Процесс родовой деятельности инициируется созреванием органов и систем плода в промежутке между 37 и 40 неделями неосложненной беременности. В целом, самопроизвольное своевременное начало родов – это состояние физиологического воспаления, обусловленное взаимодействием гормональных и механических факторов и координируемое посредством фето-плацентарной коммуникации [1].
Традиционные теории передачи сигналов об инициации родов связаны с эндокринными и иммунологическими изменениями со стороны плода, которые коррелируют с его ростом и развитием [2]. Дисбаланс, вызванный этими изменениями, может привести к воспалительной нагрузке, которая нарушает поддержание беременности [3]. Помимо плода, плодные оболочки и плацента способствуют инициации сигналов, приводящих к началу родов. Рядом авторов было описано, что старение плаценты и плодных оболочек вызывает «стерильное воспаление», которое также может увеличивать воспалительную нагрузку в миометрии и служить триггером родов [4].
Коммуникация между плодом и матерью, осуществляемая с участием внеклеточных везикул, в частности, экзосом, имеет фундаментальное значение для беременности [5]. Экзосомы являются межклеточными медиаторами сигналов и несут определенный молекулярный груз, включая различные биоактивные молекулы (белки, липиды, РНК), которые доставляются в клетки-мишени для последующей регуляции биологических функций [6].
В составе экзосом находятся также малые некодирующие молекулы – микроРНК (мкРНК) – эпигенетические модуляторы значительного числа биологических процессов [7]. В ряде исследований было продемонстрировано, что изменения экспрессии тканевых и циркулирующих материнских мкРНК могут отражать метаболическое состояние матери и использоваться в качестве биомаркеров осложнений беременности, таких как преэклампсия, гестационный сахарный диабет, задержка роста плода [8–10]. Кроме того, определена роль мкРНК в регуляции сократимости миометрия во время родов [11].
Известно, что экзосомы преодолевают гематоэнцефалический барьер, проникая через несколько слоев клеток [12]. Данное открытие может указывать на их непосредственное перемещение во время беременности от матери к плоду, и наоборот, что может свидетельствовать о существовании пока не изученных механизмов фето-материнской коммуникации.
В данном обзоре будут рассмотрены механизмы инициации родовой деятельности, в частности, воспалительная реакция в миометрии, механическое растяжение матки, передача сигналов от плода, старение плодных оболочек, а также роль мкРНК в их реализации.
Воспалительная реакция в миометрии – триггер родовой деятельности
Воспалительная реакция обычно связана с инфильтрацией лейкоцитами и продукцией цитокинов, которые модулируют функцию клеток через изменение экспрессии генов. Это тщательно скоординированный процесс, предназначенный для защиты организма от инфекции [13]. Однако, воспаление может быть вызвано воздействием других агентов, включая химические вещества и поврежденные клетки.
Воспаление, развивающееся в миометрии на доношенном сроке беременности играет физиологическую роль, которая заключается в переводе рефрактерного миометрия в состояние сократимости [14], запускает цепочку молекулярных событий, ведущих к снижению функции рецепторов прогестерона [15].
Воспалительная реакция характеризуется увеличением уровня провоспалительных цитокинов в амниотической жидкости и инвазией макрофагами/нейтрофилами плодных оболочек, шейки матки и миометрия [16]. Секреция провоспалительных цитокинов и хемокинов инвазирующими иммунными клетками [17] вызывает активацию ядерного транскрипционного фактора NF-kB, а также других факторов транскрипции, связанных с воспалением, в частности, AP-1 (активирующий белок-1) [18,19]. NF-kB является универсальным фактором транскрипции, который контролирует экспрессию генов иммунного ответа, апоптоза и клеточного цикла. В свою очередь, активированные факторы транскрипции способствуют повышенной экспрессии в миометрии генов провоспалительных цитокинов, таких как интерлейкин (IL)-1β, IL-8, а также коннексина-43 (CX43/GJA1), рецептора окситоцина (OXTR) и циклооксигеназы 2 (COX-2/PTGS2), что в итоге приводит к родам. Кроме того, NF-kB, активируя провоспалительные гены, способствует снижению функции прогестеронового рецептора (PR), что также запускает каскад событий, инициирующих родовую деятельность [20].
Эстрогены вызывают приток макрофагов и нейтрофилов в матку, нейтрализуя противовоспалительное действие прогестерона, действующего через PR [21]. Снижение функции PR может быть связано со следующими факторами: 1) снижение коактиваторов PR [22]; 2) повышенная экспрессия ингибирующих и усеченных форм PR [19]; 3) антагонистическое взаимодействие PR с фактором транскрипции NF-κB [23]; 4) усиление местного метаболизма прогестерона до неактивных продуктов [23]. Важно отметить, что повышенные уровни циркулирующего эстрадиола-17β и повышенная активность рецептора эстрогена-α к доношенному сроку беременности также способствуют каскаду провоспалительных событий, снижающих функцию PR и инициирующих начало родовой деятельности [24].
Механическое растяжение матки – триггер провоспалительных сигналов, ведущих к родовой деятельности
Растяжение матки, вызванное растущим плодом, является важным стимулом для начала родовой деятельности [25, 26]. В экспериментах на животных моделях была обнаружена повышенная экспрессия β-хемокина, моноцитарного хемотаксического протеина-1 (MCP-1), активирующего макрофаги, индукцию которого связывают с механическим растяжением матки [25]. Кроме того, показано, что экспрессия MCP-1 и инфильтрация макрофагами значительно увеличены в матке при индукции родов антагонистом прогестерона мифепристоном и ингибированы обработкой прогестином с целью задержки родов [25]. У женщин, вступивших в роды, повышена экспрессия MCP-1 в миометрии, по сравнению с женщинами без родовой деятельности [14].
Передача сигналов от созревающих органов и систем плода
На доношенном сроке беременности сигналы от плода, в частности, повышенная секреция липопротеинов сурфактанта, кортизола и эстрогена, вызывают усиление инвазии иммунных клеток в плодные оболочки и миометрий.
Сурфактантный белок (SP-A) является одной из сигнальных молекул, секретируемых плодом в амниотическую жидкость, который накапливается в повышенных количествах на поздних сроках беременности [27]. Было продемонстрировано, что продуцирование легкими плода SP-A и фактора активации тромбоцитов (PAF) приводит к увеличению их концентрации в околоплодных водах, что способствует высвобождению простагландинов из плодных оболочек и активации NF-kB в миометрии матери [28]. В результате этих паракринных эффектов повышается локальная экспрессия материнских генов, связанных с сокращением миометрия. То есть сигнал, исходящий от плода, способен изменять эндокринную ось матери, вызывая подавление прогестеронового влияния по мере приближения доношенного срока беременности и инициировать родовую деятельность [29].
Помимо SP-A и PAF, продуцируемых легкими, в качестве сигналов -активаторов воспалительных каскадов рассматриваются молекулы, связанные с созреванием других органов и систем плода, в частности, кортикотропин рилизинг-гормон, эндотелин-1, эпидермальный фактор роста (EGF), трансформирующий фактор роста (TGF), коактиваторы стероидных рецепторов 1 и 2, которые высвобождаются в амниотическую жидкость [30].
Физиологическое старение плодных оболочек
Согласно данным Menon R., главным пусковым стимулом нормальных родов является физиологическое старение плодных оболочек, что на доношенном сроке беременности рассматривается, как физиологическая реакция на окислительный стресс, возникающий в результате повышенных метаболических потребностей созревающего плода [31].
Рост плодных оболочек во время беременности тесно связан с эмбриогенезом и ростом плода. Прогрессирующее по мере роста деление клеток плодных оболочек приводит к клеточному старению, зависимому от длины теломер – концевых участков хромосом и биологических маркеров старения [32]. С прогрессированием беременности уменьшение их длины в плодных оболочках обратно коррелирует с ростом плода и его созреванием [33]. Старение клетки часто ассоциируется со стерильным воспалением, которое относится к секреторному фенотипу, связанному со старением (Senescence-Associated Secretory Phenotype, SASP) [34]. Стареющие клетки оболочек плода выполняют свою конечную функцию, распространяя сигналы, активирующие децидуальный слой матки, что приводит к отмене влияния прогестерона и генерации воспалительных сигналов в миометрии, тем самым инициируя его сокращение и созревание шейки матки [35].
Помимо SASP, клеточное повреждение, связанное со старением, приводит к производству DAMP (Damage-Аssociated Molecular Patterns). DAMP являются производными эндогенных молекул, количество которых увеличивается после клеточного или окислительного стресса, повреждения тканей; DAMP стимулируют врожденный иммунитет и инициируют стерильное воспаление [36, 37]. DAMP в составе внеклеточных везикул, в частности, экзосом из стареющих плодных оболочек действуют, как сигналы, исходящие от созревающего плода, способствуя воспалению и увеличению воспалительной нагрузки в других внутриматочных компартментах, подготавливая их к родам. В число DAMP входят: белок из группы ядерных негистоновых белков HMGB1 (high-mobility group protein B1), мочевая кислота, белок S100 (семейство, состоящее из 25 членов), интерлейкин IL-33, гистон Н3, белок теплового шока (heat-shock protein, HSP 70), фрагменты теломер и внеклеточной ДНК плода из плодных оболочек [32]. Исследованиями Menon R. на плодных оболочках (клетки амниона и хориона) было показано, в частности, что HMGB1 и компоненты внеклеточной ДНК плода индуцируют старение фетальных клеток, опосредованное стресс-ассоциированной митоген-активированной киназой p38 (MAPK) для усиления воспалительной нагрузки в ткани плода, что, в свою очередь, может способствовать изменениям, связанным с родами [32]. Следует отметить, что в зависимости от модификаций и окислительно-восстановительного состояния, HMGB1, действуя через свои рецепторы, может, как активировать воспалительные каскады, так и оказывать влияние на секрецию противовоспалительных цитокинов [38]. p38 MAPK может вызывать функциональную отмену прогестерона в клетках миометрия, а DAMP, высвобождаемые из стареющих плодных оболочек, могут обеспечивать воспалительную среду, необходимую для активации p38 MAPK в миоцитах. Таким образом, стареющие оболочки плода высвобождают DAMP и SASP, которые в петле обратной связи могут увеличивать собственное повреждение, а также приводить к изменениям в миометрии, связанным с началом родовой деятельности [32] (рис. 1).
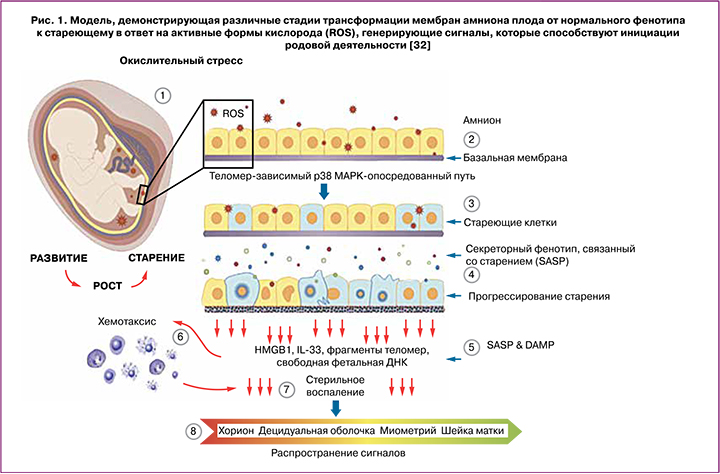
Экзосомальный сигналинг между матерью и плодом. Роль мкРНК в регуляции покоя и сократимости миометрия
Коммуникация между матерью и плодом осуществляется с участием внеклеточных везикул, в частности, экзосом. Последние содержат уникальный клеточно-специфический молекулярный груз донорской клетки, включая различные белки, РНК, липиды, а также мкРНК [39]. Последние являются малыми эндогенными некодирующими молекулами, длиной примерно 18–22 нуклеотида, которые регулируют экспрессию генов на посттранскрипционном уровне [7] и, что особенно важно, могут циркулировать во внеклеточных жидкостях в составе микровезикул, апоптотических телец, экзосом, выступая в роли медиаторов межклеточных взаимодействий и терапевтических мишеней при плаценто-ассоциированных осложнениях [40].
В последнее время активно изучается роль мкРНК в патогенезе осложнений, возникающих во время беременности, преждевременных и самопроизвольных родах. Кроме того, они рассматриваются в качестве важных регуляторов, принимающих участие в переходе миометрия из рефрактерного в состояние сократимости [11, 23, 41, 42]. В частности, во время инициации родовой деятельности в миометрии повышается экспрессия членов семейства miR-200 (miR-200b/200a/429, miR-141/200c), которые ингибируют соответствующие гены-мишени – транскрипционные факторы ZEB1 и ZEB2 [41]. При этом, высокие уровни прогестерона во время беременности поддерживают повышенную экспрессию ZEB1, что способствует подавлению экспрессии miR-200 [41]. Соответствующее снижение уровня прогестерона и функции PR к моменту наступления родов, а также увеличение активности циркулирующего эстрадиола и рецепторов эстрогенов-α вызывает подавление экспрессии ZEB1 и индукцию экспрессии семейства miR-200, дополнительно ингибирующего уровни ZEB1 и ZEB2, что приводит к активации генов, ответственных за сокращение миометрия, – OXTR и GJA1. Кроме того, miR-200a принимает участие в усилении местного метаболизма прогестерона до неактивных продуктов и в снижении функции PR в миометрии во время перехода к началу родовой деятельности [23].
Известно также, что miR-199a-3p и miR-214 принимают участие в поддержании состояния покоя миометрия путем подавления COX-2 и выработки сократительных простагландинов, поскольку COX-2 катализирует синтез простагландинов в амнионе, действуя как мощный активатор сократительной способности матки и последующего инициирования родовой деятельности [43]. Следует отметить, что ZEB1 способствует поддержанию покоя миометрия посредством подавления экспрессии семейства miR-200 [44] и усиления экспрессии miR-199a-3p/miR-214. Подавление экспрессии ZEB1 и снижение функции PR к доношенному сроку беременности вызвано повышенной активностью эстрогеновых рецепторов-α и усиленной воспалительной реакцией в миометрии. В свою очередь, это приводит к снижению экспрессии miR-199a/miR-214 и повышению COX-2, вызывая дальнейшее усиление воспалительной реакции и активацию родовой деятельности [11] (рис. 2).
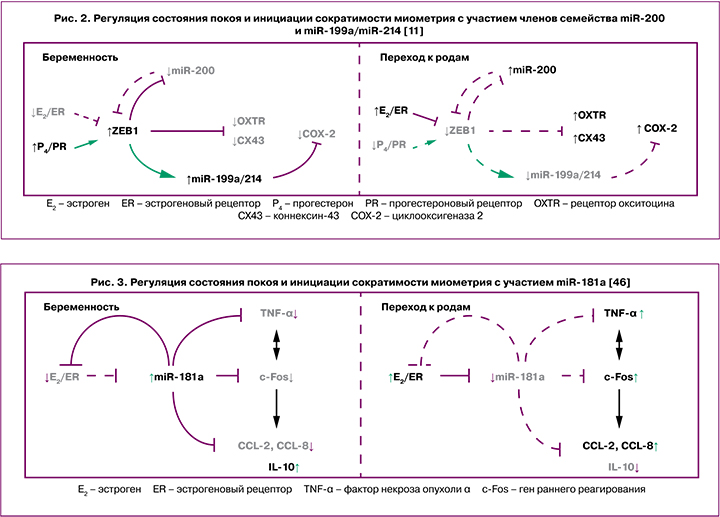
Одним из ключевых семейств мкРНК, которому отводится важная роль в регуляции воспалительных реакций в различных типах клеток, является miR-181 (miR-181a, miR-181b, miR-181c и miR-181d) [45]. Предполагается, что повышенная продукция эстрогена может активировать ряд провоспалительных механизмов в миометрии, частично опосредованных репрессией miR-181a. Снижение экспрессии miR-181a способствует повышению эстрогенового рецептора-α, который является ее непосредственной мишенью. На поздних сроках беременности, по мере увеличения уровня фактора некроза опухоли (TNF-α) и других провоспалительных цитокинов, активации генов раннего реагирования (c-Fos), которые, в свою очередь, также повышают экспрессию провоспалительных и сократительных генов, являющихся мишенями miR-181a, ее экспрессия значительно снижается [46] (рис. 3).
В исследованиях Li H. et al. была показана прямая зависимость между уровнем экспрессии эстрадиола, его ключевой мишени c-Fos, связанной с ним COX-2, и miR-144 в амнионе [47]. Низкий уровень эстрадиола до наступления доношенного срока беременности приводит к подавлению экспрессии c-Fos, что, в свою очередь, способствует снижению экспрессии COX-2 и miR-144. Усиленный воспалительный ответ к сроку своевременных родов, вызванный сигналами как от плода, так и от матери, приводит к увеличению локального уровня эстрадиола, который индуцирует повышение экспрессии miR-144 и COX-2. При этом, с целью предотвращения преждевременных родов повышенная экспрессия miR-144 частично супрессирует COX-2, что способствует небольшому подавлению секреции простагландина E2 [47].
В ряде исследований, посвященных изучению молекулярных факторов инициации родовой деятельности, показана также роль других мкРНК, в частности, miR-143 [48], miR-34b/с, miR-338, что указывает на их потенциальное взаимодействие в гормональном контроле, регуляции покоя и сократимости миометрия во время беременности и родов [49].
Заключение
Таким образом, к повышению сократимости миометрия и инициации родовой деятельности ведет сложный многофакторный процесс, тонко настраиваемый и координируемый посредством множества сигнальных молекул, секретируемых организмами матери и плода.



