Evaluation of endometrial receptivity using the level of small non-coding RNAs in uterine aspirate from women undergoing cyclic hormone therapy
Modern methods of diagnosing endometrial receptivity which have been introduced into clinical practice (ERA, Igenomix; ER-Map, iGLS; ERPeak, CooperGenomics), are based on transcriptome analysis of invasively obtained endometrial tissue; therefore, embryo cannot be transferred in the same cycle when endometrial biopsy is performed. Objective: To evaluate endometrial receptivity by determining the number of small non-coding RNAs (ncRNAs) in uterine fluid (UF) on the day of cryopreserved embryo (CE) transfer in women receiving cyclic hormone therapy (CHT) and to develop a logistic regression model for calculating the optimal endometrial receptivity for embryo implantation by comparing the UF transcriptome from patients with positive and negative outcomes of the ART program. Materials and methods: The study included 54 women whose UF was aspirated in a volume of 5–50 µl depending on the level of its secretion immediately before the CE transfer with the help of a catheter (COOK, Australia). Small ncRNAs isolated from UF with the miRNeasy Serum/Plasma Kit (Qiagen) were analyzed using deep sequencing on the NextSeq 500/550 platform (Illumina, USA); the obtained data were subsequently validated with real-time quantitative PCR using the miScript II RT Kit and miScript SYBR Green PCR Kit (Qiagen, Hilden, Germany). Results: The UF samples were classified into two groups: receptive endometrium and non–receptive endometrium depending on the results of the ART program (the presence and absence of implantation, respectively). Seven logistic regression models were developed on the basis of the small ncRNAs profile in the UF samples and endometrial thickness at the time of embryo transfer into the uterine cavity. The most accurate model appears to be the combination of content of miR-1180-3p in UF and endometrial thickness (71% sensitivity, 88% specificity) due to the lack of dependence of the variables using Spearman correlation analysis (r=0.02, p=0.9) and the statistical significance of all values included in the model (p<0.05). Conclusion: The effectiveness of ART treatment can be improved owing to the individual approach in determining the implantation window during IVF in women receiving CHT and identifying the level of small ncRNAs in UF. The use of the logistic regression model which was developed in this study is limited in clinical practice due to the lack of information about the implantation potential of the blastocyst transferred into the uterine cavity. It is the poor quality of the embryo itself rather than the absence of the receptive endometrium that may result in a negative outcome of the ART program. Simultaneous determination of endometrial receptivity and embryo implantation potential on the basis of the small ncRNA profile can lead to a decrease in the percentage of false-negative results and improve the quality of the model. It is necessary to increase the training set of participants and to check the accuracy of the constructed models on an independent test set. Authors’ contributions: Timofeeva A.V. – obtaining the experimental data, writing the article; Fedorov I.S. – obtaining the experimental data and their statistical processing; Gokhberg Ya.A. – collecting the samples of uterine aspirate, creating the clinical database of patients and processing the source material; Kalinina E.A. – editing the text and approving the manuscript. Conflicts of interest: The authors declare that there are no conflicts of interest. Funding: The study was financially supported by the Russian Science Foundation under grant No. 22-15-00363 “Epigenetic and biochemical aspects of pregnancy pathology in impairment of invasive properties of trophoblast: from early diagnosis to prevention of maternal and perinatal morbidity” under Agreement No. 22-15-00363 dated 13.05.2022 between the Russian Science Foundation, project manager Timofeeva A.V. and Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology for providing a grant to support fundamental scientific research and pilot study. Ethical Approval: The study was approved by the Ethical Review Board of Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. Patients’ Consent for Publication: All patients provided an informed consent for the publication of their data. Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator. For citation: Timofeeva A.V., Fedorov I.S., Gokhberg Ya.A., Kalinina E.A. Evaluation of endometrial receptivity using the level of small non-coding RNAs in uterine aspirate from women undergoing cyclic hormone therapy. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (7): 90-102 (in Russian) https://dx.doi.org/10.18565/aig.2023.110Timofeeva A.V., Fedorov I.S., Gokhberg Ya.A., Kalinina E.A.
Keywords
Infertility is a global problem affecting 10–15% of couples of reproductive age in most countries of the world [1, 2]. Over the past decades, infertility treatment has become more effective due to the improvement in vitro fertilization (IVF) programs, namely, the optimization of embryo cultivation conditions [3, 4], the assessment of morphological and molecular biological criteria for their quality [5–8], and the selection of the stage of embryo development suitable for the transfer to the uterine cavity [9, 10]. But not only the viability and quality of the embryo play a crucial role in successful implantation. The receptivity of the endometrium is a critical factor for successful embryo implantation during a certain period of the menstrual cycle known as the implantation window [11, 12]. This middle stage of the secretory phase occurs 6-10 days after the peak of luteinizing hormone and is regulated by cytokines, growth factors and other molecules which are important for the development of the endometrium and embryo [13, 14]. About 60% of repeated failures of assisted reproductive technologies (ART) were found to be due to the displaced implantation window [12, 15, 16]; therefore, determining the endometrial receptivity for embryo implantation is an important task in reproduction.
In order to identify the endometrial receptivity, various methods have been developed including instrumental and invasive research methods [17]. According to the data of meta-analysis, the specificity and/or sensitivity of instrumental research methods used for the analysis of thickness, volume, three layers of the endometrium, blood flow in the endometrium and hysteroscopy do not allow to assess reliably the endometrial receptivity for predicting the pregnancy in ART programs [12].
Ultrastructural markers of endometrial receptivity are pinopodes which are considered to be the preferred sites of embryo-endometrial interaction [18, 19]. Moreover, the appearance of pinopodes coincides with an increase in the expression of other biomarkers of the implantation window, such as mucin 1, leukemia inhibiting factor (LIF) and its receptor (integrin αVβ3) [20], homeodomain-containing transcription factor 10 (HOX10) [21]. The estimation of the number and shape of pinopodes during histological analysis of the endometrial biopsy was used to develop a method of personalized embryo transfer [22]. But in order to avoid mistakes in calculating the number of pinopodes in a sample of the luminal surface of the endometrium, it is necessary to examine at least 60 areas of the sample under microscope due to the high variability of the number of pinopodes (from 5 to 20%) [23]; and this is a time-consuming process and excludes its use in routine clinical practice.
The use of transcriptome analysis of endometrial biopsy (mRNA and microRNA) made it possible to identify patients with a displacement of the implantation window and increase pregnancy rate, especially in the group with repeated implantation failures in the IVF program [24–26]. Despite the great informative value and accuracy of the transcriptome analysis method in comparison with histological examination of endometrial biopsy, its high cost and invasiveness is an obstacle to routine use in clinical practice.
An alternative method for assessing the state of the endometrium is the examination of uterine fluid (UF); its aspiration is minimally invasive and safe and can be performed immediately before embryo transfer without an adverse effect on subsequent implantation in comparison with the endometrial biopsy [27, 28]. There is a change in the expression pattern of small non-coding RNAs (ncRNAs) of UF exosomes to the receptive stage in comparison with the pro-receptive stage of the secretory phase of the natural menstrual cycle and after controlled ovarian stimulation [29], but there is no association with the achievement of pregnancy. To minimize the effect of controlled ovarian stimulation on the endometrium [30], we selected cryopreserved-thawed embryo transfer model.
The aim of the study was to evaluate endometrial receptivity by determining the number of small ncRNAs in UF on the day of cryopreserved embryo (CE) transfer in women receiving cyclic hormone therapy (CHT) and to develop a logistic regression model for calculating the optimal endometrial receptivity for embryo implantation by comparing the UF transcriptome from patients with positive and negative outcomes of the ART program.
Materials and methods
Patients
Fifty-four couples presented to the Department of Assisted Reproductive Technologies in the Treatment of Infertility at the National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow to have a transfer of cryopreserved embryo in program with CHT. The criteria for inclusion in the study were the age of patients from 29 to 39 years, normal ovarian reserve in women with tubal-peritoneal infertility factor, normozoospermia or male infertility factor without severe pathozoospermia. There were the following non-inclusion criteria: 1) the presence of verified stage III–IV extragenital endometriosis; 2) oncological diseases; 3) polycystic ovarian syndrome; 4) endometrial pathology; 5) interstitial and/or subserous uterine fibroids more than 4 cm; 6) submucous fibroids deforming the uterine cavity; 7) genetic abnormalities; 8) malformations of the genitals; 9) surgical interventions on the ovaries; 10) severe forms of male infertility.
All patients had a complete clinical and laboratory examination in accordance with the Clinical Guidelines “Female infertility”, as well as the Order of the Ministry of Health of the Russian Federation dated 31.07.2020 No. 803n “On the procedure for the use of assisted reproductive technologies, contraindications and restrictions to their use”. All patients included in the study provided informed consent to have the UF aspirated on the day of embryo transfer using disposable flexible catheters.
The protocol of preparing the endometrium for the transfer of a frozen-thawed embryo in women receiving CHT
On the 2nd–4th day of the menstrual cycle, the patients were prescribed estradiol valerate at a dosage of 2 mg/day, and the endometrium reached normal thickness (>7 mm) on the 14th–15th day of the cycle, progestogens were administered (200 mg/day of micronized progesterone or 10 mg/day of dydrogesterone). The day of CE transfer into the uterine cavity was calculated by adding the number of days of embryo cultivation from the moment of fertilization of the egg in the previous ovarian hyperstimulation cycle to the date of the injection of progestogens in the current cryo-cycle as shown in Figure 1.
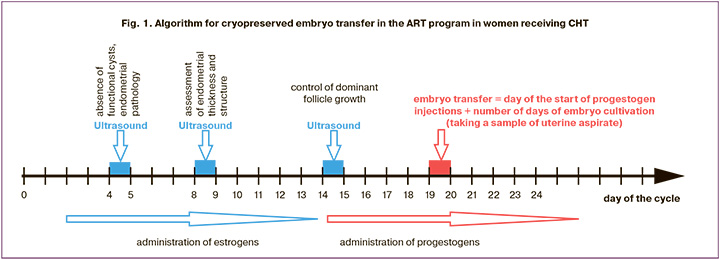
Pregnancy was diagnosed after receiving a positive result of human β-chorionic gonadotropin 14 days after embryo transfer and visualizing the gestational sac in the uterine cavity on the 21st day.
UF aspiration
UF aspiration was performed immediately before the CE transfer using a flexible catheter (COOK, Australia) which was connected to a sterile syringe (1 ml). Then 5–50 µl of the obtained UF material was placed in a sterile Eppendorf tube and 0.9% NaCl saline solution was added to the volume of 200 µl. Then the material was transported to the Laboratory of Applied Transcriptomics, Department of Systems Biology in Reproduction of National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow for its storage at -80°C and further molecular biological analysis of UF.
Isolation of small ncRNAs from the samples of UF
RNAs were isolated from the obtained samples of UF using the column technique with the help of miRNeasy Serum/Plasma Kit (Qiagen).
Deep sequencing of small ncRNAs
Seven of the fourteen microliters of column eluate miRNeasy Serum/Plasma Kit (Qiagen) containing small ncRNAs obtained from UF were used to synthesize cDNA libraries with the help of NEBNext Multiplex Small RNA Library Prep Set for Illumina (Set11 and Set2, New England Biolab®). They were amplified in 20 cycles during polymerase chain reaction (PCR), purified in 6% polyacrylamide gel and sequenced on the NextSeq 500/550 (Illumina) platform. The sequences in the range from 16 to 50 bp were mapped to human databases GRCh38.p15, miRBase v21 and piRNABase using the Bowtie algorithm [31]. The differential expression of small ncRNA was analyzed using the software package DESeq2 [32].
Reverse transcription and quantitative real-time PCR
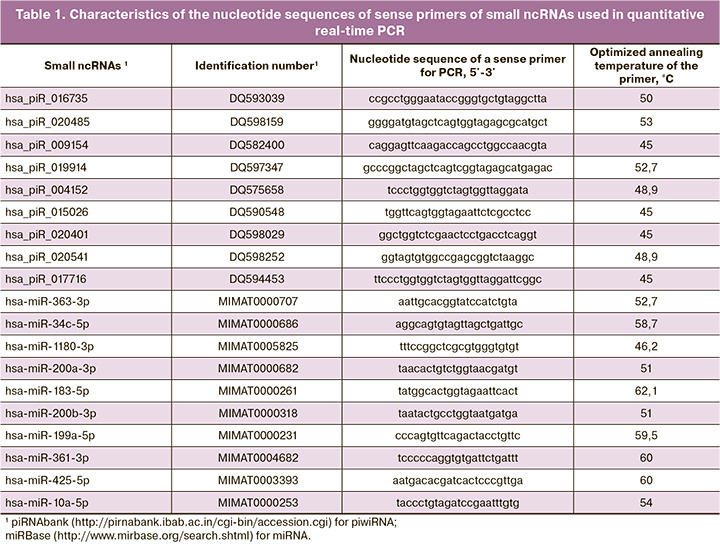
Five of the fourteen microliters of column eluate miRNeasy Serum/Plasma Kit (Qiagen) containing small ncRNAs obtained from UF were used to synthesize cDNA with the help of miScript II RT Kit (Qiagen) following the protocol of the manufacturer. Quantitative real-time PCR was performed using the miScript SYBR Green PCR Kit (Qiagen, Hilden, Germany) and sense primers specific to certain mRNAs and piwiRNAs (Table 1). The PCR program included the following: (1) 15 min at 95°C and (2) 40 cycles: 94°C for 15 s, optimized annealing temperature (45–61.6°C) for 30 s and 70°C for 30 s; (3) heating of the reaction mixture from 65 to 95 °C step 0.1°C to plot the melting curve of the PCR product in the thermal cycler StepOnePlus (Applied Biosystems). The relative expression of small ncRNAs obtained from UF was determined with the help of ∆Ct method using hsa_piR_017716 as a reference RNA.
Statistical analysis
For statistical processing of the results, we used scripts written in the R language [33] and the RStudio program [34]. The compliance of the analyzed parameters with the normal distribution was evaluated using the Shapiro–Wilk test. Statistical analysis was carried out using the Mann–Whitney U-test with a pairwise comparison when normal distribution could not be applied. The parameters which have a distribution other than normal are presented in the format Me (Q1; Q3). The significance level p was assumed to be 0.05. If the p-value was less than 0.001, it was indicated as p<0.001.
Logistic regression models were developed using the RStudio program through step-by-step inclusion and exclusion of small ncRNA predictors of receptive endometrium depending on their contribution to the model. The predictive ability of the model was evaluated using ROC analysis (Receiver operating characteristic) by the AUC (Area Under Curve) value, statistical significance, level of specificity and sensitivity.
Results
At the first stage of the study, we conducted a retrospective analysis of the anamnestic and laboratory data of the couples before they underwent IVF, embryonic stage during the ovarian hyperstimulation and the subsequent cryoprotocol in patients receiving CHT in two study groups depending on the presence or absence of embryo implantation (Table 2).
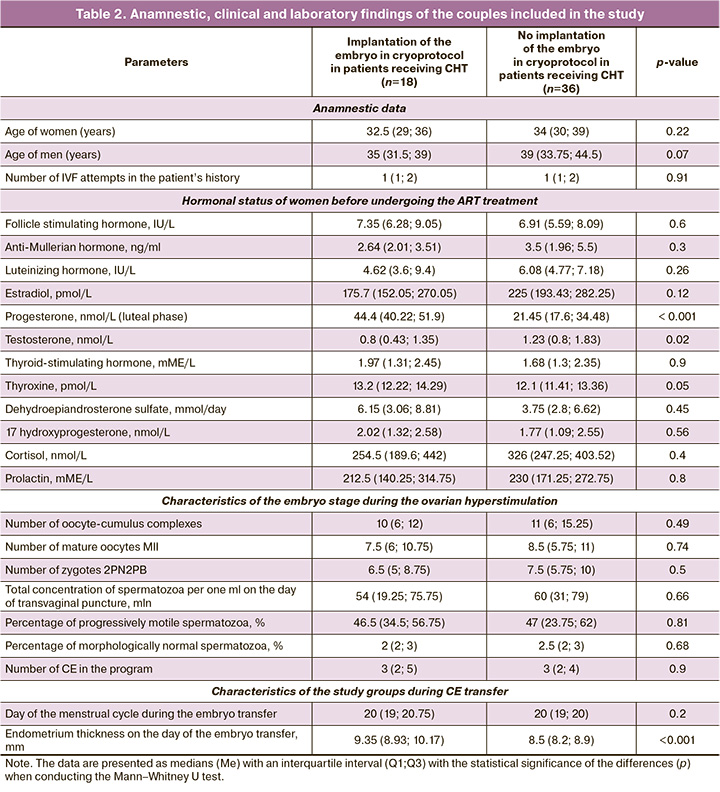
Table 2 shows that there was a statistically significant increase in testosterone levels by 1.5 times (p=0.02) and a decrease in progesterone levels by 2.1 times (p<0.001) in the group with a negative result of the ART program with CE transfer in comparison with the group where patients were pregnant before undergoing IVF treatment. The women who had a positive result of the ART program showed higher parameters of endometrial thickness on the day of embryo transfer in comparison with the group of women who did not become pregnant (p<0.001). The study groups did not differ in anamnestic data and characteristics of the embryonic stage during ovarian hyperstimulation.
At the second stage of the study, the quantitative assessment of small ncRNAs (miRNAs and piwiRNAs) was carried out in 31 out of 54 samples of UF using the deep sequencing technique; the samples were obtained immediately prior to the CE transfer. A total of 289 miRNAs and 488 piwiRNAs were identified in 15 samples of UF obtained from women who did not have embryo implantation and in 16 samples of UF obtained from women who became pregnant. Optimal combinations of small ncRNA markers of receptive endometrium were found from this list of small ncRNAs in the RStudio program via gradual inclusion and exclusion of each molecule depending on their contribution to the construction of logistic regression models (Fig. 2), where the dependent variable (response variable) was the degree of endometrial receptivity for implantation (0 refers to implantation, 1 refers to the absence of implantation). All models constructed on the basis of the content of miRNAs (Fig. 2A) and piwiRNAs (Fig. 2B) in UF were statistically significant and had high specificity (84-100%) as well as high diagnostic value of endometrial receptivity on the day of CE transfer. The lower parameters of sensitivity of the constructed models (65–87%) may be due to the lack of information about the implantation potential of the embryo transferred into the uterine cavity; this information can be obtained at the stage morula or blastocyst stage before cryopreservation [7], since the absence of implantation may be caused not only by impaired receptive endometrium, but also by the quality of the embryo itself.
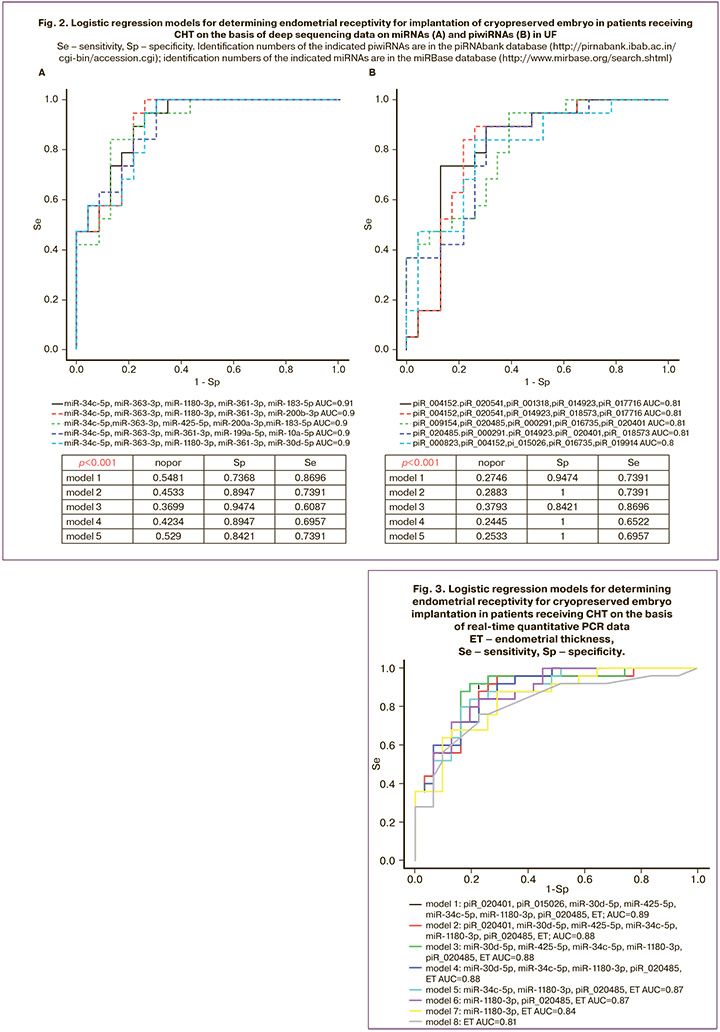
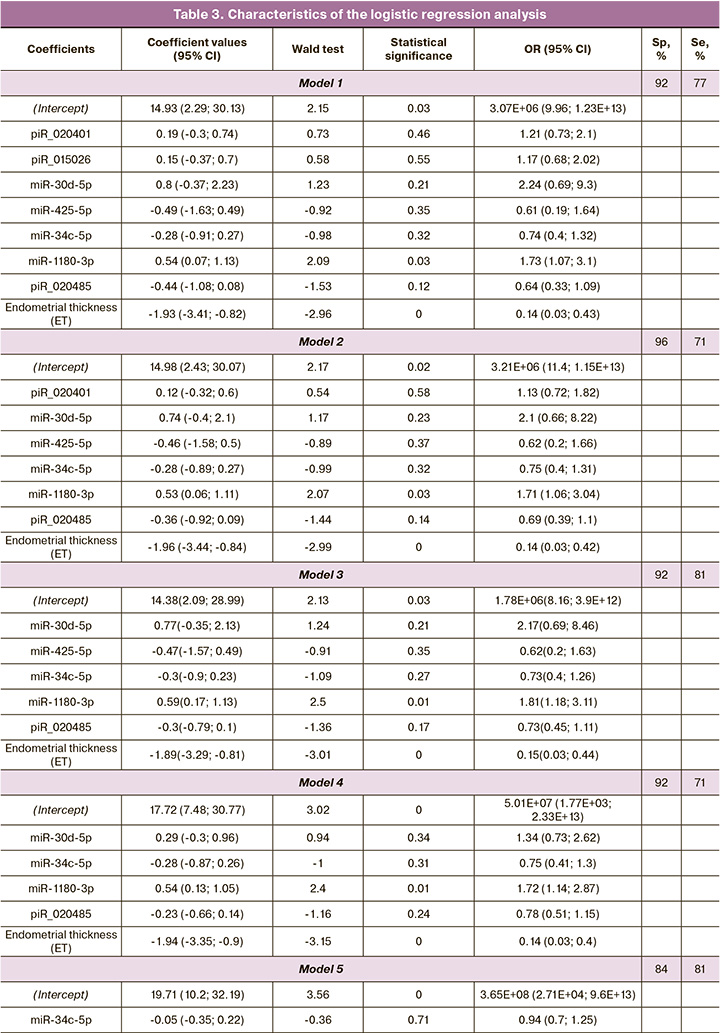
The sequencing data were validated using quantitative real-time PCR on the entire sample of patients listed in Table 2. Logistic regression models were constructed on the basis of the parameters «-ΔСt» for 18 small ncRNA molecules listed in Table 1 and endometrial thickness; these models were created in the RStudio program after finding the optimal combination of the explanatory variables with their gradual inclusion and exclusion depending on their contribution to the construction of the model, where the dependent variable (response variable) was endometrial receptivity for embryo implantation. We found a combination of explanatory variables (model 1, Figure 3) which has the largest area under the curve (AUC=0.89) and includes of seven small ncRNAs and endometrial thickness. One of the most insignificant explanatory variables was gradually eliminated from model 1, Figure 3, using backward elimination method; thus, models 2, 3, 4, 5, 6, 7, were obtained where each subsequent model was a derivative of the previous one (Table 3). In the final model 7, Figure 3, all explanatory variables turned out to be statistically significant (Table 3) and independent according to the Spearman correlation analysis (r=0.02, p=0.9). Specificity and sensitivity of model 7, Figure 3, were 88 and 71%, respectively; therefore, there is a high diagnostic value of the combined metric of miR-1180-3p level in UF and endometrial thickness in determining endometrial receptivity for implantation of CE on the day of its transfer. The formula for calculating the probability of the receptive endometrium for model 7, Figure 3, is presented below:
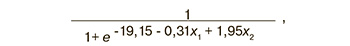
where : -ΔCt (miR-1180-3p), : endometrial thickness (mm).
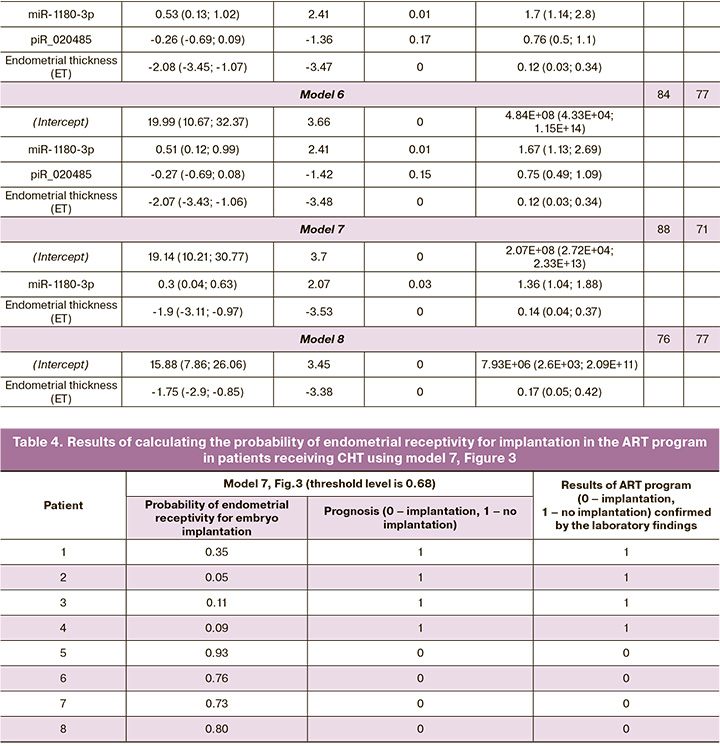
In order to assess the outcome of the study, we randomly selected eight patients with specific clinical characteristics and applied model 7. The results of the assessment are presented in Table 4.
It should be noted that one-factor model which includes only endometrial thickness is less specific for assessing its receptivity (model 8, Fig. 3, Sp=76%) as it is indicated in Table 3.
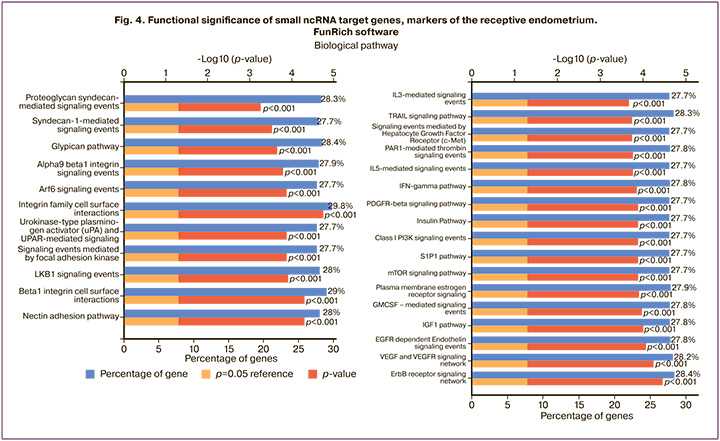
When analyzing the functional significance of small ncRNAs involved in models presented in Figure 3, potential target genes of piwiRNAs were identified using the miRanda algorithm [8], and the target genes of miRNAs were found in the Funrich program (http://www.funrich.org/). The analysis of the enrichment of signaling pathways showed that miRNAs and piwiRNAa take part in the processes of cell adhesion and signal transmission into the cells through the receptors of growth factors and interleukins which are necessary for the implantation of the embryo and its subsequent growth and development (Fig. 4).
Discussion
A significant disadvantage of modern techniques for diagnosing endometrial receptivity used in clinical practice (ERA, Igenomix; ER-Map, iGLS; ERPeak, CooperGenomics) is their invasiveness in obtaining endometrial tissue; therefore, embryo cannot be transferred in the same cycle when endometrial biopsy is performed. The implantation window is known to open at the moment of a sudden change in the transcription profile in non-ciliated epithelial cells and in stromal fibroblasts transforming into decidual cells [35]. Thus, there is a high probability of differences in the ratio of ciliated, non-ciliated epithelial cells and stromal cells in different endometrial biopsy samples obtained from the same woman. Moreover, the ratio of the density of ciliated and non-ciliated epithelial cells in various pathological conditions of the endometrium including chronic endometritis is significantly lower than the control values [36]. The above factors may lead to an incorrect interpretation of the data obtained from transcriptome analysis of the endometrial biopsy. In addition, the implantation window in the menstrual cycle during the invasive test may change its time in subsequent embryo transfer cycles due to physiological intercycle variability. Therefore, it is necessary to use a non-invasive method for analyzing endometrial receptivity which can provide a comprehensive assessment of the transformation of endometrial cells to ensure embryo implantation.
In the present study, we identified extracellular small ncRNAs in UF on the day of embryo transfer which are the markers of endometrial receptivity using a combination of methods of deep sequencing and quantitative real-time PCR. Logistic regression models are constructed in the form of a combination of quantitative characteristics of small ncRNAs and endometrial thickness. The most accurate model for the diagnosis of the receptive endometrium in women receiving CHT on the day of CE transfer turned out to be a model of combined measurement of the level of miR-1180-3p in UF and endometrial thickness due to the fact that its variables were statistically significant (p<0.05) and independent according to the Spearman correlation analysis (r=0.02, p=0.9). The limitation of this study is the small sample size based on the formula N=50+8m, where N is the required sample size, m is the number of independent variables [37]. When using two explanatory variables (the level of miR-1180-3p in UF and endometrial thickness on the day of embryo transfer, model 7, Figure 3), the sample size is close to acceptable one and deviates from the reference indicator by less than 20%. But in order to check the diagnostic significance of the original model 1, Figure 3, using eight explanatory variables, it is necessary to expand the training sample to 114 samples, which we plan to do in the future.
The analysis of the target genes of small ncRNA markers of the receptive endometrium revealed that they are involved in the following processes: 1) adhesion mediated by syndecan-1, nectin, glipican, integrin family, 2) proteolysis of extracellular matrix by urokinase-type plasminogen activator, 3) differentiation of granulocytes and monocytes-macrophages under the action of interleukins 3 and 5, granulocyte-macrophage colony stimulating factor, 4) immune reactions with the participation of interferon gamma, 5) cellular growth and proliferation mediated by the action of platelet growth factor, epidermal growth factor, vascular growth factor, insulin-like growth factor 1, estrogen, sphingosine-1-phosphate signaling system, 6) vesicular transport controlled by the ADP-ribosylation factor.
The obtained data are consistent with the results of sequencing the mRNAs of epithelial and stromal cells of the human endometrium in the receptive stage in comparison with the proreceptive stage of the secretory phase of the menstrual cycle [38]. The researchers emphasize the key role of cell adhesion proteins (including galectins, integrin beta-1, basigin and osteopontin), regulators of secretory processes and the organization of the extracellular matrix at the initial stages of embryo implantation, such as apposition, adhesion and invasion. Networks of protein-protein interactions of the blastocyst and endometrium have been constructed [38–40], where one of the main proteins is integrin beta-1. Being part of the membrane of endometrial cells, integrin beta-1 interacts with the glycoprotein BSG on the surface of the trophectoderm, where the level of integrin beta-1 is regulated by galectin-3 secreted by stromal and epithelial cells of the endometrium, which ultimately ensures the migration and invasion of trophoblast cells [41]. Due to the participation of a cascade of proteins interacting with each other in the process of embryo implantation, it is necessary not only to assess endometrial receptivity before transferring an embryo into the uterine cavity, but also to know the implantation potential of the embryo itself, focusing not only on its morphological parameters, but also on molecular biological characteristics. It should be noted that the logistic regression model for determining endometrial receptivity for embryo implantation using the level of small ncRNAs on the day of CE transfer was developed in this study without taking into account the implantation potential of the embryo which affected the number of false negative results (29%). We previously developed logistic regression models to determine the implantation potential of an embryo based on the level of small ncRNAs in the medium of its cultivation at the stage of morula and blastocyst in a cycle of controlled ovarian stimulation [7] and aimed at further transfer into the uterine cavity or cryopreservation of a high-quality embryo. Therefore, in order to improve the diagnostic ability of the constructed models, it is planned in future to carry out a simultaneous assessment of endometrial receptivity and embryo implantation potential on the basis of small ncRNA profile.
The significance of the developed models is confirmed by the literature data on the potential role of small ncRNAs included in the models (miR-30d-5p, miR-425-5p, miR-34c-5p, miR-1180-3p) in the processes associated with embryo implantation. The role of the miR-30 family in the formation of receptive endometrium was revealed in various studies [42–44]; in particular, an increase in the expression of miR-30d-5p was observed during the implantation window [43]. On the contrary, there was a sharp decrease in the expression level of miR-30d-5p and an increase in the expression level of the target suppressor
of cytokine signaling-1 (SOCS1) in the endometrium on the 7th day after the peak of luteinizing hormone [45] in patients with repeated implantation failures. The effect of miR-30d-5p deficiency in the endometrium and in the embryo on implantation and subsequent fetal development was demonstrated in miR-30d-5p knocked-out mouse models [46]. Other studies showed the role of miR-425-5p in the regulation of cell proliferation, migration and invasion and revealed an increased level of its expression in the tissues of various tumors, including kidney carcinoma [47], hepatocellular carcinoma [48], cervical cancer [49], stomach cancer [50]. As miR-34c-5p is an inhibitor of epithelial-mesenchymal transition, cell invasion and migration via suppressing the expression level of the target gene Notch1, it is involved in the pathogenesis of endometriosis when its expression level is reduced in the ectopic endometrium [51]. A significant suppression of miR-34c-5p expression in the eutopic endometrium is noted during the implantation window, during which the level of the GAS target gene protein required for embryo implantation sharply increases; while a deficiency of GAS1 expression was found in the endometrium of women with repeated implantation failures compared to women with receptive endometrium [52]. miR-1180-3p is considered to be a suppressor of many epithelial tumors, including bladder cancer [53], pancreatic cancer [54], colorectal cancer [55]; an increased expression level of miR-1180-3p causes a decrease in the proliferation and mobility of cancer cells by affecting the target gene COL12A1 [55].
Conclusion
The effectiveness of ART treatment can be improved owing to the individual approach in determining the implantation window during IVF in women receiving CHT and identifying the level of small ncRNAs in UF. The use of the logistic regression model which was developed in this study is limited in clinical practice due to the lack of information about the implantation potential of the blastocyst transferred into the uterine cavity. It is the poor quality of the embryo itself rather than the absence of the receptive endometrium that may result in a negative outcome of the ART program. Simultaneous determination of endometrial receptivity and embryo implantation potential on the basis of the small ncRNA profile can lead to a decrease in the percentage of false-negative results and improve the quality of the model. It is necessary to check the accuracy of the constructed models on an independent test set.
References
- Gerrits T., Van Rooij F., Esho T., Ndegwa W., Goossens J., Bilajbegovic A. et al. Infertility in the Global South: Raising awareness and generating insights for policy and practice. Facts Views Vis Obgyn. 2017; 9(1): 39-44.
- Sun H., Gong T.T., Jiang Y.T., Zhang S., Zhao Y. H.,Wu Q.J. Global, regional, and national prevalence and disability-adjusted life-years for infertility in 195 countries and territories, 1990-2017: results from a global burden of disease study. Aging. 2017; 11(23): 10952-91. https://dx.doi.org/10.18632/aging.102497.
- Mani S., Mainigi M. Embryo culture conditions and the epigenome. Semin. Reprod. Med. 2018; 36(3-04): 211-20. https://dx.doi.org/10.1055/s-0038-1675777.
- Simopoulou M., Sfakianoudis K., Rapani A., Giannelou P., Anifandis G., Bolaris S. et al. Considerations regarding embryo culture conditions: from media to epigenetics. In Vivo. 2018; 32(3): 451-60. https://dx.doi.org/10.21873/invivo.11261.
- Kirkegaard K., Agerholm I.E., Ingerslev H.J. Time-lapse monitoring as a tool for clinical embryo assessment. Hum. Reprod. 2012; 27(5): 1277-85.https://dx.doi.org/10.1093/humrep/des079.
- Gardner D.K., Balaban B. Assessment of human embryo development using morphological criteria in an era of time-lapse, algorithms and 'OMICS': is looking good still important? Mol. Hum. Reprod. 2016; 22(10): 704-18.https://dx.doi.org/10.1093/molehr/gaw057.
- Timofeeva A.V., Fedorov I.S., Shamina M. A., Chagovets V.V., Makarova N.P., Kalinina E.A. et al. Clinical relevance of secreted small noncoding RNAs in an embryo implantation potential prediction at morula and blastocyst development stages. life (Basel). 2021; 11(12): 1328. https://dx.doi.org/10.3390/life11121328.
- Timofeeva A.V., Drapkina Y.S., Fedorov I.S., Chagovets V.V., Makarova N.P., Shamina M.A. et al. Small noncoding RNA signatures for determining the developmental potential of an embryo at the morula ssage. Int. J. Mol. Sci. 2020; 21(24): 9399. https://dx.doi.org/10.3390/ijms21249399.
- Simopoulou M., Sfakianoudis K., Tsioulou P., Rapani A., Maziotis E., Giannelou P. et al. Should the flexibility enabled by performing a day-4 embryo transfer remain as a valid option in the IVF laboratory? A systematic review and network meta-analysis. J. Assist. Reprod. Genet. 2019; 36(6): 1049-61.https://dx.doi.org/10.1007/s10815-019-01475-0.
- Li Y.X., Wang J., Sun T.Z., Lv M.Q., Ge P., Li H.N. et al. Pregnancy outcomes after day 5 versus day 6 blastocyst-stage embryo transfer: A systematic review and meta-analysis. J. Obstet. Gynaecol. Res. 2020; 46(4): 595-605.https://dx.doi.org/10.1111/jog.14188.
- Luddi A., Pavone V., Semplici B., Governini L., Criscuoli M., Paccagnini E. et al. Organoids of human endometrium: a powerful in vitro model for the endometrium-embryo cross-talk at the implantation site. Cells. 2020 ;9(5): 1121. https://dx.doi.org/10.3390/cells9051121.
- Craciunas L., Gallos I., Chu J., Bourne T., Quenby S., Brosens J.J. et al. Conventional and modern markers of endometrial receptivity: a systematic review and meta-analysis. Hum. Reprod. Update. 2019; 25(2): 202-23.https://dx.doi.org/10.1093/humupd/dmy044.
- Massimiani M., Lacconi V., La Civita F., Ticconi C., Rago R. et al. Molecular signaling regulating endometrium-blastocyst crosstalk. Int. J. Mo.l Sci. 2019; 21(1): 23. https:/dx./doi.org/10.3390/ijms21010023.
- Kieu V., Lantsberg D., Mizrachi Y., Stern C., Polyakov A., Teh W.T. A survey study of endometrial receptivity tests and immunological treatments in in vitro fertilisation (IVF). Aust. N. Z. J. Obstet. Gynaecol. 2022; 62(2): 306-11.https://dx.doi.org/10.1111/ajo.13466.
- Sebastian-Leon P., Garrido N., Remohí J., Pellicer A., Diaz-Gimeno P. Asynchronous and pathological windows of implantation: two causes of recurrent implantation failure. Hum. Reprod. 2018; 33(4): 626-35.https://dx.doi.org/10.1093/humrep/dey023.
- Valdes C.T., Schutt A., Simon C. Implantation failure of endometrial origin: it is not pathology, but our failure to synchronize the developing embryo with a receptive endometrium. Fertil. Steril. 2017; 108(1): 15-8.https://dx.doi.org/10.1016/j.fertnstert.2017.05.033.
- Aghajanova L., Hamilton A.E., Giudice L.C. Uterine receptivity to human embryonic implantation: histology, biomarkers, and transcriptomics. Semin. Cell Dev. Biol. 2008; 19(2): 204-11. https://dx.doi.org/10.1016/j.semcdb.2007.10.008.
- Nejatbakhsh R., Kabir-Salmani M., Dimitriadis E., Hosseini A., Taheripanah R., Sadeghi Y. et al. Subcellular localization of L-selectin ligand in the endometrium implies a novel function for pinopodes in endometrial receptivity. Reprod. Biol. Endocrinol. 2012; 10: 46. Erratum in: Reprod. Biol. Endocrinol. 2021;19(1): 62. https://dx.doi.org/10.1186/1477-7827-10-46.
- Quinn K.E., Matson B.C., Wetendorf M., Caron K.M. Pinopodes: recent advancements, current perspectives, and future directions. Mol. Cell. Endocrinol. 2020; 501: 110644. https://dx.doi.org/10.1016/j.mce.2019.110644.
- Xu B., Sun X., Li L., Wu L., Zhang A., Feng Y. Pinopodes, leukemia inhibitory factor, integrin-β3, and mucin-1 expression in the peri-implantation endometrium of women with unexplained recurrent pregnancy loss. Fertil. Steril. 2012; 98(2): 389-95. https://dx.doi.org/10.1016/j.fertnstert.2012.04.032.
- Li F., Zhang M., Zhang Y., Liu T., Qu X. GnRH analogues may increase endometrial Hoxa10 promoter methylation and affect endometrial receptivity. Mol. Med. Rep. 2015; 11(1): 509-14. https://dx.doi.org/10.3892/mmr.2014.2680.
- Qiong Z., Jie H., Yonggang W., Bin X., Jing Z., Yanping L. Clinical validation of pinopode as a marker of endometrial receptivity: a randomized controlled trial. Fertil. Steril. 2017; 108(3): 513-7.e2. https://dx.doi.org/10.1016/j.fertnstert.2017.07.006.
- Jin X.Y., Zhao L.J., Luo D.H., Liu L., Dai Y.D., Hu X.X. et. al. Pinopode score around the time of implantation is predictive of successful implantation following frozen embryo transfer in hormone replacement cycles. Hum. Reprod. 2017; 32(12): 2394-403. https://dx.doi.org/10.1093/humrep/dex312.
- Hashimoto T., Koizumi M., Doshida M., Toya M., Sagara E., Oka N. et. al. Efficacy of the endometrial receptivity array for repeated implantation failure in Japan: a retrospective, two-centers study. Reprod. Med. Biol. 2017; 16(3):290-6. https://dx.doi.org/10.1002/rmb2.12041.
- Tan J., Kan A., Hitkari J., Taylor B., Tallon N., Warraich G. et. al. The role of the endometrial receptivity array (ERA) in patients who have failed euploid embryo transfers. J. Assist. Reprod. Genet. 2018; 35(4): 683-92.https://dx.doi.org/10.1007/s10815-017-1112-2.
- Altmäe S., Koel M., Võsa U., Adler P., Suhorutšenko M., Laisk-Podar T. et. al. Meta-signature of human endometrial receptivity: a meta-analysis and validation study of transcriptomic biomarkers. Sci. Rep. 2017; 7(1): 10077. https://dx.doi.org/10.1038/s41598-017-10098-3.
- van der Gaast M.H., Beier-Hellwig K., Fauser B.C., Beier H.M., Macklon N.S. Endometrial secretion aspiration prior to embryo transfer does not reduce implantation rates. Reprod. Biomed. Online. 2003; 7(1): 105-9.https://dx.doi.org/10.1016/s1472-6483(10)61737-3.
- Matorras R., Quevedo S., Corral B., Prieto B., Exposito A., Mendoza R. et. al. Proteomic pattern of implantative human endometrial fluid in in vitro fertilization cycles. Arch. Gynecol. Obstet. 2018; 297(6): 1577-86.https://dx.doi.org/10.1007/s00404-018-4753-1.
- Li T., Greenblatt E.M., Shin M.E., Brown T.J., Chan C. Cargo small non-coding RNAs of extracellular vesicles isolated from uterine fluid associate with endometrial receptivity and implantation success. Fertil. Steril. 2021; 115(5): 1327-36. https://dx.doi.org/10.1016/j.fertnstert.2020.10.046.
- Bergenheim S.J., Saupstad M., Pistoljevic N., Andersen A.N., Forman J.L., Løssl K. et al. Immediate versus postponed frozen embryo transfer after IVF/ICSI: a systematic review and meta-analysis. Hum. Reprod. Update. 2021; 27(4): 623-42. https://dx.doi.org/10.1093/humupd/dmab002.
- Langmead B., Trapnell C., Pop M., Salzberg S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009; 10: R25. https://dx.doi.org/10.1186/gb-2009-10-3-r25.
- Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014; 15(12): 550. https://dx.doi.org/10.1186/s13059-014-0550-8.
- Team R.C. A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. Available at:https://www.R-project.org Accessed 10.03. 2021.
- Team Rs. RStudio: Integrated Development for R. RStudio. Available at: http://www.rstudio.com/ Accessed 23.03.2021.
- Wang W., Vilella F., Alama P., Moreno I., Mignardi M., Isakova A. et. al. Single-cell transcriptomic atlas of the human endometrium during the menstrual cycle. Nat. Med. 2020; 26(10): 1644-53. https://dx.doi.org/10.1038/s41591-020-1040-z.
- Казачков Е.Л., Воропаева Е.Е., Казачкова Э.А., Затворницкая А.В., Дуб А.А., Мирошниченко Л.Е. Морфологическая характеристика эндометрия у пациенток с миомой матки и хроническим эндометритом при бесплодии. Архив патологии. 2019; 81(6): 41-8. [Kazachkov E.L., Voropaeva E.E., Kazachkova E.A., Zatvornitskaya A.V., Dub A.A., Miroshnichenko L.E. Endometrial morphological characteristics in patients with hysteromyoma and chronic endometritis in infertility. Archive of Pathology. 2019; 81(6): 41-8.(in Russian)]. https://dx.doi.org/10.17116/patol20198106141.
- Green S.B. How many subjects does it take to do a regression analysis. Multivariate Behav. Res. 1991; 26(3): 499-510. https://dx.doi.org/10.1207/s15327906mbr2603_7.
- Koel M., Krjutškov K., Saare M., Samuel K., Lubenets D., Katayama S. et. al. Human endometrial cell-type-specific RNA sequencing provides new insights into the embryo-endometrium interplay. Hum. Reprod. Open. 2022; 2022(4): hoac043. https://dx.doi.org/10.1093/hropen/hoac043.
- Evans J., Hutchison J., Salamonsen L.A., Greening D.W. Proteomic insights into endometrial receptivity and embryo-endometrial epithelium interaction for implantation reveal critical determinants of fertility. Proteomics. 2020; 20(1): e1900250. https://dx.doi.org/10.1002/pmic.201900250.
- Ruane P.T., Garner T., Parsons L., Babbington P.A., Wangsaputra I., Kimber S.J. et. al. Trophectoderm differentiation to invasive syncytiotrophoblast is promoted by endometrial epithelial cells during human embryo implantation. Hum. Reprod. 2022; 37(4): 777-92. https://dx.doi.org/10.1093/humrep/deac008.
- Bojić-Trbojević Ž., Jovanović Krivokuća M., Vilotić A., Kolundžić N., Stefanoska I., Zetterberg F. et. al. Human trophoblast requires galectin-3 for cell migration and invasion. Sci. Rep. 2019; 9(1): 2136. https://dx.doi.org/10.1038/s41598-018-38374-w.
- Vilella F., Moreno-Moya J.M., Balaguer N., Grasso A., Herrero M., Martínez S. et. al. Hsa-miR-30d, secreted by the human endometrium, is taken up by the pre-implantation embryo and might modify its transcriptome. Development. 2015; 142(18): 3210-21. https://dx.doi.org/10.1242/dev.124289.
- Sha A.G., Liu J.L., Jiang X.M., Ren J.Z., Ma C.H., Lei W. et. al. Genome-wide identification of micro-ribonucleic acids associated with human endometrial receptivity in natural and stimulated cycles by deep sequencing. Fertil. Steril. 2011; 96(1): 150-5.e5. https://dx.doi.org/10.1016/j.fertnstert.2011.04.072.
- Kuokkanen S., Chen B., Ojalvo L., Benard L., Santoro N., Pollard J.W. Genomic profiling of microRNAs and messenger RNAs reveals hormonal regulation in microRNA expression in human endometrium. Biol. Reprod. 2010; 82(4): 791-801. https://dx.doi.org/10.1095/biolreprod.109.081059.
- Zhao Y., He D., Zeng H., Luo J., Yang S., Chen J. et. al. Expression and significance of miR-30d-5p and SOCS1 in patients with recurrent implantation failure during implantation window. Reprod. Biol. Endocrinol. 2021; 19(1): 138. https://dx.doi.org/10.1186/s12958-021-00820-2.
- Balaguer N., Moreno I., Herrero M., Gonzáléz-Monfort M., Vilella F., Simón C. MicroRNA-30d deficiency during preconception affects endometrial receptivity by decreasing implantation rates and impairing fetal growth. Am. J. Obstet. Gynecol. 2019; 221(1): 46.e1-46.e16. https://dx.doi.org/10.1016/j.ajog.2019.02.047.
- Quan J., Li Y., Pan X., Lai Y., He T., Lin C. et. al. Oncogenic miR-425-5p is associated with cellular migration, proliferation and apoptosis in renal cell carcinoma. Oncol. Lett. 2018; 16(2): 2175-84. https://dx.doi.org/10.3892/ol.2018.8948.
- Fang F., Song T., Zhang T., Cui Y., Zhang G., Xiong Q. MiR-425-5p promotes invasion and metastasis of hepatocellular carcinoma cells through SCAI-mediated dysregulation of multiple signaling pathways. Oncotarget. 2017; 8(19): 31745-57. https://dx.doi.org/10.18632/oncotarget.15958.
- Sun L., Jiang R., Li J., Wang B., Ma C., Lv Y. et. al. MicoRNA-425-5p is a potential prognostic biomarker for cervical cancer. Ann. Clin. Biochem. 2017; 54(1): 127-33. https://dx.doi.org/10.1177/0004563216649377.
- Zhang Z., Li Y., Fan L., Zhao Q., Tan B., Li Z. et. al. microRNA-425-5pis upregulated in human gastric cancer and contributes to invasion and metastasis in vitro and in vivo. Exp. Ther. Med. 2015; 9(5): 1617-22.https://dx.doi.org/10.3892/etm.2015.2318.
- Luo Y., Wang D., Chen S., Yang, Q. The role of miR-34c-5p/Notch in epithelial-mesenchymal transition (EMT) in endometriosis. Cell. Signal. 2020; 72: 109666. https://dx.doi.org/10.1016/j.cellsig.2020.109666.
- Tan Q., Shi S., Liang J., Zhang X., Cao D., Wang Z. MicroRNAs in small extracellular vesicles indicate successful embryo implantation during early pregnancy. Cells. 2020; 9(3): 645. https://dx.doi.org/10.3390/cells9030645.
- Ge Q., Wang C., Chen Z., Li F., Hu J., Ye Z. The suppressive effects of miR-1180-5p on the proliferation and tumorigenicity of bladder cancer cells. Histol. Histopathol. 2017; 32(1): 77-86. https://dx.doi.org/10.14670/HH-11-772.
- Gu L., Zhang J., Shi M., Peng C. The effects of miRNA-1180 on suppression of pancreatic cancer. Am. J. Transl. Res. 2017; 9(6): 2798-806.
- Li C., Jin W., Zhang D., Tian S. Clinical significance of microRNA-1180-3p for colorectal cancer and effect of its alteration on cell function. Bioengineered. 2021; 12(2): 10491-500. https://dx.doi.org/10.1080/21655979.2021.1997694.
Received 03.05.2023
Accepted 28.06.2023
About the Authors
Angelika V. Timofeeva, Ph.D. (Bio), Head of the Laboratory of Applied Transcriptomics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, v_timofeeva@oparina4.ru, https://orcid.org/0000-0003-2324-9653,117997, Russia, Moscow, Academician Oparin str., 4.
Ivan S. Fedorov, Junior Researcher at the Laboratory of Applied Transcriptomics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, is_fedorov@oparina4.ru, https://orcid.org/0000-0002-2104-5887, 117997, Russia, Moscow, Academician Oparin str., 4.
Yael A. Gokhberg, postgraduate student, Professor B.V. Leonov Department of IVF, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, dr.yaelgokhberg@gmail.com, https://orcid.org/0000-0003-3637-6096, 117997, Russia, Moscow, Academician Oparin str., 4.
Elena A. Kalinina, Dr. Med. Sci., Professor, Head of Professor B.V. Leonov Department of IVF, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, e_kalinina@oparina4.ru, https://orcid.org/0000-0002-8922-2878,
117997, Russia, Moscow, Academician Oparin str., 4.



