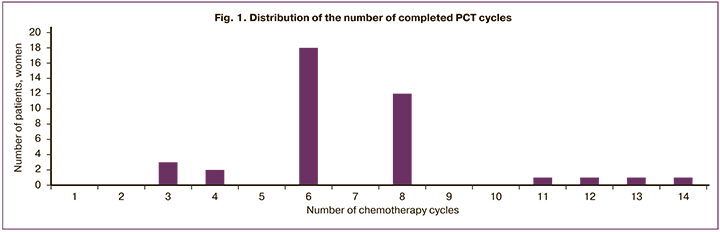
The number of PCT cycles (retrospective data from 27 women) ranged from 3 to 14. The median number of cycles was 6 (in 46.15% of patients); 5 women (30.77%) underwent 8 cycles. More than 11 cycles of PCT were applied in 4 patients (10.26%); 3–4 cycles of PCT – in 5 patients (12.82%). Recurrence after first-line PCT treatment was observed in 2 women with primary mediastinal large B-cell lymphoma and stage II small B-cell lymphoma. Hematopoietic stem cell transplantation was performed in only one patient with NHL.
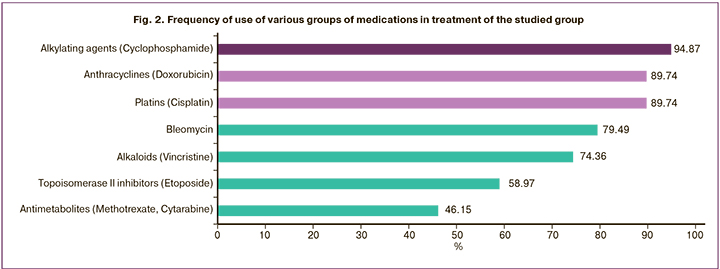
Chemotherapeutic drugs were administered according to RCHOP, R-EPOCH, R-MACOP-B, mNHL-BFM-90, and DHAP regimens. One of the most common PCT regimen for T-cell and B-cell lymphomas was R-CHOP, which consist of such components as cyclophosphamide, doxorubicin, vincristine, and prednisolone with the rituximab (a monoclonal antibody used to express the CD20+ antigen). Treatment regimen for patients with lymphoma in the majority of cases require the use of several medications [8] with very high gonadotoxicity [9]: cyclophosphamide, chlorambucil, procarbazine, ifosfamide, platins, melphalan, dacarbazine; non-alkylating medications (anthracyclines, bleomycin, etoposide, vincristine/vinblastine, gemcitabine) are not considered to have high gonadotoxicity [10].
Regarding the variety of PCT regimens, a retrospective analysis highlighted the most commonly used medication (Table 2, Fig. 2).
In the present study, among women who became pregnant after treatment, ovarian protection during gonadotoxic chemotherapy was used in 21 patients, while 18 patients were not prescribed any medication. 3 women (14.29%) out of 21 were treated with aGnRH, and 18 (85.71%) – with COCs. Menstrual cycle resumed in 32 (82.05%) patients after treatment. Among them, 15 patients (46.88%) were treated with COCs and 3 patients (9.38%) – with aGnRH. Three women who received COCs (16.66%) became permanently amenorrheic after the treatment; in the subgroup of women treated with aGnRH, amenorrhea was not noted in any patient.
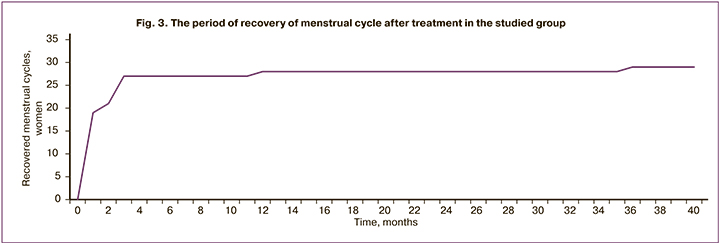
The mean period of recovery of menstrual function after treatment was 2.41 months. In the majority of women (62.07%) treated with COCs during PCT cycles, the menses resumed immediately or within 1 month. Patients treated with aGnRH had a longer period of menses recovery (1–2 month) due to mechanism of action of this medication. The recovery period in 6 women (20.69%) took 3 months, in 2 women (6.9%) – 2 months, in 1 woman (3.45%) – 12 months, in 1 woman (3.45%) – 36 months (Figure 3). Some women subsequently experienced episodic symptoms of ovarian dysfunction such as oligomenorrhea.
After treatment, the length of the menstrual cycle was 29 (28;30) days. The menstrual bleeding (data taken from 14 women) ranged from 3 to 7 days, the majority of patients (6 women, 42.9%) had 5 days of bleedings, which did not differ significantly from the baseline data.
At the time of collection of retrospective data, the mean age of women was 36 years (median 37 years), 7 women experienced an early onset of menopause.
Among women in whom menstrual function successfully restored, 82.76% became pregnant. Pregnancy occurred spontaneously between 24 and 54 months after the end of PCT treatment. The rate of sterility among the treated patients increased from 10.26 to 23.07%, including 1 case of tubal-peritoneal infertility (after salpingitisresulting from PCT). 2 patients became pregnant after the use of assisted reproductive technologies.
Discussion
The most difficult issue was to determine the degree of impact of PCT on the reproductive function of women. The difficulty in finding this relationship was due to a small number of cases and to the fact that women were treated with various PCT regimens and medications with different mechanisms of action and, accordingly, with different impact on ovarian function.
Rituximab (monoclonal antibodies to the CD20 transmembrane antigen) was prescribed and administered to 42.9% patents (in 21 women treated with PCT). Currently, data on the gonadotoxicity of monoclonal antibodies medications are limited; even the safety of rituximab intake during pregnancy remains an open question [11].
As for the stage of the disease, no correlation between the recovery of reproductive function and the severity of the disease was found. Half of the patients had stages I and II of the disease (48.72%), the other half had stages III and IV (51.28%).
The issue about the effectiveness of ovarian protection during PCT is still unsolved. Hematologists-oncologists widely use COCs and aGnRH in clinical practice, guided by the well-known studies indicating that desensitization of hypothalamus-pituitary-adrenal axis and suppression of the function of ovaries can prevent damage of ovarian reserve and preserve ovarian function. However, the unified opinion on this issue still does not exist [12].
The common characteristics of women with successful recovery of menstrual cycle were the young age and good ovarian reserve. Moreover, the medical history of patients showed that before the disease was diagnosed young women were almost healthy, except for the high incidence of hormone-dependent gynecological disorders (PCOS, uterine fibroids, uterine polyps). It may be worth to note that the women with restored menstrual cycle were young at the time of treatment (up to 27 years old) and in the majority of cases had multifollicular or polycystic ovaries. There were no statistically significant differences between the presence of ovarian protection by COCs and its absence (p=0.20).
No cases of amenorrhea or infertility were noted among the patients taking aGnRH; however, the number of young women treated with aGnRH is too small to draw a reliable conclusion about its effectiveness. Today, the use of aGnRH is increasing, and further research in this area is needed.
Considering that still quite a small number of oncological patients refer to gynecologists, there are currently no prognostic models for preservation of ovarian reserve. However, the vast majority of menstrual disorders were associated with certain features that increase the probability of depletion of ovarian reserve. These clinical observations can be described as typical cases of high-risk loss of reproductive potential.
Among women in whom their menstrual cycle failed to restore, one patient had a poor ovarian reserve and symptoms of primary ovarian insufficiency before treatment, and became menopausal after treatment. In addition to poor ovarian reserve, risk factors include recurrence of the disease, requiring repeated cycles of second-line chemotherapy, blood stem cell transplantation, and the use of other types of gonadotoxic therapy (such as antiretroviral therapy in patient with HIV).
In our study, the recovery of menstrual function occurred in the first 3 months after the end of therapy, but very rarely it could take more than a year (this was observed in 2 patients after hormone replacement therapy). According to the data, the more time had passed since the end of treatment, the lower are the chances of recovery of menstrual function.
Dependence of recovery of menstrual function from age and ovarian reserve is confirmed by Russian studies [5, 13, 14] and studies from other countries [15, 16]. The older patients with malignant diseases are at the time of diagnosis of the disease and treatment initiation, the lower are their chances of becoming parents [17, 18]. Moreover, women have lower chances than men, which, taking into account the difficulty of cryopreservation of genetic material of women, emphasizes the need to develop unified algorithms for the management of oncological patients by obstetricians-gynecologists.
Conclusion
The crucial factors for restoration of menstrual function in patients after NHL therapy are the age, the presence of multifollicular ovaries with high ovarian reserve, and the duration and doses of chemotherapy. An important point is that the restoration of menstrual function does not always mean recovery of reproductive function. Some patients had to undergo ART to get pregnant, and some had to use donor oocytes due to diminished ovarian reserve. Our study showed that protection of the ovaries with medicinal treatment does not completely eliminate the gonadotoxic effects of PCT. Thus, it is important to refer oncological patients to an obstetrician-gynecologist not only after diagnosis of the disease, but also after treatment. Only joint efforts of oncologists and obstetricians-gynecologists in patients management can ensure correct prognosis, reduce the loss of reproductive function, and fully inform the patient about reproductive prospects.
References
- Campo E., Swerdlow S.H., Harris N.L., Pileri S., Stein H., Jaffe E.S. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011; 117(19): 5019-32. https://dx.doi.org/10.1182/blood-2011-01-293050.
- Howlader N., Noone A.M., Krapcho M., Garshell J., Miller D., Altekruse S.F. et al. (eds.). SEER Cancer Statistics Review, 1975-2011, National Cancer Institute. Bethesda, MD, based on November 2013 SEER data submission, posted to the SEER web site, April 2014. Available at: http://seer.cancer.gov/csr/1975_2011
- Каприн А.Д., Старинский В.В., Шахзадова А.О., ред. Состояние онкологической помощи населению России в 2019 году. М.: МНИОИ им. П.А. Герцена – филиал ФГБУ "НМИЦ радиологии" Минздрава России; 2020. 239с. [Kaprin A.D., Starinskiy V.V., Shakhzadova A.O., ed. The state of cancer care for the population of Russia in 2019. M.; 2020. 239 p. (in Russian)].
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020; 70(1): 7-30. https://dx.doi.org/10.3322/caac.21590.
- Сухих Г.Т., Давыдов М.И., Савченко В.Г., ред. Репродуктивное здоровье женщин с онкогематологическими заболеваниями. М.; 2012. 310с. [Sukhikh G.T., Davydov M.I.,Savchenko V.G., ed. Reproductive health in women diagnosed with hematologic malignancies. M.; 2012. 310 p. (in Russian)].
- Coleman C.E.M., Pudwell J., McClintock C., Korkidakis A., Green M., Velez M.P. Modest increase in fertility consultations in female adolescents and young adults with lymphoma: a population-based study. J. Adolesc. Young Adult Oncol. 2021; 10(3): 342-5. https://dx.doi.org/10.1089/jayao.2020.0101.
- Kelvin J.F., Thom B., Benedict C., Carter J., Corcoran S., Dickler M.N. et al. Cancer and fertility program improves patient satisfaction with information received. J. Clin. Oncol. 2016; 34(15): 1780-6. https://dx.doi.org/10.1200/JCO.2015.64.5168.
- Румянцев А.Г., Масчан А.А., Самочатова Е.В. Федеральные клинические рекомендации по диагностике и лечению неходжкинских лимфом. М.; 2015. 47с. [Rumyantsev A.G., Maschan A.A., Samochatova E.V. Federal clinical recommendations for the diagnosis and treatment of non-Hodgkin's lymphomas. M.: 2015. 47 p. (in Russian)].
- Meirow D., Biederman H., Anderson R.A., Wallace W.H. Toxicity of chemotherapy and radiation on female reproduction. Clin. Obstet. Gynecol. 2010; 53(4):727-39. https://dx.doi.org/10.1097/GRF.0b013e3181f96b54.
- Brydøy M., Fosså S.D., Dahl O., Bjøro T. Gonadal dysfunction and fertility problems in cancer survivors. Acta Oncol. 2007: 46(4): 480-9. https://dx.doi.org/10.1080/02841860601166958.
- Pavanello F., Zucca E., Ghielmini M. Rituximab: 13 open questions after 20 years of clinical use. Cancer Treat. Rev. 2017; 53: 38-46. https://dx.doi.org/10.1016/j.ctrv.2016.11.015.
- Blumenfeld Z., Avivi I., Linn S., Epelbaum R., Ben-Shahar M., Haim N. Prevention of irreversible chemotherapy-induced ovarian damage in young women with lymphoma by a gonadotrophin-releasing hormone agonist in parallel to chemotherapy. Hum. Reprod. 1996; 11(8): 1620-6. https://dx.doi.org/10.1093/oxfordjournals.humrep.a019457.
- Пылова И.В., Шмаков Р.Г., Демина Е.А., Любимова Н.В., Сметник В.П., Самойлова Т.Е., Махова Е.Е. Эффективность защиты яичников при проведении химиотерапии у женщин с лимфомой Ходжкина. Акушерство и гинекология. 2011; (7-1): 40-5. [Pylova I.V., Shmakov R.G., Demina E.A., Lyubimova N.V., Smetnik V.P., Samoilova T.E., Makhova E.E. Efficiency of ovarian protection during chemotherapy in women with Hodgkin’s lymphoma. Obstetrics and Gynecology. 2011; 7-1: 40-5. (in Russian)].
- Пылова И.В., Демина Е.А., Шмаков Р.Г., Перилова Е.Е. Репродуктивная функция пациенток с лимфомой Ходжкина и возможности ее сохранения. Онкогематология. 2006; (1-2): 113-20. [Pylova I.V., Demina E.A., Shmakov R.G., Perilova E.E. Reproductive function in females with Hodgkin's lymfoma and possibilities of its protection. Oncogematology. 2006; 1-2: 113-20. (in Russian)].
- Elis A., Tevet A., Yerushalmi R., Blickstein D., Bairy O., Dann E.J. et al. Fertility status among women treated for aggressive non-Hodgkin’s lymphoma. Leuk. Lymphoma. 2006; 47(4): 623-7. https://dx.doi.org/10.1080/10428190500353877.
- Akhtar S., Youssef I., Soudy H., Elhassan T.A.M., Rauf S.M., Maghfoor I. Prevalence of menstrual cycles and outcome of 50 pregnancies after high-dose chemotherapy and auto-SCT in non-Hodgkin and Hodgkin lymphoma patients younger than 40 years. Bone Marrow Transplant. 2015; 50(12): 1551-6. https://dx.doi.org/10.1038/bmt.2015.178.
- Fossa S.D., Magelssen H., Melve K., Jacobsen A.B., Langmark F., Skjaerven R. Parenthood in survivors after adulthood cancer and perinatal health in their offspring: a preliminary report. J. Natl. Cancer Inst. Monogr. 2005; (34): 77-82. https://dx.doi.org/10.1093/jncimonographs/lgi019.
- Viviani S., Caccavari V., Gerardi C., Ramadan S., Allocati E., Minoia C. et al. Male and female fertility: prevention and monitoring Hodgkin’ lymphoma and diffuse large B-сell lymphoma adult survivors. A systematic review by the Fondazione Italiana Linfomi. Cancers (Basel). 2021; 13(12): 2881. https://dx.doi.org/10.3390/cancers13122881.
Received 08.10.2021
Accepted 13.10.2021
About the Authors
Irina Ye. Dmitrieva, PhD candidate, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology,Ministry of Health of the Russian Federation, +7(916)089-08-09, nika06@inbox.ru, https://orcid.org/0000-0001-5119-3816, 117997, Russia, Moscow, Ac. Oparin str., 4.
Tatiana A. Nazarenko, Dr. Med. Sci., Professor, Head of the Institute of Reproductive medicine, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, +7(985)998-74-12, t_nazarenko@oparina4.ru, https://orcid.org/0000-0002-5823-1667,
117997, Russia, Moscow, Ac. Oparin str., 4.
Anna G. Burduli, PhD/Med, Senior Researcher of the Professor Leonov Department of Assistive Reproductive Technologies, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation,
+7(985)120-36-14, burdulianna@gmail.com, https://orcid.org/0000-0002-2849-5426, 117997, Russia, Moscow, Ac. Oparin str., 4.
Evgeniya S. Polushkina, PhD/Med, Senior Researcher of the 2nd Maternity Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, +7(903)154-74-13, e_polushkina@oparina4.ru,
https://orcid.org/0000-0002-1945-0154, 117997, Russia, Moscow, Ac. Oparin str., 4.
Svetlana V. Khokhlova, PhD/Med, Head of the Oncology Department of Anticancer Drug Therapy, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, +7(903)170-98-02, s_hohlova@oparina4.ru,
https://orcid.org/0000-0002-4121-7228, 117997, Russia, Moscow, Ac. Oparin str., 4.
Authors’ contributions: Dmitrieva I.E., Nazarenko T.A., Burduli A.G. – the concept and design of the study, material collection and processing, writing and editing the article; Polushkina E.S. – the concept and design of the study, material collection and processing; Khokholova S.V. – the concept and design of the study, editing the article.
Conflicts of interest: The authors have no conflicts of interest.
Funding: The study was carried out without any sponsorship.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Dmitrieva I.E., Nazarenko T.A., Burduli A.G., Polushkina E.S., Khokhlova S.V. Restoration of fertility in women after treatment for non-Hodgkin lymphoma.
Akusherstvo i Ginekologiya/ Obstetrics and Gynecology. 2021; 11: 187-193 (in Russian)
https://dx.doi.org/10.18565/aig.2021.11.187-193



