Comparative analysis of clinical and anamnestic data from couples undergoing frozen-thawed embryo transfer in the natural and hormone replacement therapy cycle
Objective: To analyze clinical and anamnestic data of patients undergoing ART and subsequent frozen-thawed embryo transfer in the natural and hormone replacement therapy (HRT) cycle. Materials and methods: The study included 102 infertile couples with women aged from 23 to 43 years. Of these, 42 and 60 underwent embryo transfer in a natural and HRT cycle, respectively. The baseline evaluation consisted of a complete clinical and laboratory examination, assessment of the hormonal profile, and ultrasound examination. The study analyzed the results of in-vitro fertilization (IVF) and frozen-thawed embryo transfer. Results: Patients in the HRT cycle group who achieved and did not achieve pregnancy had statistically significant differences in progesterone level (p<0.001), testosterone level (p=0.02) and endometrial thickness on date of embryo transfer (p<0.001). Positive correlations were also found between endometrial thickness on the day of embryo transfer and baseline progesterone levels (p=0.04, r=0.28). In the natural cycle group, there were statistically significant differences between patients who achieved and did not achieve pregnancy in progesterone levels (p<0.001). Conclusion: The natural cycle and HRT cycle groups did not differ statistically significantly in pregnancy rates in frozen-thawed embryo transfer (p=0.70). High baseline progesterone levels before IVF were associated with pregnancy onset in both natural cycle and HRT groups, which suggests its possible association with the effectiveness of ART programs and requires further study. Therefore, an individualized approach to luteal phase support during cryopreserved embryo transfer in both natural cycle and HRT cycle according to serum progesterone levels may play an important role in increasing the effectiveness of ART programs.Gokhberg Ya.A., Fedorov I.S., Timofeeva A.V., Kalinina E.A.
Keywords
Female and male infertility remains an important and unresolved problem in the modern world. In regions of Russia, the prevalence of infertility ranges from 17.2 to 24.0% [1]. The impairment of embryo implantation is one of the possible causes for the decrease in the effectiveness of assisted reproductive technology (ART). Successful pregnancy requires the simultaneous presence of two factors: a morphologically and genetically normal embryo and an endometrium with high implantation potential [2]. Statistically, 1/3 of the failed protocols correlate directly with embryo quality, while 2/3 are due to the condition of the endometrium [3]. The main regulators of morphological and functional changes in the endometrium during the menstrual cycle are estrogens and progesterone. Sex steroids exert their effect by binding specific receptors to the surface of the functional layer, which contributes to the change in endometrial properties [4].
One of the main causes of impaired implantation is the shift in the window of implantation in time, when the endometrium is most receptive and ready for embryo implantation [5]. Endometrial receptivity and selectivity are two complementary concepts used to describe the endometrium. Selectivity is the internal programmed function of recognizing and rejecting embryos with reduced developmental potential [6]. On the contrary, endometrial receptivity is a set of structural and functional characteristics of the uterine mucosa that provide optimal conditions for blastocyst implantation. In most women, the window of implantation covers the 7th–10th day after the luteinizing hormone peak in the secretory phase of the menstrual cycle [7]. Currently, research evidence indicates that the best endometrial receptivity can be achieved with frozen-thawed embryo transfer, compared to a stimulated cycle [8]. Cycle segmentation allows the prevention of ovarian hyperstimulation syndrome and the transfer of embryos to a better intrauterine environment, without the possible adverse effects of hormonal stimulation on endometrial receptivity. There are various protocols for preparing the endometrium for embryo transfer, including cryopreservation in the natural cycle and the hormone replacement therapy (HRT) cycle. The choice of protocol is based on the results of the clinical and anamnestic characteristics of the patients, the hormonal profile, and ultrasound examination. To date, many studies have discussed the lack of differences in efficacy between endometrial preparation protocols [9, 10].
This study aimed to compare clinical and anamnestic data from patients undergoing frozen-thawed embryo transfer in the natural and hormone replacement therapy (HRT) cycle and to evaluate the efficacy of the program.
Materials and methods
The study analyzed clinical data on 102 couples referred to the B.V. Leonov Department of IVF, V.I. Kulakov NMRC of OG&P, Ministry of Health of Russia. All patients underwent a full course of treatment. All patients underwent a complete clinical and laboratory examination in accordance with Order No. 803n of the Russian Ministry of Health of 31.07.2020 «On the procedure for using assisted reproductive technologies, contraindications and limitations to their use». The baseline evaluation consisted of the hormonal status prior to entering the IVF program. It included follicle stimulating hormone (FSH), anti-Müllerian hormone (AMH), luteinizing hormone (LH), progesterone in the luteal phase of the menstrual cycle, estradiol, testosterone (T), thyroid-stimulating hormone (TSH), thyroxine (T4), dehydroepiandrosterone sulfate (DHEA-S), 17-hydroxyprogesterone (17-OH), cortisol, prolactin (PRL)), and pelvic ultrasonography (US) for antral follicle count (AFC) on day 2 to 3 of the menstrual cycle. Patients included in the study provided informed consent for the IVF program.
Ovarian stimulation protocol
Ovarian stimulation protocols for embryo vitrification for subsequent transfer were retrospectively analyzed.
On days 2–3 of the menstrual cycle, ovarian stimulation was started according to the standard protocol with human gonadotropin-releasing hormone antagonists and menopausal gonadotropin. As soon as 1 follicle reached 14–15 mm in size, ovulation was triggered by a human chronic gonadotropin injection at a dose of 0.25 mg/day to prevent a surge in LH. For final oocyte maturation, ovulation was triggered with 10,000 IU of human chronic gonadotropin once the leading follicle reaches a mean diameter of 18–19 mm. Thirty-six hours after ovulation initiation, transvaginal follicle puncture (TVP) with follicular fluid sampling was performed and the quality of the oocyte was evaluated. The semen analysis parameters were evaluated during the preliminary examination before entering the IVF program and on the day of the TVP. Sperm selection for intracytoplasmic sperm injection (ICSI) was performed according to morphological parameters (standard ICSI). After fertilization with ICSI, the oocytes were transferred to culture medium for further culture. 14–16 h after fertilization, pronuclear formation was evaluated. After fertilization, embryo quality was evaluated on days 3 and 5 according to the Istanbul Consensus. Embryos with the best morphological characteristics were transferred to the uterus under ultrasound guidance. Luteal phase and posttransfer support were performed with progesterone supplementation. The remaining embryos after the IVF/ECO+ICSI program, suitable for transfer into the uterine cavity, were cryopreserved.
After both positive and negative stimulation results, these couples applied for cryopreserved embryo transfer.
Patients were divided into two groups, depending on the method of endometrial preparation. Group I patients (n=42) underwent frozen-thawed embryo transfer in the natural cycle and Group II patients (n=60) in HRT cycle. The algorithm for selecting an endometrial preparation protocol for frozen-thawed embryo transfer in natural cycle included regular menstrual cycle, ovulatory cycle, and endometrial thickness ≥ 8 mm in the 1st or 2nd phase of the menstrual cycle; in HRT cycle the algorithm included irregular menstrual cycle, persistent anovulation, and endometrial thickness ≤ 8 mm in the 1st or 2nd phase of the cycle.
Endometrial preparation protocol for frozen-thawed embryo transfer in the natural cycle
Ultrasound monitoring of dominant follicle growth and ovulation was performed in the natural cycle group of patients, after which micronized progesterone in a dose of 200 mg/day was administered to support the luteal phase of the menstrual cycle and the posttransfer period. The day of transfer corresponded to the day of spontaneous ovulation + the number of days of embryo cultivation.
Endometrial preparation protocol for frozen-thawed embryo transfer in HRT cycle
In HRT cycles, estradiol valerate was administered from days 2–4 of the menstrual cycle with an initial dose of 2 mg with a gradual increase according to the individual patient's parameters, When a normal endometrial thickness and structure (>7 mm) was reached on day 14–15 of the menstrual cycle, gestagens (micronized progesterone 200 mg/day or dydrogesterone 10 mg/day) were injected, also with a gradual increase according to the individual parameters.
Under favorable conditions, the embryos were transferred using disposable flexible catheters after obtaining the informed consent. The pregnancy was diagnosed by a positive human β-chorionic gonadotropin test 14 days after transfer. Clinical pregnancy was confirmed on day 21 after embryo transfer by ultrasound visualization of a gestational sac.
Statistical analysis
Statistical analysis was performed using Microsoft Excel spreadsheets and RStudio software. It was found that numerical variables were not normally distributed and they were described as median (Me) and quartiles of Q1 and Q3 in Me (Q1; Q3) format. Continuous variables were compared with a nonparametric Mann-Whitney test. Pearson's χ2 test was used to compare categorical variables. Correlation analysis was conducted by calculating Spearman's rank correlation coefficients. Differences were considered statistically significant at p<0.05.
Results
At the first stage, the study retrospectively analyzed of the data of patients who underwent the frozen-thawed embryo transfer in the natural cycle and HRT cycle (Table 1). No statistically significant differences were found in the age characteristics of the couples. The mean age of the women in the natural cycle group was 33 (23–42) years and that of the HRT cycle group was 34 (26–42) years. The mean age of men in the natural cycle group was 35 (26–54) years and in the HRT cycle group 37 (25–55) years. Comparative analysis of the two groups revealed no statistically significant differences in the number of oocyte-cumulus complexes obtained on day of the HRT cycle, the number of mature oocytes (MII) and the number of fertilized oocytes (2PN2PB zygotes). Analysis of the ejaculate on the day of TVP showed that the groups did not differ statistically significantly in sperm analysis parameters.
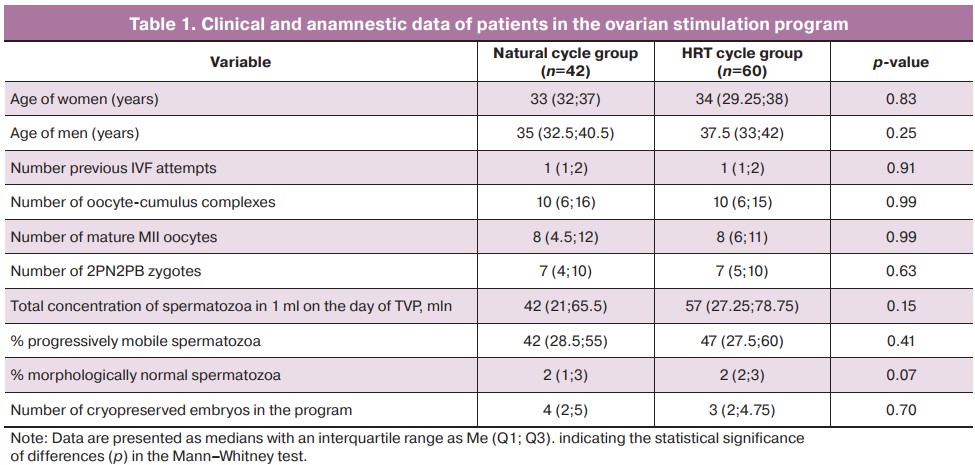
The parameters of the hormonal status before entering the IVF program corresponded to the reference values (Table 2). It should be noted that the patients in the natural cycle group had a mean progesterone level of 38.9 nmol/L, while in the HRT cycle group it was 33.5 nmol/L (p=0.02). The decreased level of progesterone secretion in the HRT cycle group may be related to the insufficiency of the luteal phase, which was maintained with hormones, compared to the natural cycle, where patients had independent ovulation and their own corpus luteum.
We analyzed the conception rate in patients in the ovarian stimulation protocol. In the natural cycle group (n=42), 11/42 patients became pregnant (conception rate =26.19%); in the HRT cycle group (n=60), 17/60 patients became pregnant (conception rate =28.33%).
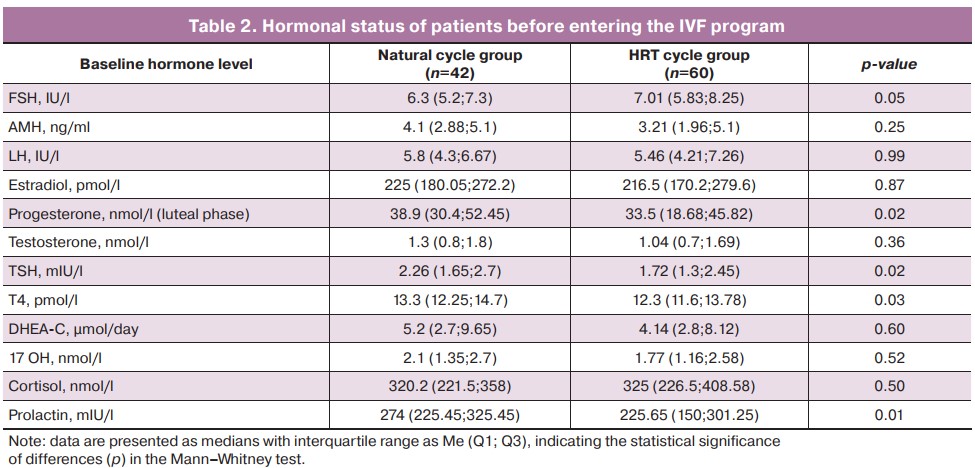
A subsequent analysis of the patient who underwent a cryopreserved embryo transfer program (Table 3) revealed statistically significant differences between the natural cycle and HRT cycle groups in endometrial thickness on the day of embryo transfer (p<0.001). There were no statistically significant differences between the natural cycle and HRT cycle groups in such parameters as the day of menstrual cycle on the day of embryo transfer, their number, as well as the total number of embryos obtained during ovulation stimulation.
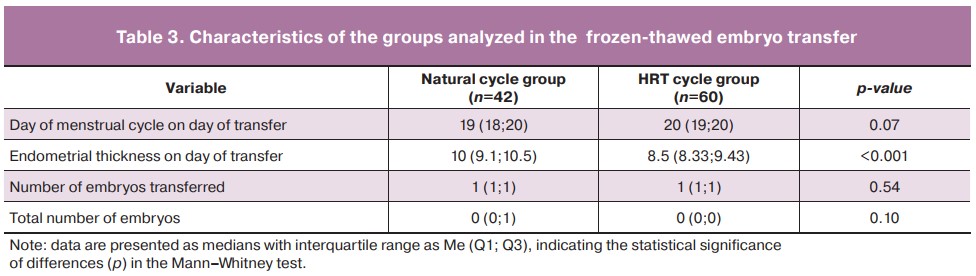
We analyzed conception rates in patients in frozen-thawed embryo transfer. In the natural cycle group (n=42), pregnancy occurred in 19/42 patients (conception rate=40.48%); in the HRT cycle group (n=60), pregnancy occurred in 24/60 patients (conception rate=41.67%).
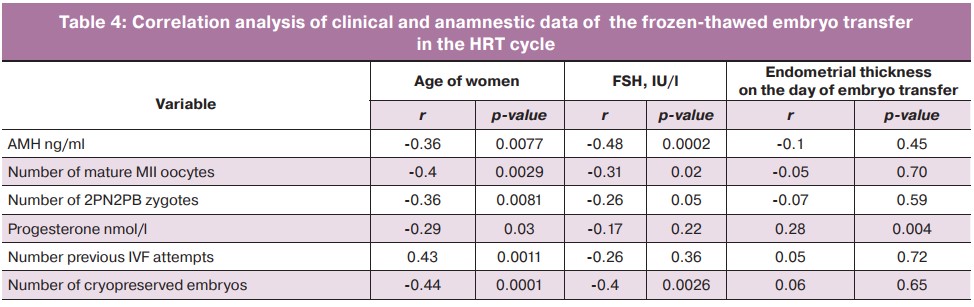
In the HRT cycle, we performed a correlation analysis of quantitative variables, taking into account the statistical significance of each of them (Table 4). The thickness on the day of embryo transfer was directly correlated with the progesterone level (p=0.004). There was a statistically significant negative correlation between the age and the number of mature MII oocytes, the number of zygotes of 2PN2PB, the level of AMH and progesterone, as well as a positive correlation between the patients' age of the patients and the number of previous IVF attempts. There also was a statistically significant negative correlation between FSH levels and the number of mature MII oocytes, the number of 2PN2PB, the number of cryopreserved embryos obtained, and AMH level.
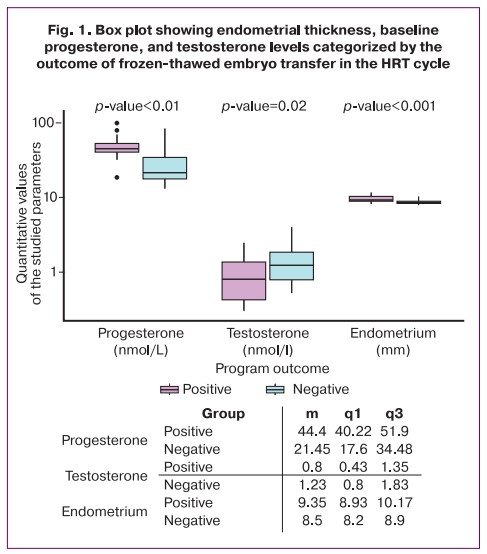
A comparison of patients with the onset of pregnancy and women who did not achieve pregnancy found statistically significant differences in progesterone levels (p<0.001), testosterone levels (p=0.02), and endometrial thickness on the embryo transfer date (p<0.001). The median values and the first and third quartiles of the parameters numerical data of the studied are shown in the insert in Figure 1. Patients with a positive result had higher values of baseline progesterone level and endometrial thickness, but lower testosterone level compared to patients with a negative cryopreservation program, as evidenced by the data presented in Figure 1.
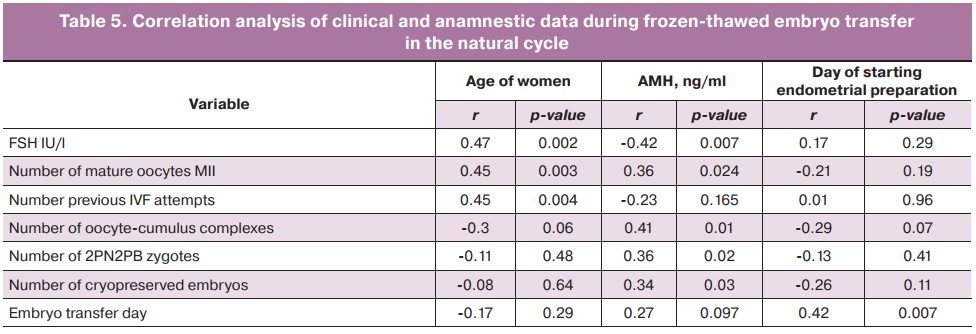
During cryopreserved embryo transfer in a natural cycle, correlation coefficients were obtained for quantitative clinical and the anamnestic data and statistical significance of each of them was evaluated (Table 5). The age of women who underwent frozen-thawed embryo transfer had a statistically significant positive correlation with FSH levels, the number of IVF attempts in history, and the number of mature MII oocytes. AMH level had a statistically significant direct correlation with the number of oocyte-cumulus complexes, the number of 2PN2PB zygotes, and the number of cryopreserved embryos. There was also a direct correlation between the day of endometrial preparation and the choice of the optimal day for frozen-thawed embryo transfer (p=0.007).
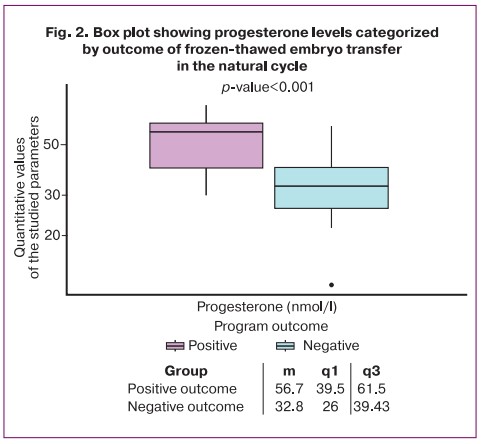
In the natural cycle group, there were statistically significant differences in progesterone levels between patients who became pregnant and those who failed the frozen-thawed embryo transfer (p<0.001). The median values, as well as the first and third quartiles of the numerical values of the parameters under investigation are shown in Figure 2. The patients with positive outcomes had higher baseline progesterone levels compared to those with failed frozen-thawed embryo transfer, as evidenced by the data in Figure 2.
Discussion
A prerequisite for pregnancy involves normal endometrial development in the follicular and luteal phases of the menstrual cycle under the action of estrogen and progesterone, as well as synchronization between a good quality blastocyst and normal thickness endometrial epithelium.
In our study, patients who achieved and did not achieve pregnancy in the natural cycle and HRT cycle groups statistically had significant differences in progesterone levels (p<0.001). Volovsky M. et al. reported a large retrospective study [11] that analyzed 2010 cycles with frozen-thawed embryo transfer. There were no statistically significant differences in pregnancy rates (20.82 vs 40.98%, p=0.30) and overall fertility (14.25 and 16.21%, p=0.23) in patients with progesterone threshold levels <10 ng/ml and >10 ng/ml, and at 20 ng/ml progesterone levels. On the contrary, Labarta E. et al. [12] showed that progesterone levels <9.2 ng/ml on the day of embryo transfer significantly reduced pregnancy rates compared to patients with progesterone levels 9.2–11.7 ng/ml, 11.8–15.7 ng/ml 32.7% vs 52.8%; p=0.016; RR (95% CI): 0,62 (0,41–0,94). The study of Tihomirova T. et al. [13] also showed that low progesterone levels were associated with no pregnancy compared to women who had threshold progesterone levels above 20.08 ng/mL ± 9.44 and 27.9 ng/mL ± 10.52, respectively (p<0.034). It should be noted that low progesterone levels may be associated with lower pregnancy rates and live births in general. Therefore, progesterone levels measured on the day of embryo transfer can be used as a predictor of success in HRT programs.
In our study, patients who achieved and did not achieve pregnancy in the HRT cycle had statistically significant differences in testosterone levels (p=0.02). Mokhtar M. et al. [14] showed the effect of testosterone level on pregnancy. The authors demonstrated that when exogenous testosterone was administered at a dose of 500 µg/kg/day for 3 days, there was a sufficient decrease in the expression of MECA-79 and pinopodes, which are present during the implantation window and are associated with the highest concentration of protein receptivity markers. Besides, Huang L. et al. [15] in their study proved that baseline testosterone levels <0.305 ng/mL have an adverse effect on pregnancy outcomes after IVF. Additionally, when designing an individualized stimulation protocol, measuring testosterone levels may also be helpful. Consequently, fluctuations in testosterone levels towards higher or lower values can adversely affect endometrial receptivity and embryo implantation in general.
Our findings revealed correlations in the HRT group: patient age was inversely correlated with AMH level (p=0.007, r=-0.36), number of mature MII oocytes (p=0.002, r=-0.4), number of 2PN2PB zygotes (p=0.008, r=-0.36), while directly correlated with number of previous IVF attempts (p=0.001, r=0.43). FSH levels were inversely correlated with AMH levels (p=0.0002, r=-0.48), the number of mature MII oocytes (p=0.0217, r=-0.31), the number of 2PN2PB zygotes (p=0.05, r=-0.26), and the number of cryopreserved embryos obtained (p=0.002, r=-0.4). Similarly, in the natural cycle group: women's age was positively correlated with FSH levels (p=0.002, r=0.47), with the number of IVF attempts (p=0.004, r=0.45), and with the number of mature MII oocytes (p=0.003, r=0.45). AMH levels were directly correlated with the number of oocyte-cumulus complexes obtained (p=0.01, r=0.41), the number of 2PN2PB, zygotes (p=0.002, r=0.36), and the number of cryopreserved embryos (p=0.03, r=0.34). AMH and FSH levels as well as patient age are the main predictors of ovarian reserve. Scheffer J.A.B. et al. [16] found significant correlations between AMH levels and patient age (r=-0.34, p<0.01), FSH blood levels (r=-0.32, p<0.01), AFC (r=0.81, p<0.00001), and conception rate (p<0.05). The age correlated with FSH (r=0.46, p<0.01), AFC (r=-0.34, p<0.00001), and the conception rate (p<0.04). Similar data was presented in the study of Zhu J. et al. [17], where the level of AMH was negatively correlated with the age of the patients (r=-0.606, p<0.001), thereby reducing the cells quality of the obtained and the effectiveness of ART programs.
Therefore, it is essential to perform a thorough hormonal examination of the patients before entering an IVF program. Although baseline hormone levels in all patients before entering the IVF program in our study were within the reference range, they had influence on the parameters and results of the ART programs.
Additionally, in this study, statistically significant differences were found between patients who achieved and did not achieve pregnancy in the HRT cycle group in terms of endometrial thickness on the day of embryo transfer (p<0.001). In 2021, Shalom-Paz E. et al. [18] conducted a large study in which Group A included patients with an endometrial thickness ≤ 8 mm and Group B > 8 mm. Compared with group A, group B had significantly higher biochemical and clinical pregnancy rates (30.3 vs 24.6%, p=0.046 and 24.0 vs 18.6%, p=0.036, respectively). The authors showed that endometrial thickness was the only parameter that affected the probability of pregnancy with an OR of 1.54 (95% CI 1.07–2.22, p=0.019). Similar data were presented in the analysis of Bu Z. et al. [19] when comparing patients with endometrial thickness >7 mm and a «thin» endometrium according to ultrasound data, the clinical pregnancy was (56.21% vs 47.13%, P=0.00 in CGT cycles; 55.15% vs 49.55%, P=0.00 in the natural cycle). These data underline the importance of estimating endometrial thickness when choosing between the natural and HRT cycle.
Conclusion
The findings of our study showed that the natural cycle and HRT cycle groups did not differ statistically significantly in pregnancy rates in frozen-thawed embryo transfer. High baseline progesterone levels before IVF were associated with pregnancy onset in both natural cycle and HRT groups, which suggests its possible association with the effectiveness of ART programs and requires further study. Therefore, an individualized approach to luteal phase support during cryopreserved embryo transfer in both natural cycle and HRT cycle according to serum progesterone levels may play an important role in increasing the effectiveness of ART programs.
References
- Ministry of Healthcare of the Russian Federeation. Clinical guidelines. Female infertility. : Russian Society of Obstetricians-gynecologists; 2021. Access mode: https://rd1.medgis.ru/uploads/userfiles/shared/StandartMed/Protokol-acusher/jenskoe-besplodie-2021.pdf. (in Russian)].
-
Gokhberg Ya.A., Makarova N.P., Babayan A.A., Kalinina E.A. The role of various factors affecting the endometrium in enhancing the effectiveness of assisted reproductive technology programs. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 1: 28-34. (in Russian).
https://dx.doi.org/10.18565/aig.2021.1.28-34.
- Craciunas L., Gallos I., Chu J., Bourne T., Quenby S., Brosens J.J., Coomarasamy A. Conventional and modern markers of endometrial receptivity: a systematic review and meta-analysis. Hum. Reprod. Update. 2019; 25(2): 202-23. https://dx.doi.org/10.1093/humupd/dmy044.
- Massimiani M., Lacconi V., La Civita F., Ticconi C., Rago R., Campagnolo L. Molecular signaling regulating endometrium-blastocyst crosstalk. J. Mol. Sci. 2019; 21(1): 23. https://dx.doi.org/10.3390/ijms21010023.
- Kibanov M.V., Makhmudova G.M., Gokhberg Y.A. In search for an ideal marker of endometrial receptivity: from histology to comprehensive molecular genetics-based approaches. Almanac of Clinical Medicine. 2019; 47(1): 12-25. (in Russian)]. https://dx.doi.org/10.18786/2072-0505-2019-47-005.
-
Babayan A.A., Makarova N.P., Kondakova N.V., Gokhberg Ya.A., Nepsha O.S., Kalinina E.A. Uterine fluid analysis as a new opportunity to increase implantation ratesin assisted reproductive technology programs. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 11: 32-40
(in Russian)]. https://dx.doi.org/10.18565/aig.2021.11.32-40.
- Gokhberg Ya.A., Timofeeva A.V., Kalinina E.A. Molecular markers for endometrial receptivity in assisted reproductive technology programs. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 11: 56-62 (in Russian). https://dx.doi.org/10.18565/aig.2021.11.56-62.
- Mumusoglu S., Polat M., Ozbek I.Y., Bozdag G., Papanikolaou E.G., Esteves S.C. et al. Preparation of the endometrium for frozen embryo transfer: A systematic review. Front. Endocrinol. (Lausanne). 2021; 12: 688237. https://dx.doi.org/3389/fendo.2021.688237.
- Kalem Z., Namlı Kalem M., Bakırarar B., Kent E., Gurgan T. Natural cycle versus hormone replacement therapy cycle in frozen-thawed embryo transfer. Saudi Med. J. 2018; 39(11): 1102-8. https://dx.doi.org/10.15537/smj.2018.11.23299.
- Demirdağ E., Güler İ., Cevher Akdulum M.F., Şahin E., Tufan A.D., Erdem A., Erdem M. Comparison of natural and artificial cycles in frozen-thawed embryo transfer: A retrospective analysis of 1696 cycles. Turk. J. Obstet. Gynecol. 2022; 19(1): 28-34. https://dx.doi.org/10.4274/tjod.galenos.2021.17981.
- Volovsky M., Pakes C., Rozen G., Polyakov A. Do serum progesterone levels on day of embryo transfer influence pregnancy outcomes in artificial frozen-thaw cycles? J. Assist. Reprod. Genet. 2020; 37(5): 1129-35. https://dx.doi.org/1007/s10815-020-01713-w.
- Labarta E., Labarta E., Mariani G., Holtmann N., Celada P., Remohí J., Bosch E. Low serum progesterone on the day of embryo transfer is associated with a diminished ongoing pregnancy rate in oocyte donation cycles after artificial endometrial preparation: a prospective study. Hum. Reprod. 2017; 32(12): 2437-42. https://dx.doi.org/10.1093/humrep/dex316.
- Tihomirova T., Parvanov D., Stamenov G., Chaushev T.A. Measurement of serum progesterone levels on the day of embryo transfer is a useful tool in prediction of successful pregnancy. Annuaire de l'Afrique du Nord. 2018; 103(4): 53-9.
- Mokhtar M.H., Giribabu N., Salleh N. Testosterone decreases the number of implanting embryos, expression of pinopode and L-selectin ligand (MECA-79) in the endometrium of early pregnant rats. Int. J. Environ. Res. Public Health. 2020; 17(7): 2293. https://dx.doi.org/10.3390/ijerph1707229.
- Huang L., Chen M., Long L., Tuo Y., Wang Z., Zhou C., Li Y. Low basal serum testosterone level is detrimental to the embryo implantation in the patients with severe endometriosis. J. Obstet. Gynaecol. Res. 2021; 47(6): 2166-74. https://dx.doi.org/10.1111/jog.14791.
- Scheffer J.A.B., Scheffer B., Scheffer R., Florencio F., Grynberg M., Lozano D.M. Are age and anti-Müllerian hormone good predictors of ovarian reserve and response in women undergoing IVF? JBRA Assist. Reprod. 2018; 22(3): 215-20. https://dx.doi.org/10.5935/1518-0557.20180043.
- Zhu J., Li T., Xing W., Lin H., Ou J. Chronological age vs biological age: a retrospective analysis on age-specific serum anti-Müllerian hormone levels for 3280 females in reproductive center clinic. Gynecol. Endocrinol. 2018; 34(10): 890-4. https://dx.doi.org/10.1080/09513590.2018.1462317.
- Shalom-Paz E., Atia N., Atzmon Y., Hallak M., Shrim A. The effect of endometrial thickness and pattern on the success of frozen embryo transfer cycles and gestational age accuracy. Gynecol. Endocrinol. 2021; 37(5): 428-32. https://dx.doi.org/10.1080/09513590.2020.1821359.
- Bu Z., Yang X., Song L., Kang B., Sun Y. The impact of endometrial thickness change after progesterone administration on pregnancy outcome in patients transferred with single frozen-thawed blastocyst. Reprod. Biol. Endocrinol. 2019; 17(1): 99. https://dx.doi.org/10.1186/s12958-019-0545-0.
Received 24.05.2022
Accepted 10.08.2022
About the Authors
Yael A. Gokhberg, PhD Student at the Professor B.V. Leonov Department of IVF, Academician V.I. Kulakov National Medical Research Center for Obstetrics,Gynecology and Perinatology, Ministry of Health of the Russian Federation, dr.yaelgokhberg@gmail.com, 117997, Russia, Moscow, Academician Oparin str., 4.
Ivan S. Fedorov, Researcher at the Laboratory of Applied Transcriptomics, Academician V.I. Kulakov National Medical Research Center for Obstetrics,
Gynecology and Perinatology, Ministry of Health of the Russian Federation, is_fedorov@oparina4.ru, 117997, Russia, Moscow, Academician Oparin str., 4.
Angelika V. Timofeeva, PhD (Bio. Sci.), Head of the Laboratory of Applied Transcriptomics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, avtimofeeva28@gmail.com, 117997, Russia, Moscow, Academician Oparin str., 4.
Elena A. Kalinina, Dr. Med. Sci., Professor, Head of the Professor B.V. Leonov Department of IVF, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, e_kalinina@oparina4.ru, 117997, Russia, Moscow, Academician Oparin str., 4.
Authors' contributions: Gokhberg Ya.A. – data collection and analysis, review of the relevant literature, manuscript drafting; Fedorov I.S. – statistical analysis; Timofeeva A.V., Kalinina E.A. – manuscript editing and approval of publication.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Gokhberg Ya.A., Fedorov I.S., Timofeeva A.V., Kalinina E.A. Comparative analysis of clinical and anamnestic data from couples undergoing frozen-thawed embryo transfer in the natural and hormone replacement therapy cycle.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 9: 72-80 (in Russian)
https://dx.doi.org/10.18565/aig.2022.9.72-80



