За последнее десятилетие эффективные и безопасные методы витрификации, наряду с увеличением количества циклов «замораживания всех», способствовали заметному увеличению числа циклов переноса замороженных эмбрионов (FET, frozen embryo transfer) во всем мире [1]. В цикле переноса размороженного эмбриона были выявлены более благоприятные исходы по увеличению числа живорождений, снижению частоты осложнений беременности за счет адекватной рецептивности эндометрия и возможности проведения переноса эмбрионов после предимплантационного генетического тестирования [2, 3].
Экзогенный микронизированный прогестерон, который играет важную роль в репродуктивной функции женщин, все чаще применяется в программах вспомогательных репродуктивных технологий (ВРТ) – как в стимулированных циклах экстракорпорального оплодотворения (ЭКО), так и в циклах заместительной гормональной терапии (ЗГТ) при подготовке эндометрия к переносу размороженных эмбрионов [4]. В циклах ЗГТ прогестерон можно вводить перорально, интравагинально или внутримышечно. Интравагинальное применение препаратов прогестерона в виде капсул, геля или таблеток – наиболее распространенный способ поддержки лютеиновой фазы [5]. Основное обоснование преимущества интравагинального применения заключается в том, что прогестерон при нанесении геля или при введении во влагалище капсулы/таблетки будет абсорбироваться слизистой оболочкой влагалища и поступать в лимфатические сосуды посредством механизма, называемого «эффектом первого прохода через матку», при котором быстро достигается эффективная концентрация в эндометрии [6]. Благодаря этому механизму не ожидается, что в середине лютеиновой фазы концентрация прогестерона в сыворотке значительно повысится из-за его местного действия.
Согласно крупному исследованию, проведенному в 2014 г. с участием 82 центров во всем мире, вагинальный прогестерон был наиболее предпочтительным препаратом в программах ВРТ [7].
Учитывая риск возможного дефицита лекарственных препаратов зарубежного производства, государственную направленность на импортозамещение, сравнимую эффективность зарубежных воспроизведенных препаратов микронизированного прогестерона, применяемых в программах ВРТ [8–11], с 2022 г. на российском фармацевтическом рынке появился отечественный воспроизведенный препарат микронизированного прогестерона.
Доказательной базы терапевтической эквивалентности воспроизведенного препарата микронизированного прогестерона российского производства по сравнению с применением оригинального препарата для посттрансферной поддержки после переноса размороженных эмбрионов в настоящее время нет. В связи с этим важной задачей является подтвердить терапевтическую эквивалентность отечественного препарата в программах ВРТ.
Цель исследования: сравнительный анализ эффективности программ переноса размороженных эмбрионов и исходов беременностей при интравагинальном применении воспроизведенного и оригинального препаратов микронизированного прогестерона с целью секреторной трансформации эндометрия и посттрансферной поддержки.
Материалы и методы
Была проведена ретроспективная оценка исходов программ переноса размороженных собственных эмбрионов в цикле ЗГТ в период с января 2023 г. по май 2023 г. на базе отделения вспомогательных репродуктивных технологий ФГБУ «НИИ ОММ» Минздрава России.
В исследование были включены 93 женщины с диагнозом «женское бесплодие», которым с целью подготовки эндометрия к переносу размороженных эмбрионов были назначены гормональные препараты: для достижения пролиферативных изменений эндометрия – эстрогены (эстрадиола валерат в дозировке 6 мг/сут со 2–4-го дня менструального цикла). При достижении М-эхо эндометрия более 7 мм с целью формирования секреторной трансформации и децидуализации эндометрия 45 пациенткам 1-й группы был назначен интравагинально воспроизведенный препарат микронизированного прогестерона «ДляЖенс про» российского производства в дозе 600 мг/сут до 12 недель беременности; 48 пациенткам 2-й группы назначен интравагинально оригинальный препарат микронизированного прогестерона 600 мг/сут до 12 недель беременности.
Основные показатели эффективности программы ВРТ: частота имплантации (содержание хорионического гонадотропина человека более 15 МЕ/мл в сыворотке крови на 10-й день после переноса эмбрионов), наличие клинической беременности (по результатам ультразвукового исследования). Проведена оценка частоты наступления многоплодных беременностей при переносе размороженных эмбрионов.
Статистический анализ
Cтатистическую обработку результатов исследований проводили с использованием пакетов прикладных программ Microsoft Excel (2010), Stat Soft Statistica 6.0 (Stat Soft, США), SPSS Statistics версия 22.0 (IBM Microsoft, США). Оценку соответствия выборки нормальному распределению проводили с использованием критерия Колмогорова–Смирнова. В случае подчинения признака закону нормального распределения данные представляли в виде средней величины (М) и стандартного отклонения (SD). При отклонении распределения признака от закона нормального распределения данные представляли в виде медианы (Ме) и нижнего и верхнего квартилей (25-го и 75-го процентилей, Р25–Р75).
Качественные признаки представляли в виде абсолютного значения и относительной величины в процентах; оценку статистической значимости осуществляли с использованием критерия хи-квадрат (χ2), если абсолютные частоты были меньше 10, то применяли поправку Йейтса. Нулевая гипотеза отклонялась при р<0,05. Для оценки первичных итоговых результатов каждого из изучаемых клинических исходов переноса размороженных эмбрионов был рассчитан размер эффекта показателем отношения шансов (OШ) с 95% доверительным интервалом (ДИ).
Результаты
В 1-й группе средний возраст пациенток составил 34,13 (5,13) года, во 2-й группе – 34,58 (6,63) года, демографические показатели были сравнимы в исследуемых группах (р=0,464).
В результате анализа анамнестических данных не было выявлено статистически значимых различий по структуре соматической патологии (p>0,05). В обеих группах ведущее место занимают доброкачественные заболевания молочной железы: 12/45 – в 1-й группе, что составляет 26,67%, и 5/48 (10,42%) – во 2-й группе. На втором месте по частоте встречаемости соматической патологии – заболевания эндокринной системы, расстройства питания и нарушения обмена веществ: 11/45 – в 1-й группе, что составляет 24,4%, и 8/48 (16,67%) – во 2-й группе. Болезни желудочно-кишечного тракта в 1-й группе встречались у 5/45 (11,11%) пациенток, во 2-й группе – у 2/48 (4,17%) (табл. 1).
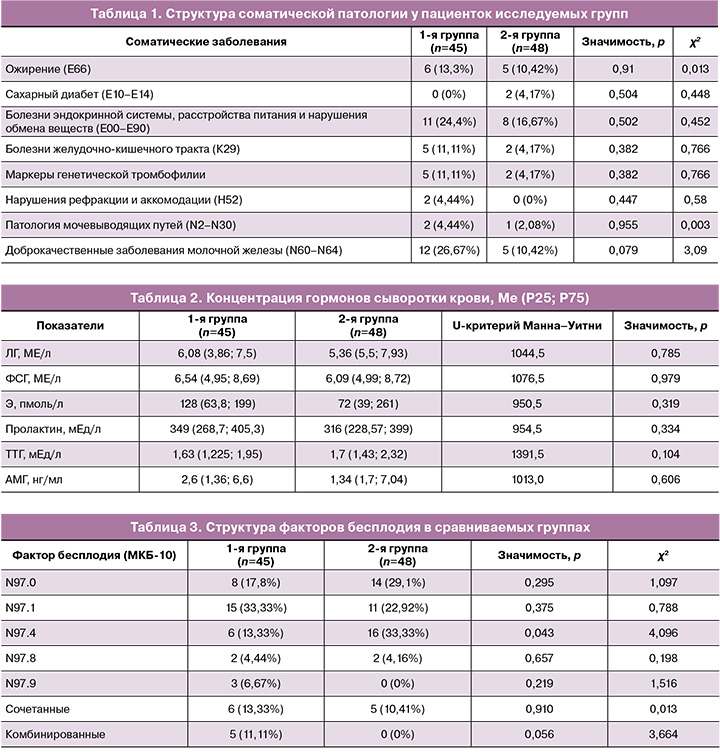
Гормональный статус всех пациенток был исследован на содержание следующих гормонов: лютеинизирующий гормон (ЛГ), фолликулостимулирующий гормон (ФСГ), эстрадиол (Э), тиреотропный гормон (ТТГ), пролактин, антимюллеров гормон (АМГ). Статистически значимых различий в уровнях гормонов, характеризующих репродуктивную функцию пациенток, не выявлено (табл. 2).
В ходе работы проанализирована структура бесплодия в каждой группе пациенток (табл. 3).
Наиболее частым фактором бесплодия у пациенток 1-й группы, применяющих воспроизведенный препарат микронизированного прогестерона для поддержки лютеиновой фазы, являлось женское бесплодие трубного происхождения (N97.1) – 15/45 (33,33%); однако статистических различий в частоте встречаемости во 2-й группе не было выявлено. Во 2-й группе у супружеских пар наиболее частой формой являлось бесплодие, связанное с мужским фактором (N97.4), – 16/48 (33,33%), что статистически значимо различалось с частотой данной формы бесплодия в 1-й группе – 6/45 (13,33%) (р=0,043) (табл. 3).
Учитывая структуру бесплодия в обеих группах, их статистическую значимость по различиям в структуре, был проведен анализ эмбриологического этапа программ переноса размороженных эмбрионов в изучаемых группах. Статистически значимых различий в количестве переносимых эмбрионов у пациенток 1-й и 2-й групп не было выявлено. В группе применения воспроизведенного препарата микронизированного прогестерона в 26/45 (57,78%) случаев осуществляли перенос одного эмбриона, а в группе применения оригинального препарата микронизированного прогестерона – в 22/48 (45,83%) случаев (р=0,34); в остальных случаях проводили перенос двух размороженных эмбрионов в полость матки (табл. 4).
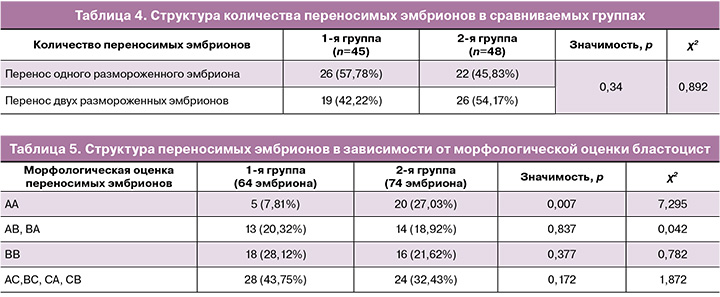
Переносимые эмбрионы были криоконсервированы в программах овариальной стимуляции ЭКО на 5-е или 6-е сутки развития и соответствовали стадии бластоцисты. Оценку качества бластоцист после разморозки проводили по системе оценки Gardner et al. (1999). При анализе структуры переносов эмбрионов по качеству бластоцист были выявлены особенности, представленные в таблице 5. Частота переносимых эмбрионов отличного и хорошего качества (АА, АВ, ВА, ВВ) в группе применения воспроизведенного препарата микронизированного прогестерона составила 36/64 (56,25%), во 2-й группе – 50/74 (67,57%) (р>0,05). При этом частота переноса эмбрионов отличного качества АА в группе применения оригинального препарата микронизированного прогестерона у пациенток 2-й группы была статистически значимо выше, чем в 1-й группе, и составила 20/74 (27,03%) (р=0,007). Остальные эмбрионы были удовлетворительного качества: в 1-й группе – 28/64 (43,75%) случаев, во 2-й – 24/74 (32,43%) (р=0,172).
Были оценены исходы программ ВРТ по частоте имплантаций (биохимические беременности) и наступлению клинических беременностей (ультразвуковая фиксация плодного яйца в полости матки) в исследуемых группах.
Данные, полученные в ходе анализа исходов программ переноса размороженных эмбрионов, показали сопоставимую эффективность применения воспроизведенного и оригинального препаратов микронизированного прогестерона по частоте имплантаций и наступления клинической беременности. В 1-й группе частота имплантаций составила 22/45 (48,89%), у пациенток 2-й группы, применявших оригинальный препарат микронизированного прогестерона, – 21/48 (43,75%) (р=0,62; ОШ=0,813; ДИ 0,359–1,84). У пациенток, применявших воспроизведенный препарат микронизированного прогестерона, частота наступления клинических беременностей, зафиксированных через 21 день после переноса эмбрионов, была выше, чем у пациенток во 2-й группе при применении оригинального препарата микронизированного прогестерона, и составила 19/45 (42,22%); однако статистических различий не было выявлено (р=0,796; ОШ=0,897; ДИ 0,329–2,051). В обеих группах не были зафиксированы случаи внематочных беременностей.
Учитывая, что перенос двух эмбрионов проводился более чем в 40% случаев, была проведена оценка наступления многоплодных беременностей.
Статистически значимых различий в частоте наступления многоплодных беременностей в обеих группах не было выявлено (р=0,462; ОШ=0,578; ДИ 0,133–2,506). В 1-й группе у 4/19 (21%) пациенток были выявлены многоплодные маточные беременности, одна из них –монохориальная двойня. Во 2-й группе у пациенток, которые применяли оригинальный препарат микронизированного прогестерона, частота многоплодных беременностей составила 6/19 (31,6%), в 2 случаях были монохориальные двойни.
Заключение
Проведенное исследование было направлено на оценку эффективности программ переноса размороженных эмбрионов в зависимости от применения воспроизведенного и оригинального препаратов микронизированного прогестерона, который назначался пациенткам для формирования секреторной трансформации, децидуализации эндометрия и посттрансферной поддержки.
Данные, полученные в исследовании, по исходам программ переноса размороженных эмбрионов показали сопоставимую эффективность воспроизведенного и оригинального препаратов микронизированного прогестерона при интравагинальном применении.
Проведенное исследование демонстрирует возможность интравагинального применения как оригинального, так и воспроизведенного препаратов микронизированного прогестерона российского производства в циклах ЗГТ для подготовки эндометрия к переносу размороженных эмбрионов в циклах ВРТ. Это приобретает особую важность в условиях импортозамещения. Результаты нашего исследования показали необходимость продолжения накопления знаний о применении воспроизведенного препарата микронизированного прогестерона в программах ВРТ, а также оценки исходов и осложнений беременностей у пациенток с бесплодием.



