Возможность реализации репродуктивных планов посредством применения гормональных контрацептивов появилась у женщин в 60-х гг. XX в. Одна из глобальных целей создания надежных методов контрацепции – это снижение числа незапланированных беременностей, большинство из которых заканчиваются абортами. Каждый год в мире проводится порядка 73 млн искусственных абортов. Ими заканчиваются 6/10 (61%) всех случаев нежелательной беременности и 3/10 (29%) всех случаев беременности [1]. Последствия абортов известны вплоть до фатальных (от 4,7 до 13,2% ежегодных случаев материнской смерти), а в ряде случаев будет нанесен непоправимый вред репродуктивному здоровью женщины [2–5]. Гормональные контрацептивы представлены в виде пероральных таблетированных препаратов, вагинальных колец, пластырей, имплантатов и внутриматочных спиралей. Два последних типа относятся к методам долгосрочной обратимой контрацепции. Наиболее популярным вариантом предохранения от беременности являются обратимые методы контрацепции короткого действия, в частности комбинированные оральные контрацептивы (КОК) [6]. Из негормональных методов чаще всего применяются презервативы, которые по-прежнему являются единственным методом контрацепции, предотвращающим заражение инфекциями, передаваемыми половым путем.
Грамотный подбор гормональных препаратов для предохранения от беременности – одна из сложнейших задач гинеколога. Основная цель консультирования – это выбор надежной контрацепции, эффективность которой, в случае пероральных препаратов, зависит от приверженности пациентки к терапии и/или ее правильного применения [7]. Под приверженностью понимают ежедневный прием таблетки в одно и то же время и соблюдение ряда мер для предотвращения беременности при приеме препаратов, которые снижают их эффективность [7]. Известно, что у 8 из 100 женщин, принимающих КОК, наступает нежелательная беременность, в том числе из-за снижения приверженности [7, 8]. Учитывая, что именно надежность контрацептивного эффекта КОК зависит от самой пациентки, на этапе консультирования важно объяснить женщине необходимость ежедневного приема препарата и, желательно, в одно и то же время.
В марте 2023 г. опубликованы Национальные медицинские критерии приемлемости методов контрацепции, что значительно облегчило работу клиницисту [9]. При обсуждении безопасности приема комбинированных гормональных контрацептивов (КГК) одним из актуальных вопросов являются венозные тромбоэмболические осложнения. Действительно риск таких осложнений существует, известна статистика по рискам венозной тромбоэмболии (ВТЭ). У женщин репродуктивного возраста ВТЭ встречается редко (частота 1–5/10 000 женщино-лет), прием КОК увеличивает риск ВТЭ, повышая ее частоту примерно до 10–15/10 000 женщино-лет. Однако риск ВТЭ на фоне приема КОК намного ниже, чем риски при таких физиологических состояниях, как беременность (примерно 5–20/10 000 женщино-лет) и послеродовой период (40–65/10 000 женщино-лет) [10, 11]. В сравнении риски уже не столь высоки. Риск ВТЭ снижается в течение 1 года от начала приема КОК [12, 13].
При грамотном консультировании многих неблагоприятных событий при применении КОК можно избежать [9]. На элементарном этапе сбора анамнеза будет понятно, что пациентке нельзя принимать КОК, если женщина старше 35 лет, курит или у нее мигрень без ауры (шестикратное увеличение риска острого нарушения мозгового кровообращения). Мигрень с аурой будет противопоказанием независимо от возраста. Чем выше индекс массы тела, тем выше риск ВТЭ (до 24-кратного увеличения риска) [14]. Важен семейный анамнез, а именно случаи инфаркта миокарда, инсульта, тромбоэмболической болезни в возрасте до 45 лет. Для каждой женщины риски индивидуальны, со временем они меняются, поэтому важна постоянная и своевременная переоценка факторов риска.
КГК обладают неконтрацептивными эффектами и рекомендованы в качестве терапии синдрома поликистозных яичников и для лечения эндометриоз-ассоциированной боли, также это вариант лечения предменструального синдрома и, по некоторым данным, – функциональных кист яичников. КГК назначаются для терапии обильных менструальных кровотечений пациенткам, нуждающимся в контрацепции, что опосредованно положительно влияет на такую распространенную проблему именно у женщин, как железодефицитная анемия [15].
По данным масштабных исследований показано, что прием КОК приводит к снижению риска развития рака эндометрия и рака яичников, также обсуждается положительный эффект в отношении колоректального рака, рака легких и поджелудочной железы [16–18].
Основные контрацептивные механизмы — это подавление овуляции и повышение вязкости цервикальной слизи, посредством чего снижается ее проницаемость для сперматозоидов.
Существует несколько режимов приема КОК. Монофазный 21+7 и 24+4, цифры 21/24 обозначают количество таблеток, содержащих гормоны; 7 – это количество дней перерыва между началом приема новой упаковки или 4 – плацебо-таблетки, так же существуют двухфазный и трехфазный режимы. Одним из преимуществ монофазного режима является возможность приема препарата в непрерывном режиме для отсрочки менструальноподобной реакции.
КГК содержат комбинацию эстрогена и прогестагена, оба из которых чаще всего являются синтетическими стероидными гормонами. Этинилэстрадиол является синтетическим эстрогеном, который действует аналогично эстрадиолу. Более 60 лет он широко применяется в качестве эстрогенного компонента большинства КОК. Известна более высокая частота ВТЭ у женщин, принимавших КОК, содержащих 50 мкг этинилэстрадиола (отношение шансов 2,65) [19]. С момента появления КОК в 1960-х гг. первоначальная доза этинилэстрадиола 50 мкг была снижена до наиболее часто назначаемой в настоящее время дозы 15–35 мкг [20]. Разработка низкодозированных препаратов преследует цель улучшения показателей безопасности и переносимости гормональной контрацепции без влияния на ее эффективность.
Большинство препаратов КОК в качестве эстрогенного компонента содержат 20 или 30 мкг этинилэстрадиола. В 2011 г. появился КОК с эстрадиола валератом, у которого, помимо показания «контрацепция», указана «терапия обильных менструальных кровотечений», позже был зарегистрирован КОК с 17β-эстрадиолом, а в 2022 г. – эстроген IV поколения – эстетрол.
Различные эффекты КОК определяются свойствами прогестагенного компонента. Например, антиандрогенным и антиминералокортикоидным эффектами обладает прогестин IV поколения – дроспиренон, минимальные андрогенный и антиминералокортикоидный эффекты определяются у гестодена (III поколение).
Фармацевтическая компания ООО «Фармасинтез-Тюмень», Россия, разработала воспроизведенные КГК линейки «ПланиЖенс»: «ПланиЖенс гесто 20», «ПланиЖенс гесто 30», «ПланиЖенс дроспи».
Лекарственный препарат «ПланиЖенс гесто 20» (гестоден+этинилэстрадиол), таблетки, покрытые оболочкой, 0,075 мг+0,02 мг, является воспроизведенным препаратом по отношению к оригинальному (референтному) препарату «Логест», таблетки, покрытые оболочкой, 0,075 мг+0,02 мг («Байер Фарма АГ», Германия).
Лекарственный препарат «ПланиЖенс гесто 30» (гестоден+этинилэстрадиол), таблетки, покрытые оболочкой, 0,075 мг+0,03 мг, является воспроизведенным препаратом по отношению к оригинальному (референтному) препарату «Фемоден», таблетки, покрытые оболочкой, 3 мг+0,03 мг («Байер Фарма АГ», Германия).
Комбинированный препарат гестодена+этинилэстрадиол – низкодозированный монофазный пероральный эстроген-гестагенный контрацептив. Гестоден – прогестаген III поколения, обладающий высокой прогестагенной активностью. Важное преимущество гестодена – 100% биодоступность и отсутствие активных метаболитов. Благодаря этому возможно применение гестодена в сравнительно более низких дозах для подавления овуляции [21]. Стабильная фармакокинетическая кривая обеспечивает хороший контроль менструального цикла при применении гестодена даже с низкой дозой эстрогена. Гестоден обладает антиминералокортикоидной активностью, что противодействует возникновению отеков, вызванных действием эстрогенов. В отличие от других прогестагенов гестоден не влияет на активность эстрогеновых рецепторов в клетках органов-мишеней, в связи с чем представляется более безопасным по влиянию на пролиферативные процессы. В клинических исследованиях показано, что гестоденсодержащие контрацептивы не оказывают значимого влияния на метаболизм липидов, углеводов, систему гемостаза и имеют хорошую переносимость [21–23].
Лекарственный препарат «ПланиЖенс дроспи» (дроспиренон+этинилэстрадиол), таблетки, покрытые пленочной оболочкой, 3 мг+0,03 мг, является воспроизведенным препаратом по отношению к оригинальному (референтному) препарату «Ярина», таблетки, покрытые пленочной оболочкой, 3 мг+0,03 мг («Байер Фарма АГ», Германия). Дроспиренон+этинилэстрадиол – КГК с антиминералокортикоидным и антиандрогенным действиями. Дроспиренон – синтетический прогестаген IV поколения, по фармакологическим свойствам максимально приближен к эндогенному прогестерону, обладает, наряду с прогестагенным и антиандрогенным, антиминералокортикоидным действием, способен оказывать антиадипогенный эффект, положительно влияет на липидный профиль, уровень некоторых адипокинов, без значимого воздействия на чувствительность к инсулину. Активность дроспиренона в составе КОК проявляется в клинических эффектах на физиологические параметры, массу тела, эмоциональное состояние и симптомы, связанные с задержкой жидкости [21, 24]. Антиандрогенная активность обеспечивает дополнительную пользу применения у женщин с проявлениями гиперандрогении.
Препарат «ПланиЖенс дроспи», таблетки, покрытые пленочной оболочкой, 3 мг+0,03 мг, предназначен для применения женщинами в качестве средства пероральной контрацепции с приемом 1 таблетки 1 раз в сутки на протяжении 21 суток с последующим 7-дневным перерывом.
Высокая эффективность комбинаций гестодена/этинилэстрадиола и дроспиренона/этинилэстрадиола доказана в клинических исследованиях и подтверждена многолетним обширным опытом в пострегистрационном периоде.
Внедрение в клиническую практику воспроизведенных препаратов, обладающих системной биодоступностью (за исключением водных растворов), предусматривает предварительное проведение исследования биоэквивалентности. Биоэквивалентность лекарственных препаратов предполагает их терапевтическую эквивалентность. Исследование биоэквивалентности является основным видом медико-биологического контроля качества воспроизведенных препаратов, содержащих такое же количество действующего вещества, как и соответствующий оригинальный лекарственный препарат, и регулируется нормативными документами [25–27].
С целью оценки биоэквивалентности новых воспроизведенных лекарственных препаратов «ПланиЖенс гесто 20», «ПланиЖенс гесто 30», «ПланиЖенс дроспи» компанией ООО «Фармасинтез-Тюмень», Россия, проведены исследования их биоэквивалентности в сравнении с соответствующими оригинальными (референтными) препаратами.
Материалы и методы
Каждое из исследований биоэквивалентности было проведено в одном клиническом центре в Российской Федерации.
Исследования биоэквивалентности препаратов «ПланиЖенс гесто 20» (гестоден+этинилэстрадиол), «ПланиЖенс гесто 30» (гестоден+этинилэстрадиол), «ПланиЖенс дроспи» (дроспиренон+этинилэстрадиол) были проведены в Российской Федерации в соответствии с принципами, изложенными в Хельсинкской декларации Всемирной медицинской ассоциации «Рекомендации для врачей, занимающихся биомедицинскими исследованиями с участием людей» (Бразилия, Форталеза, 2013 г.); принципами Надлежащей клинической практики; международными правилами проведения клинических исследований (ICH GCP); требованиями к проведению исследований биоэквивалентности российского законодательства и ЕАЭС, а также в соответствии с утвержденными в установленном порядке протоколами исследований биоэквивалентности.
В каждое исследование биоэквивалентности препаратов гестодена+этинилэстрадиол («ПланиЖенс гесто 20», «ПланиЖенс гесто 30») и дроспиренона+этинилэстрадиол («ПланиЖенс дроспи», таблетки, покрытые пленочной оболочкой, 3 мг+0,03 мг) было рандомизировано по 32 добровольца.
В исследования биоэквивалентности включались здоровые женщины в возрасте 18–45 лет, добровольно изъявившие желание участвовать в исследовании, прошедшие физикальное и лабораторно-инструментальное обследование, подписавшие письменное информированное согласие и соответствующие всем критериям включения/невключения в исследование.
Цель исследований биоэквивалентности
Целью проведенных исследований биоэквивалентности были изучение сравнительной фармакокинетики и оценка биоэквивалентности комбинированных препаратов гестодена+этинилэстрадиол и дроспиренона+этинилэстрадиол и оригинальных (референтных) препаратов у здоровых добровольцев женского пола при однократном приеме их натощак.
Дизайн исследований
Исследования биоэквивалентности препаратов гестодена+этинилэстрадиол и дроспиренона+этинилэстрадиол проводились как открытые рандомизированные перекрестные сравнительные с двумя периодами и двумя последовательностями исследования с однократным приемом натощак каждого из препаратов исследования здоровыми добровольцами женского пола.
Обоснование выбора препаратов сравнения
Выбор лекарственных препаратов «Логест», «Фемоден», «Ярина» в качестве препаратов сравнения (R) для препаратов «ПланиЖенс гесто 20», «ПланиЖенс гесто 30», «ПланиЖенс дроспи» (таблетки, покрытые пленочной оболочкой, 3 мг+0,03 мг) соответственно в исследованиях биоэквивалентности обоснован следующим: препараты сравнения являются оригинальными препаратами по отношению к исследуемым препаратам (T); исследуемые препараты (T) и препараты сравнения (R) имеют одинаковую лекарственную форму; содержат равную дозу действующего вещества; вспомогательные вещества, входящие в состав сравниваемых препаратов, хорошо изучены и не должны оказывать влияния на фармакокинетику данных препаратов.
График исследований
Клиническая фаза исследований состояла из периода скрининга, двух периодов исследования, когда осуществлялся прием исследуемых препаратов (по 1 таблетке однократно), отбора образцов крови и «отмывочного» периода между периодами исследования. Длительность периода скрининга в исследованиях биоэквивалентности препаратов гестодена+этинилэстрадиол – 8 дней; длительность периода скрининга в исследованиях биоэквивалентности препаратов дроспиренона+этинилэстрадиол в дозировке 3 мг+0,03 мг – от 3 до 5 дней. Длительность каждого периода исследования – около 3,5 суток (приблизительно 84 ч). Длительность «отмывочного» периода – 28 суток от момента первого приема одного из исследуемых препаратов.
Этап скрининга включал набор добровольцев и их обследование с целью определения критериев включения/невключения. Каждый доброволец был госпитализирован в клинический центр для проведения первого периода исследования не позднее чем за 12 ч до момента приема одного из исследуемых препаратов. Госпитализация в первом периоде исследования длилась около 36 ч, после чего каждый доброволец был отпущен домой. Амбулаторные визиты осуществлялись через 36, 48 и 72 ч после приема препарата. После чего, в случае отсутствия показаний для медицинского наблюдения и при условии хорошего самочувствия, в случае исследования биоэквивалентности препаратов гестодена+этинилэстрадиол каждый доброволец был отпущен домой до проведения контрольного визита за 1 день до начала второго периода исследования.
В исследованиях биоэквивалентности препаратов дроспиренона+этинилэстрадиол добровольцы прибывали на амбулаторный визит в клинический центр за 1–3 дня до начала второго этапа исследования для сдачи промежуточных анализов крови и мочи (для оценки критериев включения/невключения), электрокардиографии (ЭКГ). В случае получения нормальных результатов анализов, при наличии критериев включения в отсутствие критериев невключения, добровольцы приглашались для госпитализации на второй этап. Процедуры второго периода исследования были идентичны таковым первого периода.
После проведения всех процедур второго периода исследования каждому добровольцу был проведен завершающий осмотр, затем исследование для добровольцев считалось завершенным.
На каждом этапе исследования, согласно схеме рандомизации, которая определяла последовательность приема исследуемого препарата (Т) и препарата сравнения (R), в исследованиях биоэквивалентности препаратов гестодена+этинилэстрадиол каждый доброволец принимал внутрь натощак 0,075 мг+0,02 мг гестодена+этинилэстрадиол (T) и 0,075 мг+0,02 мг препарата «Логест» (R) или 0,075 мг+0,03 мг гестодена+этинилэстрадиол (T) и 0,075 мг+0,03 мг препарата «Фемоден» (R); в исследовании биоэквивалентности препаратов дроспиренона+этинилэстрадиол каждый доброволец принимал внутрь натощак 3 мг+0,03 мг дроспиренона+этинилэстрадиол (T) и 3 мг+0,03 мг препарата «Ярина» (R).
На каждом этапе исследований биоэквивалентности препаратов гестодена+этинилэстрадиол и дроспиренона+этинилэстрадиол образцы крови для фармакокинетического анализа отбирались до приема исследуемых препаратов и препаратов сравнения и в определенных временных точках в течение 72 ч после приема препаратов.
Общая продолжительность исследования для добровольца в исследованиях биоэквивалентности препаратов гестодена+этинилэстрадиол составила 38 дней; общая продолжительность исследования для добровольца в исследованиях биоэквивалентности препаратов дроспиренона+этинилэстрадиол в дозировке 3 мг+0,03 мг – 33–35 дней.
Фармакокинетические параметры
Для каждого добровольца рассчитывались следующие фармакокинетические параметры, необходимые для оценки биоэквивалентности сравниваемых лекарственных препаратов: AUC0–72 – площадь под кривой «плазменная концентрация-время» с момента приема препарата до 72 ч; AUC0–∞ – площадь под кривой «плазменная концентрация-время» с момента приема препарата до бесконечности; Cmax – максимальная плазменная концентрация действующего вещества; Tmax – время достижения Cmax; T½ – период полувыведения из плазмы; f’ – относительная биодоступность исследуемого препарата по отношению к препарату сравнения AUC0-t(T)/AUC0-t(R); f’’ – относительная степень всасывания, определяемая отношением Cmax(T)/Cmax(R); Cmax/AUC – относительная скорость всасывания; kel – константа скорости терминальной элиминации; MRT – среднее время удерживания препарата в организме.
Биоаналитический метод
Для определения гестодена, дроспиренона и этинилэстрадиола в плазме крови здоровых добровольцев был использован биоаналитический метод высокоэффективной жидкостной хроматографии с тандемной масс-спектрометрией.
Статистический анализ данных
Для проверки гипотез о статистической значимости вклада различных факторов (различия между препаратами, различия между добровольцами, последовательность приема препаратов, периоды исследования) в наблюдаемую вариабельность применялся дисперсионный анализ. Расчет фармакокинетических параметров и статистический анализ полученных данных выполнены в предположении о логнормальном распределении параметров AUC и Cmax. Полученная с помощью дисперсионного анализа оценка остаточной вариации использовалась при расчете доверительного интервала для отношения средних геометрических значений соответствующего параметра.
Критерии оценки биоэквивалентности
Вывод о биоэквивалентности сравниваемых препаратов был сделан на основе оценки 90% доверительных интервалов для отношений средних геометрических значений параметров AUC0-t и Сmax исследуемых и референтных препаратов. После проведения логарифмического преобразования эти показатели анализировались с помощью дисперсионного анализа (ANOVA; параметрический метод). Полученные результаты остаточной вариации использовались при расчете доверительного интервала для отношения средних значений соответствующего параметра с обратным преобразованием из логарифмических в исходные единицы.
Препараты считались биоэквивалентными, если границы оцененного 90% доверительного интервала для отношений средних геометрических значений параметров AUC0-t и Cmax исследуемых и референтных препаратов гестодена+этинилэстрадиол и дроспиренона+этинилэстрадиол находились в пределах 80,00–125,00%.
Биоэквивалентность исследуемых препаратов считалась доказанной при подтверждении биоэквивалентности по обоим компонентам.
Результаты и обсуждение
В ходе трех исследований биоэквивалентности определение концентраций гестодена+этинилэстрадиол после однократного перорального приема сравниваемых препаратов в дозе 0,075 мг+0,02 мг или 0,075 мг+0,03 мг (1 таблетка) и дроспиренона+этинилэстрадиол после однократного перорального приема сравниваемых препаратов в дозе 3 мг+0,03 мг (1 таблетка) было проведено в плазме крови 29, 31 и 28 здоровых добровольцев женского пола соответственно.
В каждом периоде исследований, согласно схеме рандомизации, которая определяла последовательность приема исследуемого препарата (Т) и препарата сравнения (R), на основании полученных данных можно сделать вывод, что исследуемые препараты характеризуются высокой степенью сходства показателей фармакокинетики.
В исследовании биоэквивалентности препаратов гестодена+этинилэстрадиол после однократного перорального приема сравниваемых препаратов в дозе 0,075 мг+0,02 мг индивидуальные и усредненные профили фармакокинетических кривых гестодена и этинилэстрадиола исследуемого препарата и препарата сравнения имеют совпадающие формы (табл. 1).
На рисунках 1–4 представлены усредненные фармакокинетические профили концентрации гестодена и этинилэстрадиола в плазме крови добровольцев после однократного приема исследуемого препарата гестодена+этинилэстрадиол (T) и препарата сравнения «Логест» (R).
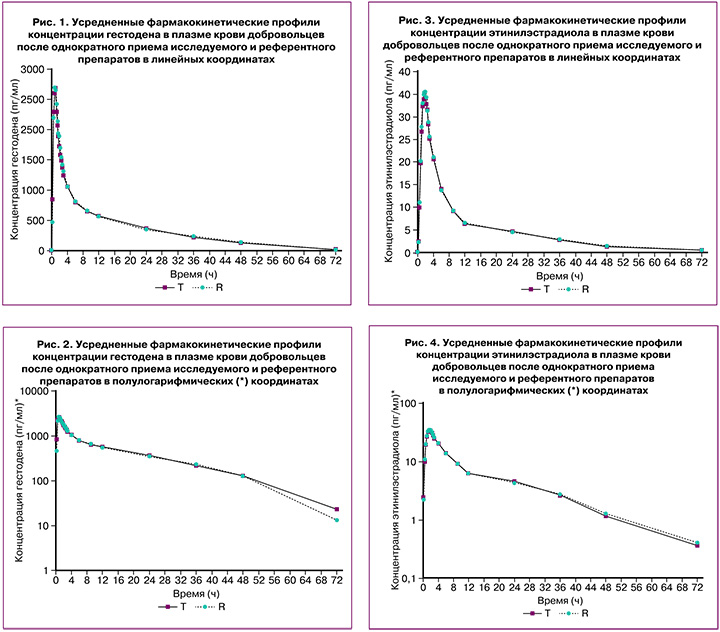
Препараты характеризуются близкими значениями относительной биодоступности и максимальной концентрации гестодена и этинилэстрадиола. Доверительные интервалы для отношений средних геометрических значений AUC0-72 и Cmax гестодена и этинилэстрадиола исследуемого и референтного препаратов полностью соответствуют допустимым пределам 80,00–125,00%.
Таким образом, препараты «ПланиЖенс гесто 20» (гестоден+этинилэстрадиол), таблетки, покрытые оболочкой, 0,075 мг+0,02 мг (ООО «Фармасинтез-Тюмень», Россия) и «Логест», таблетки, покрытые оболочкой, 0,075 мг+0,02 мг («Байер Фарма АГ», Германия), являются биоэквивалентными.
В исследовании биоэквивалентности препаратов гестодена+этинилэстрадиол после однократного перорального приема сравниваемых препаратов в дозе 0,075 мг+0,03 мг индивидуальные и усредненные профили фармакокинетических кривых гестодена и этинилэстрадиола исследуемого препарата и препарата сравнения имеют совпадающие формы (табл. 2).
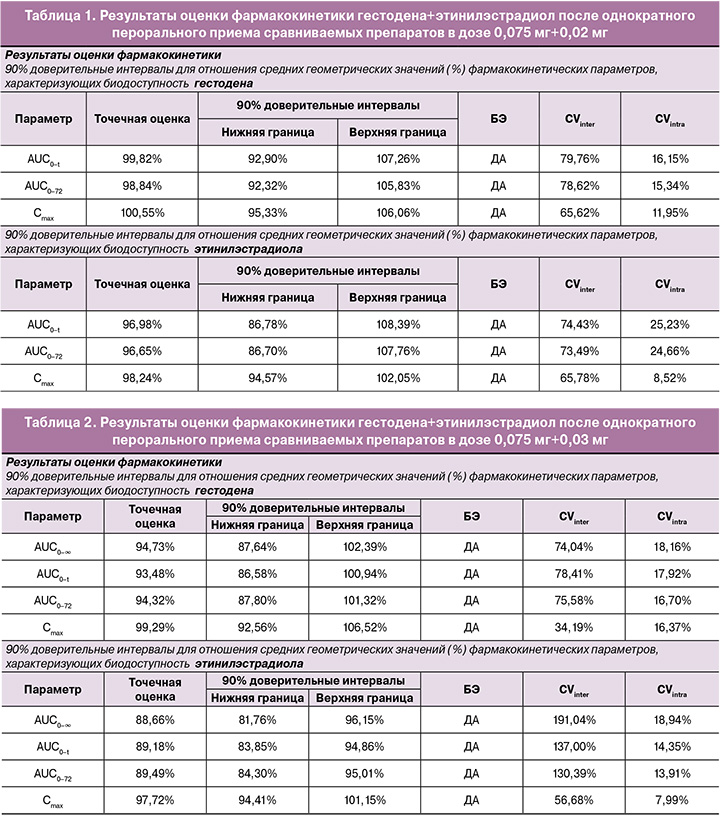
На рисунках 5–8 представлены усредненные фармакокинетические профили концентрации гестодена и этинилэстрадиола в плазме крови добровольцев после однократного приема исследуемого препарата гестодена+этинилэстрадиол (T) и препарата сравнения «Фемоден» (R).
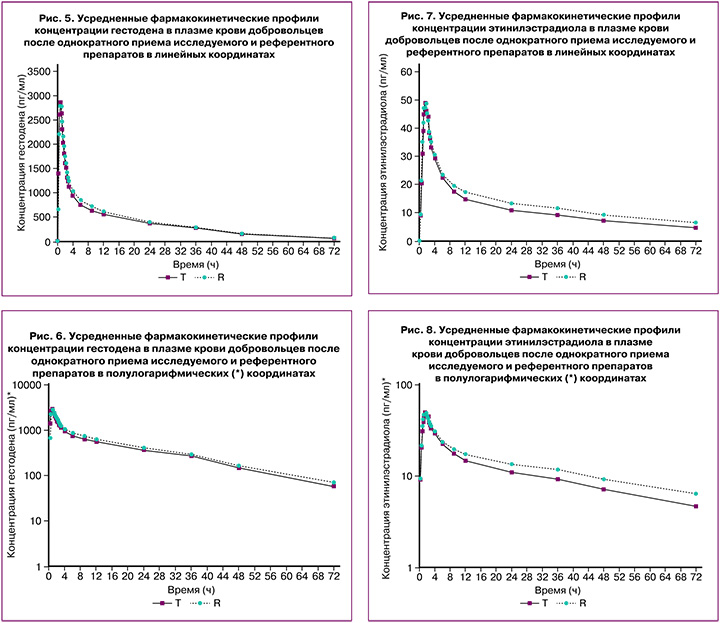
Препараты характеризуются близкими значениями относительной биодоступности и максимальной концентрации гестодена и этинилэстрадиола. Доверительные интервалы для отношений средних геометрических значений AUC0-72 и Cmax гестодена и этинилэстрадиола исследуемого и референтного препаратов полностью соответствуют допустимым пределам 80,00–125,00%.
Таким образом, препараты «ПланиЖенс гесто 30» (гестоден+этинилэстрадиол), таблетки, покрытые оболочкой, 0,075 мг+0,03 мг (ООО «Фармасинтез-Тюмень», Россия), и «Фемоден», таблетки, покрытые оболочкой, 0,075 мг+0,03 мг («Байер Фарма АГ», Германия), являются биоэквивалентными.
В исследовании биоэквивалентности препаратов дроспиренона+этинилэстрадиол после однократного перорального приема сравниваемых препаратов в дозе 3 мг+0,03 мг индивидуальные и усредненные профили фармакокинетических кривых дроспиренона и этинилэстрадиола исследуемого препарата и препарата сравнения имеют совпадающие формы (табл. 3).
На рисунках 9–11 представлены усредненные фармакокинетические профили концентрации дроспиренона и этинилэстрадиола в плазме крови добровольцев после однократного приема исследуемого препарата дроспиренона+этинилэстрадиол (T) и препарата сравнения «Ярина» (R).
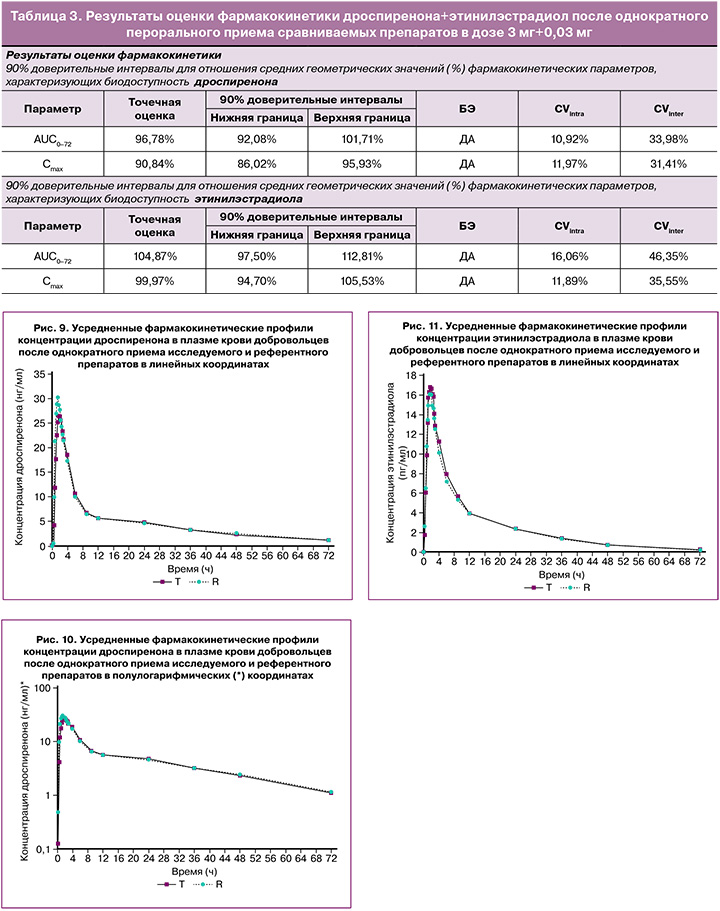
На основании полученных данных можно констатировать, что исследуемые препараты характеризуются высокой степенью сходства показателей фармакокинетики. Индивидуальные и усредненные профили фармакокинетических кривых дроспиренона и этинилэстрадиола тестируемого и референтного препаратов имеют совпадающие формы. Препараты характеризуются близкими значениями относительной биодоступности и максимальной концентрации дроспиренона и этинилэстрадиола. Доверительные интервалы для отношений средних геометрических значений AUC0-72 и Cmax дроспиренона и этинилэстрадиола тестируемого и референтного препаратов полностью соответствуют допустимым пределам 80,00–125,00%.
Таким образом, препараты «ПланиЖенс дроспи» (дроспиренон+этинилэстрадиол), таблетки, покрытые пленочной оболочкой, 3 мг+0,03 мг (ООО «Фармасинтез-Тюмень», Россия), и «Ярина», таблетки, покрытые пленочной оболочкой, 3 мг+0,03 мг («Байер Фарма АГ», Германия), являются биоэквивалентными.
Оценка безопасности
Данные по безопасности анализировались на основании следующих показателей:
- клинический осмотр;
- измерение жизненных показателей: артериальное давление, частота сердечных сокращений (ЧСС), частота дыхательных движений и температура тела;
- лабораторные обследования по окончании второго периода исследования;
- мониторинг нежелательный явлений (НЯ)/серьезных НЯ.
В исследовании биоэквивалентности препаратов гестодена+этинилэстрадиол при однократном применении их в дозе 0,075 мг+0,02 мг после приема исследуемого препарата гестодена+этинилэстрадиол зарегистрировано 3 НЯ: головная боль, острая респираторная вирусная инфекция (ОРВИ), головокружение. После приема препарата сравнения «Логест» зарегистрировано 2 НЯ: головная боль и ОРВИ. В 3 случаях (головокружение, головная боль – 2 случая) связь НЯ с приемом препарата отмечена как сомнительная (маловероятная), в 2 случаях (ОРВИ) – связь отсутствует. В 2 случаях НЯ (ОРВИ) потребовалось назначение дополнительной терапии. Исходом всех НЯ являлось разрешение без последствий для здоровья.
В исследовании биоэквивалентности препаратов гестодена+этинилэстрадиол при однократном применении их в дозе 0,075 мг+0,03 мг после приема исследуемого препарата гестодена+этинилэстрадиол зарегистрировано 7 НЯ: артериальная гипотензия – 4 случая, головная боль – 2 случая, рвота – 1 случай. После приема препарата сравнения «Фемоден» зарегистрировано 5 НЯ: артериальная гипотензия – 4 случая, головная боль – 1 случай. Во всех случаях связь НЯ с приемом препарата отмечена как возможная. Все НЯ разрешились самостоятельно, дополнительных мер не предпринималось.
В исследовании биоэквивалентности препаратов дроспиренона+этинилэстрадиол при однократном применении их в дозе 3 мг+0,03 мг после приема исследуемого препарата дроспиренона+этинилэстрадиол возникло 32 НЯ: анемия – 1, артериальная гипотензия – 1, герпетические высыпания на губах – 1, головокружение – 1, головная боль – 9, лимфопения – 1, межменструальное кровотечение – 8, моноцитоз – 1, нейтропения – 1, неполная блокада правой ножки пучка Гиса – 1, повышенное количество незрелых гранулоцитов в общем анализе крови – 1, рвота однократно – 2, тошнота – 3, повышение скорости оседания эритроцитов – 1. После приема препарата «Ярина» возникло 8 НЯ: головная боль – 1, задержка менструального цикла – 1, затруднение носового дыхания – 1, межменструальное кровотечение – 1, тошнота – 1, тромбоцитоз – 1, умеренные изменения реполяризации миокарда в области верхушки левого желудочка по ЭКГ – 1, учащение ЧСС – 1. После приема исследуемого препарата дроспиренона+этинилэстрадиол НЯ имели связь с препаратом: вероятную – в 7 случаях, возможную – в 17 случаях, сомнительную – в 2, а отсутствие связи – в 6 случаях. После приема препарата «Ярина» НЯ имели связь с препаратом: вероятную – в 3 случаях, возможную – в 4, сомнительную – в 1. В 1 случае исход НЯ неизвестен (неполная блокада правой ножки пучка Гиса), во всех остальных случаях исходом НЯ было разрешение без последствий. Действий в отношении НЯ не предпринималось.
Во всех исследованиях биоэквивалентности препаратов гестодена+этинилэстрадиол и дроспиренона+этинилэстрадиол во всех случаях тяжесть НЯ оценена как легкая. Серьезных НЯ зарегистрировано не было. Случаев беременности выявлено не было.
По результатам исследования можно сделать вывод о схожем хорошем профиле безопасности исследуемых препаратов.
Заключение
Границы оцененных 90% доверительных интервалов для отношений средних геометрических значений фармакокинетических параметров AUC0-t и Cmax исследуемых и референтных препаратов гестоден+этинилэстрадиол и дроспиренон+этинилэстрадиол находятся в пределах 80,00–125,00%. Эти данные подтверждают, что лекарственные препараты «ПланиЖенс гесто 20», таблетки, покрытые оболочкой, 0,075 мг+0,02 мг (ООО «Фармасинтез-Тюмень», Россия), и «Логест», таблетки, покрытые оболочкой, 0,075 мг+0,02 мг («Байер Фарма АГ», Германия); «ПланиЖенс гесто 30», таблетки, покрытые оболочкой, 0,075 мг+0,03 мг (ООО «Фармасинтез-Тюмень», Россия), и «Фемоден», таблетки, покрытые оболочкой, 0,075 мг+0,03 мг («Байер Фарма АГ», Германия); «ПланиЖенс дроспи», таблетки, покрытые оболочкой, 3 мг+0,03 мг (ООО «Фармасинтез-Тюмень», Россия), и «Ярина», таблетки, покрытые пленочной оболочкой, 3 мг+0,03 мг («Байер Фарма АГ», Германия), биоэквивалентны.
Исследуемые препараты обладают сопоставимыми профилями безопасности в сравнении с оригинальными препаратами.



