Indications and effectiveness of technique for immature oocyte-cumulus complexes retrieval from ovarian tissue followed by their in vitro maturation
Objective: To develop tactics for preserving genetic material in patients with malignant ovarian neoplasms using the technique for immature oocyte retrieval from ovarian tissue, ovarian tissue oocyte in vitro maturation (OTO IVM) and cryopreservation; to identify the indications and limitations of OTO IVM.Bunyaeva E.S., Kirillova A.O., Nazarenko T.A., Dzhanashvili L.G., Gadzhimagomedova K.K., Khabas G.N., Biryukova A.M., Gavisova A.A.
Materials and methods: Seventy-two patients diagnosed with ovarian cancer or borderline ovarian tumors were examined prior to surgical treatment. The patients were evaluated for clinical and anamnestic characteristics, reproductive function and ovarian reserve, and characteristics of the oncological process; risk factors that could influence OTO IVM outcome were determined as well. Each patient underwent retrieval of oocyte-cumulus complexes (OCC) from one or two ovaries, followed by their identification and in vitro maturation. Depending on the patient’s desire, the obtained oocytes were cryopreserved or fertilized with biological material of the partner.
Results: The patients were divided into two groups depending on the outcome of the OTO IVM program. Group 1 consisted of 17 patients who had a successful outcome of the program, namely, at least five mature oocytes and/or one or more embryos were preserved. Group 2 included 55 patients with an unsuccessful outcome. The main factors that could influence OTO IVM outcome were identified.
Conclusion: It was possible to determine the criteria for the selection of patients for using the OTO IVM technique. The results of the study showed that the use of OTO IVM can provide a sufficient number of OCC in young patients in case of good indicators of ovarian reserve, no preliminary resection of the ovaries, and if sufficient amount of ovarian tissue not affected by tumor was sent to the laboratory of embryology. This technique continues to be experimental: in spite of successful retrieval of OCC and oocyte in vitro maturation, high-quality blastocysts are cultivated less frequently than in assisted reproductive technologies and this may affect negatively the result of the treatment, namely, establishing pregnancy in this cohort of patients.
Keywords
Techniques for preserving fertility of cancer patients have been of great interest over the past few years. The growing interest could be due to the rise in cancer survival rates, increased attention to the quality of life of cancer patients in remission, constant progress in the field of assisted reproductive technologies (ART), as well as greater awareness of doctors and patients about the possibil-ities for preserving reproductive material [1].
Among 100,000 cases of gynecological malignant tumors diagnosed eve-ry year, 15–20% occur in women under 40 years of age [2]. Early identification and improvement in treatment protocols have significantly increased survival. Thus, the 5-year survival rate reaches 75% for oncological diseases of all types and localizations in women in the reproductive and pubertal periods [3]. Along with that, postponing motherhood to a late reproductive age which is typical for modern society has led to the fact that a lot of women are diagnosed with cancer earlier than the first child is born, and this situation is unfortunately getting worse. In this regard, preserving reproductive material of young wom-en for delayed motherhood is a relevant and extremely difficult issue of mod-ern medicine.
Nowadays, clinicians of different countries use several techniques aimed at preserving reproductive material and protecting the reproductive function of patients from the negative effect of gonadotoxic therapy [4]. Some of them are still experimental, while others are certainly clinically effective [5].
There are the following techniques:
- preliminary cryopreservation of oocytes/embryos (clinically proven technique);
- preliminary cryopreservation of spermatozoa (clinically proven tech-nique);
- cryopreservation of ovarian tissue/testicles with subsequent autotrans-plantation (the technique is not sufficiently developed);
- obtaining immature oocytes during transvaginal puncture in an un-stimulated cycle, maturation in vitro, cryopreservation of oocytes/embryo (the technique is not enough clinically effective)
- obtaining immature oocytes from the tissue of the removed ovary or part of it, maturation in vitro, cryopreservation of oocytes/embryos (experi-mental method);
- medication protection of the ovaries using aGnRH (clinical technique, effectiveness is not clear enough);
- ovarian transposition during surgical intervention (clinical technique, effectiveness in terms of reproductive function is not clear);
- combination of techniques (it is used individually).
This graduation is largely conditional, because multidisciplinary team considers the possibility and techniques of preserving reproductive material in each individual case. Nevertheless, making a decision is based on the primary use of clinical methods and the search for alternative often experimental meth-ods in case if clinical techniques cannot be used.
A number of papers have identified the main indications for ovarian stimulation for the collection and cryopreservation of oocytes/embryos before gonadotoxic treatment [6]. These are young women with any localization of stage I cancer, except for cancer and recurrent borderline ovarian tumors. The stimulation of the ovaries can be performed after oncologist’s evaluation of the condition of the ovaries, estimating indicators of the ovarian reserve, and ob-taining the informed consent of the patient. The following factors should be in-cluded: individual modified programs, a combination of effectiveness (a suffi-cient number of oocytes/embryos), a decrease in the level of sex steroids during stimulation, and the duration of treatment not exceeding 14–16 days [7–9]. Thus, malignant neoplasms of the ovaries are a contraindication to ovarian stimulation and puncture, as well as for cryopreservation of ovarian tissue. This fact explained the need to develop a new OTO IVM (Ovarian Tissue Oo-cyte In Vitro Maturation) technique, namely the retrieval of oocyte-cumulus complexes (OCCs) from ovarian tissue after ovariectomy followed by their maturation in vitro. OTO IVM technique implies retrieval and manipulation of OCCs in vitro; it does not pose additional risks for patients in terms of transfer of atypical cells which may not be excluded during autotransplantation of ovarian tissue [10, 11]. The technique is experimental and it is performed in cases when other methods cannot be applied, mainly in ovarian cancer which according to the US National Institute of Health is diagnosed in 12% of repro-ductive-aged women [12]. There are few publications evaluating the effective-ness of the technique for obtaining and cryopreservation of oocytes/embryos after OTO IVM [10, 11]. The cases of the birth of children after using the OTO IVM technique are obviously few as well [13].
Therefore, the aim of this study was to determine the indications, limita-tions, and effectiveness of the OTO IVM technique.
Materials and methods
The study was approved by the Ethical Review Board of National Medi-cal Research Center for Obstetrics, Gynecology and Perinatology, Moscow, Russia (protocol No. 11 dated 13.12.2018). Informed consent to participate in the study and for the processing of personal data was obtained from all the pa-tients. The OTO IVM technique was used in 72 cases: 60/72 (83.3%) patients underwent surgical treatment for ovarian cancer and 12/72 (16.7%) patients were operated on for borderline ovarian tumors. Embryology laboratory re-ceived 89 removed ovaries and 3 samples of ovaries.
The ovaries were delivered from the operating room in sterile containers, OCCs were retrieved from ovarian tissue by aspiration using 21G needle con-nected to 10 ml syringe or by crushing ovarian tissue with scalpels.
The obtained OCCs matured in vitro for 30–48 hours in the medium for oocyte maturation. In the standard IVM protocol, immature OCCs were ma-tured in an IVM medium (Origio, Denmark) with the addition of human albu-min (Alburex, Germany), hormones FSH and LH for 48 hours. The obtained oocytes were evaluated for maturity and quality, mature oocytes were cryo-preserved using the generally accepted vitrification technique. If the patients wanted to fertilize the obtained oocytes, fertilization was performed using the ICSI technique; fertilization was evaluated based on the presence of two pro-nuclei; embryos were cultured to the blastocyst stage in a one-step GTL (Vitro-life) medium covered with Ovoil (Vitrolife) oil in an incubator at 37°C, in 6% CO2 and 5% O2 up to 5 days of development. The presence and quality of the obtained blastocysts were evaluated using Gardner’s blastocyst morphology criteria, and blastocysts suitable for cryopreservation were vitrified. A total of 92/141 (65.2%) stage III oocytes were cryopreserved in 26/72 (36.1) patients and 11 embryos in 6/72 (8.3%) patients.
The work of the embryologist consisted of several stages. The first stage was the preparation of media and materials before the planned work with the ovary. The preparation of the specimen, the search and extraction of OCC from the ovary takes approximately 1.5 hours and requires certain skills. Cul-tivation in the medium for maturation takes an average of 35 hours, whereas the identification of oocytes, the selection of mature ones and their subsequent cryopreservation takes no more than one hour. Further, mature oocytes are fer-tilized with biological material of a partner or a donor via ICSI and embryo cultivation is carried out within 5 days if desired by the patient. The above in-formation is given to emphasize the complexity of the work of a highly quali-fied embryologist.
Statistical analysis
Statistical data were processed using an R software package [14]. Spearman rank correlation coefficient (Rs) was used to find the relationship between two quantitative variables. The relationship between two qualitative variables was determined using the Fisher exact test. Normal distribution for quantitative variables was checked using the Kolmogorov–Smirnov test. In case of an ab-normal distribution, nonparametric Mann–Whitney U test (for comparing two groups) or Kruskal–Wallis test (for comparing more than two groups) were used. To compare groups with normally distributed variables, two sample Student’s t-criterion was used. In case of normal distribution, the data are pre-sented as the mean (SD); in case of abnormal distribution, the median (Q1; Q3) is indicated.
Results and discussion
Since the study aimed to determine the indications for the OTO IVM program, the patients were divided into two groups. Group 1 consisted of 17/72 (23.6%) women whose program was considered successful and at least five mature oocytes or one or more embryos of good quality were preserved; group 2 included 55/72 (76.4%) patients with an unsuccessful attempt of ob-taining and cryopreservation of oocytes, when the oocytes were not obtained, they were not mature, or only 1–2 oocytes were suitable for cryopreservation.
Such a division into groups was due to the complexity of the method and the purpose of preventing possible discrediting of this direction, as the failure to obtain the necessary materials is most often associated not with de-fects of the method, but with the incorrect selection of patients.
Among the patients included in the study, 60/72 (83.3%) women were diagnosed with ovarian cancer, 12/72 (16.7%) had borderline ovarian tumors. The age of the patients ranged from 14 to 43 years; the average patient age was 33 (6.25) years. The table shows a comparison of anamnestic, clinical and laboratory characteristics of patients with successful and unsuccessful out-comes of OTO IVM program.
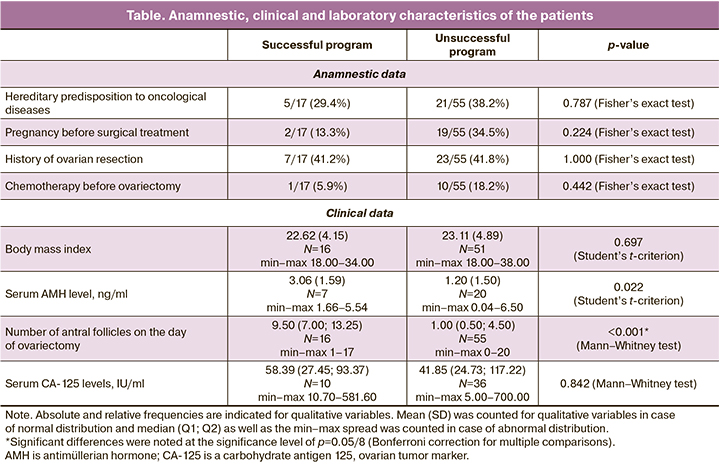
According to the data of the patients’ case histories, a hereditary predis-position to oncological diseases was revealed in 26/72 (36.1%) of the patients. Pregnancy was detected in 21/72 (29.1%) women before the diagnosis of the disease, and 13/72 (18%) of them gave birth to a child. The correlation between BMI and OCC was not significant (Rs=-0.160, p=0.196).
Ovarian resection was performed in 31/72 (43%) cases, and the presence of a growth in the ovary was the initial diagnosis in 19/72 (26.4%) of them; histological examination confirmed a malignant or borderline tumor which re-quired repeated radical surgery.
The average age of the patients was 33 (6.25) years which corresponds to the active reproductive period, but the age range varied from 14 to 43 years. The average AMH level in the blood serum was 1.68 (1.7) ng/ml with values from 0 to 6.5 ng/ml, respectively, the average number of antral follicles was 4.52 (4.88) ranging from 0 to 20.
According to the presented data, the group of patients was very hetero-geneous in anamnestic and clinical characteristics. The criterion of success or failure of the OTO IVM program was cryopreservation of five or more oocytes of stage MII and/or one or more embryos. The results of the study showed that these indicators were achieved only in 17/72 (23.6%) women and the program was unsuccessful for the rest 55/72 (76.4) patients. We tried to determine the predictors of a positive result and those factors that limit the use of the OTO IVM technique.
Age of patients, AMH level, number of antral follicles
The groups with successful and unsuccessful programs were significantly different in age (p=0.008; Student’s t-criterion): the average age of patients with obtained OCCs was significantly lower and amounted to 28.9 (6.9) years compared to 34.3 (5.5) years in patients with an unfavorable outcome.
The average AMH level was also significantly higher in patients with a successful outcome (p=0.022; Student’s t-criterion) and amounted to 3.06 (1.59) compared to 1.20 (1.50) in patients with an unsuccessful outcome of the program.
The groups with successful and unsuccessful programs were significantly different in the number of antral follicles (p<0.001; Mann-Whitney test): the median (Q1; Q3) of the number of antral follicles in patients with a successful outcome was higher and amounted to 9.50 (7.00; 13.25) versus 1.00 (0.50; 4.50) in patients with an unsuccessful program.
Thus, the age of the patient and the associated indicators of ovarian re-serve showed a strong correlation with the outcome of the OTO IVM program. A significant negative correlation was found between the number of the ob-tained oocyte-cumulus complexes and age (Rs=-0.442, p<0.001), namely, older patients had a lower number of OCCs. The number of OCCs obtained from ovarian tissue is also significantly associated with AMH (Rs=0.822, p<0.001), namely, the patients with a higher AMH level had a higher number of OCCs.
Ovarian resection and a history of chemotherapy
Both ovarian resection and chemotherapy are known to have a negative effect on the ovarian reserve and the possibility of obtaining a sufficient num-ber of oocytes [15–17]. Our study did not reveal a significant relationship be-tween the success or failure of the OTO IVM program and the presence or ab-sence of ovarian resection in the history (p=1; Fischer’s exact test). There are no significant differences in the number of OCCs among the groups of patients with resection of one ovary, two ovaries and the absence of resections (p=0.458; Kruskal–Wallis test).
However, there was a weak but significant negative correlation between obtained OCCs and a history of chemotherapeutic treatment (Rs=-0.340, p=0.003): patients with a higher number of chemotherapy courses had a lower number of obtained OCCs (there were only 11/72 (15.3%) patients who un-derwent chemotherapy).
There were significant differences (p=0.004; Mann–Whitney test) be-tween patients with a history of treatment, where the median (Q1; Q3) number of obtained OCCs was 0.6 (1.5), and patients without gonadotoxic treatment was 3.0 (0.0; 8.0).
Day of the menstrual cycle
Some articles provide information that the effectiveness of obtaining OCCs and the subsequent maturation of immature oocytes do not depend on the day of the menstrual cycle [18]. We tried to compare the results of treat-ment in patients depending on the day of the cycle when the ovary was sam-pled.
All patients underwent surgery in different phases of the cycle: 42/72 (58.3%) of them were operated on in the follicular phase, 20/72 (27.8%) had an operation in the ovulatory phase, and 10/72 (13.9%) underwent surgery in the luteal phase. There is no significant relationship between the success of the OTO IVM program and the phase of the cycle (p=0.720; Fisher’s exact test). The results of the study showed no significant differences in the number of ob-tained OCCs among three groups of patients whose ovary sampling was per-formed at different phases of the cycle (p=0.885; Kruskal–Wallis test); no ef-fect of the cycle phase on the amount of MII was found (p=0.779; Kruskal–Wallis test).
Adverse factors limiting the possibility of obtaining OCCs
During the study, we identified factors unfavorable for the OTO IVM program.
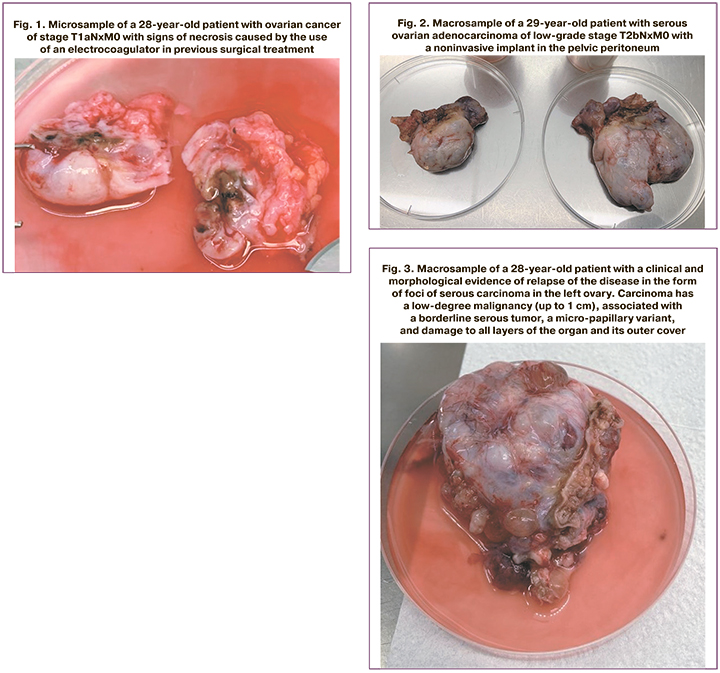
Unfortunately, a significant coagulation of the ovary including coagula-tion of the ovary with unipolar current is often used during ovariectomy. This is naturally due to the surgical situation, bleeding and difficulties in sampling the ovary, but ovarian coagulation fatally affects the possibility of obtaining oocytes (Fig. 1).
There were three cases when ovarian tissue measuring 0.5x0.5 cm was delivered to the IVF laboratory after ovarian resection. It was not possible to obtain OCC in any of the cases because the functional tissue was replaced by fibrous and sclerosed one. The cells from ovarian biopsy specimens were not obtained in any of the cases either.
It was previously shown that there was no significant relationship be-tween the success of the OTO IVM program and ovarian resection in the histo-ry (p=1, Fisher’s exact test). However, further analysis proved the relationship between the size of the delivered ovarian sample and the possibility of obtain-ing OCC. The patients with a smaller size of ovarian tissue had a lower proba-bility of obtaining oocytes; although the fact of resection itself did not show a reliable correlation with the possibility of obtaining oocytes, the volume of the ovary after resection plays a decisive role.
OCC could not be obtained from the ovary in the presence of tumors larger than 4 cm (Fig. 2 and Fig. 3) because the functional tissue of the ovary was affected by the tumor.
A total of 92/141 (65.2%) mature oocytes obtained from 26/72 (36.1%) patients were cryopreserved. The maturation of immature cells occurred in 47.96% of cases.
It is well known that embryos cultivated on the 5th day are better for cryopreservation and subsequent defrosting, and they have greater chances to ensure the onset of pregnancy [19]. In the present study, 14/72 (19.4%) pa-tients wanted to have their oocytes fertilized with the sperm of their partner.
Fertilization of 49/141 (34.7%) mature oocytes was performed with the ICSI method. The partner’s sperm was fertile or demonstrated a slight degree of pathozoospermia. Fertilization was diagnosed in 27.6% of cases, however, the formation of blastocysts was observed only in 17.95% of the fertilized em-bryos. The results obtained in this study were significantly lower than the av-erage parameters in the IVF programs [20].
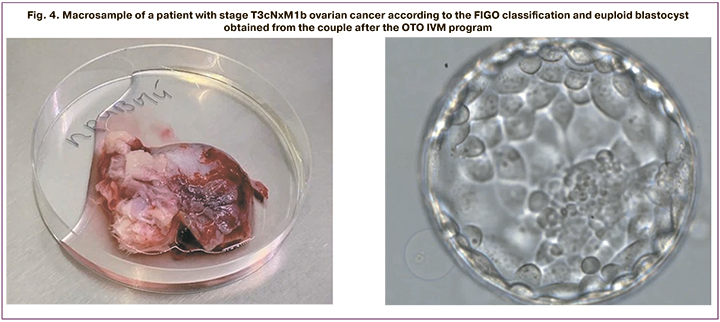
Figure 4 shows a macrosample of a patient with stage T3cNxM1b ovari-an cancer according to the FIGO classification and euploid blastocyst obtained from this couple after the OTO IVM program.
Despite the effectiveness of the stages of retrieval and maturation of im-mature oocytes obtained from the tissue of the removed ovary, the presented data showed that the processes of fertilization, early embryogenesis, the num-ber and quality of blastocysts are unsatisfactory. This may be due to genetic factors, for example, aneuploidy, which impairs the development of the em-bryo and, more likely, to epigenetic causes leading to the arrest in early em-bryogenesis.
In any case, the first and very important step in the development of the OTO IVM technique has been taken, but there is a lot of work to be done eval-uating the effectiveness and safety of the technique. According to the recom-mendations of the European Society of Human Reproduction and Embryology (ESHRE), further studies are necessary to analyze the quality of oocytes after IVM, as well as long-term follow-up of children born after the use of this tech-nique [10, 13]. In addition, further development and standardization of oocyte maturation and cryopreservation protocols are required to increase the effec-tiveness of the IVM program.
There is no doubt that the technique of maturing immature OCC in vitro has great prospects as a method of preserving fertility in cancer patients. It should be noted that the method is used in cases where there are no alternative options to have a genetically related child, primarily in young women with ovarian cancer. This method is their only hope and it is necessary to continue working in this direction.
Therefore, it is important to determine the criteria for selecting patients with a promising prognosis for using the OTO IVM technique. Unfortunately, it is recommended to exclude unpromising patients at this stage, as it is neces-sary for further improvement of the method.
Conclusion
It was possible to determine the criteria for the selection of patients for using the OTO IVM technique. Women over 40 years of age with AMH level below 1.0 ng/ml, a history of ovarian resection and chemotherapy, the use of ovarian coagulation during its removal, sending an ovarian sample and tissue smaller than 3 cm to the IVF laboratory should be considered unpromising. If the size of a tumor exceeds 4 cm in both or a single ovary, it is difficult to ob-tain OCC as well. The cases are considered to be favorable when ovariectomy is performed in young women with good indicators of ovarian reserve, the size of the tumor does not exceed 2–3 cm, ovarian coagulation should not be used. This technique continues to be experimental: in spite of successful retrieval of OCC and oocyte in vitro maturation, high-quality blastocysts are cultivated less frequently than in assisted reproductive technologies and this may affect negatively the result of the treatment, namely, establishing pregnancy in this cohort of patients. But this is the only opportunity for many young women to bear a child in the future.
References
- Santos M.L., Pais A.S., Almeida Santos T. Fertility preservation in ovarian cancer patients. Gynecol. Endocrinol. 2021; 37(6): 483-9.https://dx.doi.org/10.1080/09513590.2021.1872534.
- Ferlay J., Ervik M., Lam F., Colombet M., Mery L., Piñeros M. et al. Global cancer observatory: cancer today. Lyon: IARC; 2020.
- Xu Y., Zhang M., Zhang J., Ng D.M., Chen X., Si Y. et al. Neoadjuvant chemiotherapy increases the 5-year overall survival of patients with resectable cervical cancer: A systematic review and meta-analysis. Taiwan. J. Obstet. Gy-necol. 2021; 60(3): 433-41. https://dx.doi.org/10.1016/j.tjog.2021.03.008.
- Alexander V.M., Martin C.E., Schelble A.P., Laufer A.B., Hardi A., McKenzie L.J. et al. Ovarian stimulation for fertility preservation in women with cancer: A systematic review and meta-analysis comparing random and conventional starts. J. Gynecol. Obstet. Hum. Reprod. 2021; 50(8): 102080. https://dx.doi.org/10.1016/j.jogoh.2021.102080.
- Massarotti C., Kohlhepp F., Liperis G., Ammar O.F., Mincheva M.N., Ali Z.E. et al. #ESHREjc report: Is OTO-IVM the future fertility preservation alternative for urgent cancer patients? Hum. Reprod. 2021; 36(9): 2631-3. https://dx.doi.org/10.1093/humrep/deab180.
- Chen C.N., Chang L.T., Chen C.H., Tam K.W. Fertility preservation for women with breast cancer before chemotherapy: a systematic review and meta-analysis. Reprod. Biomed. Online. 2022; 44(2): 357-69. https://dx.doi.org/10.1016/j.rbmo.2021.08.003.
- Fatemi H.M., Kyrou D., Al-Azemi M., Stoop D., De Sutter P., Bourgain C., Devroey P. Ex-vivo oocyte retrieval for fertility preservation. Fertil. Steril. 2011; 95(5): 1787.e15-1787.e17. https://dx.doi.org/10.1016/j.fertnstert.2010.11.023.
- Moragón S., Di Liello R., Bermejo B., Hernando C., Olcina E., Chirivella I. et al. Fertility and breast cancer: A literature review of counseling, preservation options and outcomes. Crit. Rev. Oncol. Hematol. 2021; 166: 103461. https://dx.doi.org/10.1016/j.critrevonc.2021.103461.
- Kappy M., Lieman H.J., Pollack S., Buyuk E. Fertility preservation for cancer patients: treatment gaps and considerations in patients' choices. Arch. Gynecol. Obstet. 2021; 303(6): 1617-23. https://dx.doi.org/10.1007/s00404-021-05985-0.
- Ковальская Е.В., Кириллова А.О., Буняева Е.С., Хабас Г.Н., Камалетдинов Н.С., Назаренко Т.А., Абубакиров А.Н. Эффективность дозревания ооцитов, полученных в ходе овариэктомии, у онкологических пациенток. Акушерство и гинекология. 2019; 9: 87-91. [Kovalskaya E.V., Kirillova A.O., Bunyaeva E.S., Khabas G.N., Kamaletdinov N.S., Nazarenko T.A., Abubakirov A.N. Efficiency of maturation of oocytes obtained from cancer patients during ovariectomy. Obstetrics and Gynecology. 2019; 9: 87-91. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.9.87-91.
- Kirillova A., Bunyaeva E., Van Ranst H., Khabas G., Farmakovskaya M., Kamaletdinov N., Nazarenko T., Abubakirov A., Sukhikh G., Smitz J.E.J. Improved maturation competence of ovarian tissue oocytes using a biphasic in vitro maturation system for patients with gynecological malignancy: a study on sibling oocytes. J. Assist. Reprod. Genet. 2021; 38(6): 1331-40. https://dx.doi.org/10.1007/s10815-021-02118-z.
- Howlader N., Noone A.M., Krapcho M., Miller D., Brest A., Yu M. et al.; eds. SEER Cancer Statistics Review, 1975-2018. Bethesda, MD: National Cancer Institute. Available at: https://seer.cancer.gov/csr/1975_2018/, based on November 2020 SEER data submission, posted to the SEER web site, April 2021.
- Назаренко Т.А., Ашрафян Л.А., Бирюкова А.М., Кириллова А.О., Мартиросян Я.О., Джанашвили Л.Г., Буняева Е.С. Характеристики и тактика ведения онкологических больных, нуждающихся в сохранении репродуктивного материала. Акушерство и гинекология. 2020; 11: 93-9. [Nazarenko T.A., Ashrafyan L.A., Biryukova A.M., Kirillova A.O., Martirosyan Ya.O., Dzhanashvili L.G., Bunyaeva E.S. Characteristics and management of cancer patients who wish to preserve their reproductive capacity. Obstetrics and Gynecology. 2020; 11: 93-9. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.11.93-99.
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria; 2020.
- Berjeb K.K., Debbabi L., Braham M., Zemni Z., Chtourou S., Hannachi H. et al. Evaluation of ovarian reserve before and after chemotherapy. J. Gynecol. Obstet. Hum. Reprod. 2021; 50(5):102035. https://dx.doi.org/10.1016/j.jogoh.2020.102035.
- Romito A., Bove S., Romito I., Zace D., Raimondo I., Fragomeni S.M. et al. Ovarian reserve after chemotherapy in breast cancer: A systematic review and meta-analysis. J. Pers. Med. 2021; 11(8): 704. https://dx.doi.org/10.3390/jpm11080704.
- Hopeman M.M., Cameron K.E., Prewitt M., Barnhart K., Ginsberg J.P., Sammel M.D., Gracia C.R. A predictive model for chemotherapy-related diminished ovarian reserve in reproductive-age women. Fertil. Steril. 2021; 115(2): 431-7. https://dx.doi.org/10.1016/j.fertnstert.2020.08.003.
- Cakmak H., Rosen M.P. Random-start ovarian stimulation in patients with cancer. Curr. Opin. Obstet. Gynecol. 2015; 27(3): 215-21. https://dx.doi.org/10.1097/GCO.0000000000000180.
- Wang N., Zhao X., Ma M., Zhu Q., Wang Y. Effect of Day 3 and Day 5/6 embryo quality on the reproductive outcomes in the single vitrified embryo transfer cycles. Front. Endocrinol. (Lausanne). 2021; 12: 641623. https://dx.doi.org/10.3389/fendo.2021.641623.
- Anderson R.A., Amant F., Braat D., D'Angelo A., de Sousa Lopes S.M.C., Demeestere I., Dwek S. et al.; ESHRE Guideline Group on Female Fertility Preservation. ESHRE guideline: female fertility preservation. Hum. Reprod. Open. 2020; 2020(4): hoaa052. https://dx.doi.org/10.1093/hropen/hoaa052.
Received 25.05.2022
Accepted 14.06.2022
About the Authors
Ekaterina S. Bunyaeva, gynecologist, 1st Gynecology Department, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology, and Perinatology,Ministry of Health of Russia, +7(495)531-44-44, e_bunyaeva@oparina4.ru, es_bunyaeva@mail.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Anastasia O. Kirillova, PhD, Senior Researcher, Skoltech Center of Life Sciences, +7(495)280-14-81, stasia.kozyreva@gmail.com, 121205, Russia, Moscow, Nobelya str, 1.
Tatiana A. Nazarenko, Dr. Med. Sci., Professor, Director of Institute of Reproductive Medicine, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia, +7(495)531-44-44, t_nazarenko@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Lana G. Dzhanashvili, PhD, obstetrician-gynecologist at Scientific and Clinical Department of ART named after F. Paulsen, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia, +7(495)531-44-44, l_dzanashvili@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Karina K. Gadzhimagomedova, embryologist at Scientific and Clinical Department of ART named after F. Paulsen, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia, +7(495)531-44-44, k_gadzhimagomedova@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Grigory N. Khabas, PhD, Head of the Department of Innovative Oncology and Gynecology, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology,
and Perinatology, Ministry of Health of Russia, +7(495)531-44-44, khabas@list.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Almina M. Birukova, PhD, gynecologist at Scientific and Clinical Department of ART named after F. Paulsen, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia, +7(495)531-44-44, a_birukova@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Alla A. Gavisova, PhD, Acting Head of the 1st Gynecology Department, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology, and Perinatology,
Ministry of Health of Russia, +7(495)531-44-44, a_gavisova@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Authors’ contributions: Bunyaeva E.S., Kirillova A.O., Nazarenko T.A., Dzhanashvili L.G., Gadzhimagomedova K.K., Khabas G.N., Biryukova A.M., Gavisova A.A. – developing research design, obtaining data for analysis, review of publications on the topic of the article, analysis of the obtained findings, writing the text of the manuscript.
Conflicts of interest: The authors declare that they have no competing interests.
Funding: The study was performed without external funding.
Ethical Approval: The study was approved by the Ethical Review Board of Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow, Russia (Ref. No. 11 dated 13.12.2018).
Patient Consent for Publication: All patients provided informed consent for the publication of their data and associated images.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Bunyaeva E.S., Kirillova A.O., Nazarenko T.A., Dzhanashvili L.G., Gadzhimagomedova K.K., Khabas G.N., Biryukova A.M., Gavisova A.A.
Indications and effectiveness of technique for immature oocyte-cumulus complexes retrieval
from ovarian tissue followed by their in vitro maturation.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 6: 75-82 (in Russian)
https://dx.doi.org/10.18565/aig.2022.6.75-82



