Expression patterns of maternal and fetal tissue and exosomal microRNAs during pre-induction of labor (pilot study)
Gaidarova A.R., Gusar V.A., Chagovets V.V., Edilberg I.V., Kan N.E., Baev O.R.
Successful preparation of the female body for childbirth, carried out through various mechanisms of labor initiation and activities aimed at early delivery, is necessary to reduce obstetric and neonatal complications. These mechanisms, which combine the inflammatory response in the myometrium, fetal signaling, and physiological aging of membranes, are based on processes and signaling pathways. Coordinated regulation of these processes is performed using microRNAs (miRNAs).
Objective: This study aimed to investigate the expression of maternal and fetal tissues and exosomal miRNAs as signaling molecules for the initiation of labor during cervical ripening.
Materials and methods: This pilot study assessed maternal and fetal tissue and exosomal miRNA expression using quantitative real-time reverse transcription-PCR in 22 pregnant women divided into two cohorts: «labor without pre-induction» (cohort I, n=10) and «pre-induction of labor» (cohort II, n=12). Cohort I was further divided into two groups: pregnant women with spontaneous labor (group Ia, n=5) and pregnant women who underwent a planned cesarean section before the onset of labor (group Ib, n=5). Cohort II included pregnant women with a positive response to pre-induction of labor (group IIa, n=5) and pregnant women who did not achieve this effect (group IIb, n=7).
Results: The study found that the initial level of exosomal miR-181a-5p in the blood plasma before the pre-induction of labor was significantly different (p = 0.03) in pregnant women with a good response (cervical ripening and onset of labor) compared to those with no response. The level of exosomal miR-92a-3p in umbilical cord blood was higher than that in maternal blood before pre-induction (p=0.009). The expression of miR-454-3p and miR-548g-5p was detected only in umbilical cord blood samples, indicating their fetal origin. A pairwise comparison between the groups revealed a significantly higher level of let-7b-5p expression in the membranes in the successful pre-induction group than in the planned cesarean section group (p=0.02) and the group with no effect from pre-induction of labor (p=0.01). Similarly, in the myometrium, the expression of let-7b-5p was significantly higher in the successful pre-induction group than in the planned cesarean section group (p = 0.05).
Conclusion: The identified differences in the expression of tissue and exosomal miRNAs may be due to the highly coordinated regulation of different signaling pathways and the corresponding target genes involved in the mechanism of communication between the maternal and fetal compartments. This provides a basis for further research using larger cohorts of pregnant women.
Authors’ contributions: Baev O.R., Gusar V.A., Gaidarova A.R. – conception and design of the study; Gusar V.A., Gaidarova A.R., Edilberg I.V. – data collecting and processing; Chagovets V.V., Gaidarova A.R. – statistical analysis; Gusar V.A., Gaidarova A.R. – drafting of the manuscript; Gusar V.A., Kan N.E., Baev O.R. – editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors’ Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Gaidarova A.R., Gusar V.A., Chagovets V.V., Edilberg I.V., Kan N.E., Baev O.R. Expression patterns of maternal and fetal tissue and exosomal microRNAs during pre-induction of labor (pilot study).
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (2): 62-71 (in Russian)
https://dx.doi.org/10.18565/aig.2024.7
Keywords
The rates of pre-induction/induction of labor have recently increased worldwide [1–3]. However, despite the widespread use of this procedure, there remains a lack of consensus in the scientific literature regarding many aspects.
An indisputable factor in reducing obstetric and neonatal complications is the successful preparation of the female body for childbirth, which is achieved through various mechanisms that initiate labor. These mechanisms, which involve the inflammatory response in the myometrium, fetal signaling, and physiological aging of membranes, are based on processes and signaling pathways regulated by microRNAs (miRNAs).
MicroRNAs are small endogenous non-coding molecules, approximately 18–22 nucleotides in length, that regulate gene expression at the post-transcriptional level [4, 5]. Furthermore, their ability to be secreted as part of extracellular microvesicles, apoptotic bodies, and exosomes enables them to act as mediators of intercellular interactions [6–9]. Previously, a change in expression during the initiation of labor in the myometrium was shown in members of the miR-200 family (miR-200b/200a/429, miR-141/200c) [7], miR-199a-3p/miR-214 [7, 9, 10], and miR-181 (miR-181a, miR-181b, miR-181c, and miR-181d), which play an important role in the regulation of inflammatory reactions in various cell types [11, 12], and miR-144 [13]. Additionally, some studies have indicated the potential role of miR-143, miR-34b/c, and miR-33 in hormonal control, regulation of rest, and myometrial contractility during pregnancy and childbirth [14, 15]. However, there is currently a lack of literature on miRNA expression during pre-induction of labor.
In consideration of the above, we used the miRTarBase 9.0 database to search for and select miRNAs involved in the mechanisms of initiation of labor and the regulation of the expression of a number of key genes, in particular, the transcription factor NFKB1, lung surfactant protein SFTPA1, prostaglandin synthase (cyclooxygenase) PTGS2, and progesterone receptor PGR, to evaluate the expression of selected miRNAs in the mother and fetus as circulating and tissue signaling molecules for the initiation of labor during cervical labor (pre-induction of labor).
Materials and methods
Formation of clinical groups of patients
This pilot prospective study included 22 pregnant women who were observed at Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation.
The inclusion criteria for the study and control groups were age 18 to 45 years, singleton pregnancy, cephalic presentation, full-term pregnancy, “immature” birth canal, pre-induction of labor, spontaneous onset of labor (physiological readiness for childbirth), and indications for elective caesarean section (lack of readiness for childbirth). The study did not include women with multiple pregnancies, severe extragenital diseases, pregnancy complications (preeclampsia, HELLP syndrome, and antiphospholipid syndrome), or fetal malformations.
The sample of pregnant women included in the study was divided into two cohorts: “labor without pre-induction” (cohort I, n=10) and “pre-induction of labor” (cohort II, n=12). Cohort I was divided into two groups: pregnant women with spontaneous labor (group Ia, n=5) and pregnant women who underwent a planned cesarean section before the onset of labor (group Ib, n=5). Cohort II included pregnant women with a positive effect of pre-induction (group IIa, n=5) and pregnant women with no effect (group IIb, n=7) (Fig. 1).
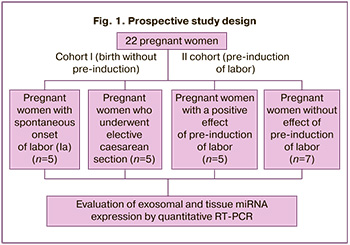
In patients in cohort II, pre-induction and subsequent induction of labor were performed according to the 2021 Clinical Guidelines “Failed attempt to induce labor (preparing the cervix for labor and inducing labor)”.
Preparation of the birth canal for childbirth began with an “immature” cervix (from 0 to 5 points on the Bishop scale); labor induction was performed upon reaching the “mature” birth canal (from 8 points on the Bishop scale and above).
For preinduction, a 200 mg tablet of mifepristone was used. In the case of a “response” to pre-induction in the form of achieving a birth canal maturity of 8 points or higher on the Bishop scale, or the appearance of regular labor within 24 hours, this category of women was assigned to group Ia; in the absence of this effect, to group IIb.
The study was reviewed and approved by the Research Ethics Committee of V.I. Kulakov NMRC for OG&P. Written informed consent was obtained from all the patients.
Obtaining exosomes from the blood plasma of pregnant women and fetal umbilical cord blood
Venous maternal blood was collected once for the control group during hospitalization and twice for the study group before pre-induction of labor and after induction of labor. Cord blood was collected from the umbilical cord artery and vein during cesarean section between two clamps placed on the umbilical cord. Blood samples were collected in VACUETTE tubes containing EDTA (Becton Dickinson). Whole blood was centrifuged at 300×g and 4°C for 20 min, and the collected plasma phase was centrifuged at 16,000×g for 10 min. The supernatant, without contact with the sediment, was transferred to a clean test tube. To obtain exosomes from the blood plasma (0.5 ml of blood plasma, the miRCURY Exosome Serum/Plasma Kit (Qiagen, Germany) was used in accordance with the manufacturer's instructions. Exosome samples were stored at -75°C before isolation and assessment of miRNA expression.
Isolation of miRNA from exosomes
To isolate miRNAs, 200 μl of a suspension of the obtained exosomes was used, to which QIAzol Lysis Reagent (1 ml) and chloroform (200 μl) were added with successive incubations. They were then centrifuged at 12,000 g (+4°C) for 15 min. Up to 600 μl of the aqueous phase was transferred to a clean tube, and subsequent miRNA isolation was carried out using the miRNeasy Serum/Plasma kit (Qiagen, Germany) using a QIAcube automatic station (Qiagen).
Isolation of total RNA from myometrial tissue and fetal membranes, enrichment of the miRNA fraction
Samples of myometrial tissue (taken from the upper part of the incision on the uterus in the lower uterine segment) and membranes were collected during cesarean section no later than 10 min after delivery. The samples were placed in a cryovial and immersed in liquid nitrogen, where they were transported for further storage at -75°C.
Tissue samples were homogenized in QIAzol Lysis Reagent for further isolation of total RNA from to 5-10 mg of tissue using the miRNeasy Micro Kit (Qiagen, Germany), followed by enrichment with the low-molecular-weight miRNA fraction (RNeasy MinElute Cleanup Kit, Qiagen, Germany) using an automatic station QIAcube (Qiagen). All the procedures were performed in accordance with the manufacturer's protocol. The quality of the isolated samples was checked on an Agilent 2100 bioanalyzer (Agilent Technologies) using an RNA 6000 Nano Kit, and the concentration was measured on a Qubit 3.0 fluorometer (Invitrogen). Samples falling within the range of ribosomal RNA molar concentration ratios (28S and 18S) of 1.5–1.8 were used for further research.
Real-time quantitative reverse transcription polymerase chain reaction
Using a reaction mixture (“miRCURY LNA RT Kit,” Qiagen), the miRNAs from each sample were converted into cDNA. UniSp6 RNA Spike-in was used as an internal control for cDNA synthesis efficiency and real-time quantitative polymerase chain reaction (PCR) according to the manufacturer's protocol. To determine the level of miRNA expression in myometrial tissue, fetal membranes, and exosomes isolated from maternal blood plasma and fetal cord blood, quantitative real-time PCR (miRCURY LNA SYBR Green PCR Kit, Qiagen) and miRCURY LNA miRNA PCR Assay primers were used. specific for the miRNAs under study: hsa-let-7b-5p (00204750), hsa-miR-9-5p (00204513), hsa-miR-26b-5p (00204172), hsa-miR-92a-3p (00204258), hsa-miR-146a-5p (00204688), hsa-miR-181a-5p (00206081), hsa-miR-301b-3p (00204390), hsa-miR-454-3p (00205663), hsa-miR-548g-5p (02114059). The reaction was performed in a StepOnePlus thermal cycler (Applied Biosystems, USA). Comparison of miRNA expression levels in the samples relative to the reference molecule (UniSp6 RNA Spike-in) was carried out using the 2(-ΔΔCT) method [16].
Statistical analysis
The statistical significance of the differences in miRNA expression levels between the study groups was assessed using the Wilcoxon–Mann–Whitney test with scripts written in the R language (https://www.R-project.org/). The non-parametric Spearman’s rank correlation method was used to assess the relationship between miRNA expression and clinical parameters in pregnant women. In the case of a non-normal distribution, the parameter was described as the median (Me) and quartiles (Q1; Q3).
Results
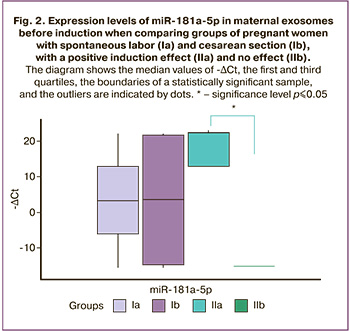
A pairwise comparison between groups showed that the expression level of miR-181a-5p in maternal exosomes before induction was significantly higher in group IIa than in group IIb (p=0.03) (Fig. 2).
The expression level of exosomal miR-92a-3p in umbilical cord blood (umbilical cord artery) was higher than that in the blood of pregnant women before induction in groups IIa (p=0.009) and IIb (p=0.03). At the same time, a higher level of miR-181a-5p expression was noted in exosomes of umbilical cord blood relative to the blood of pregnant women obtained before induction in group IIb (p=0.01) (Fig. 3) (Table 1).
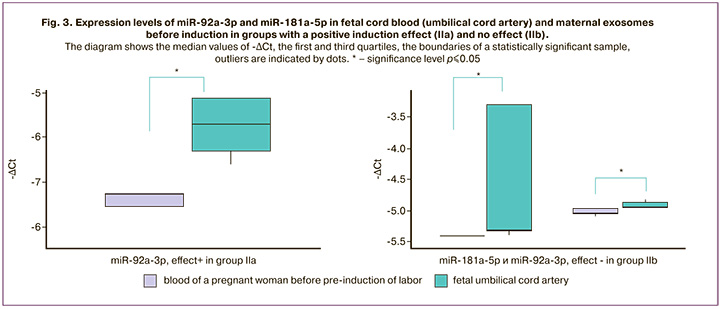
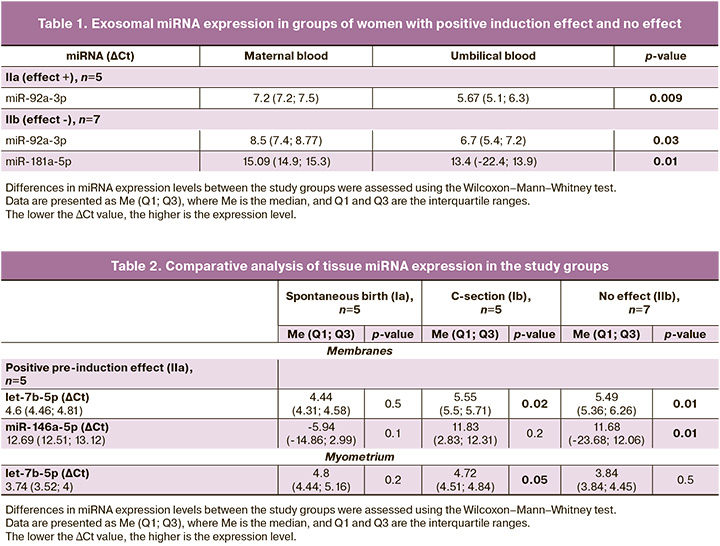
The expression of other miRNAs did not show any significant changes in exosomes. However, it is interesting that some miRNAs, in particular miR-454-3p and miR-548g-5p, were detected only in umbilical cord blood samples, but not in the blood of women. Notably, these miRNAs regulate the expression of the lung surfactant protein SFTPA1. Most likely, their lack of expression in the blood of pregnant women is because of their secretion by the fetus.
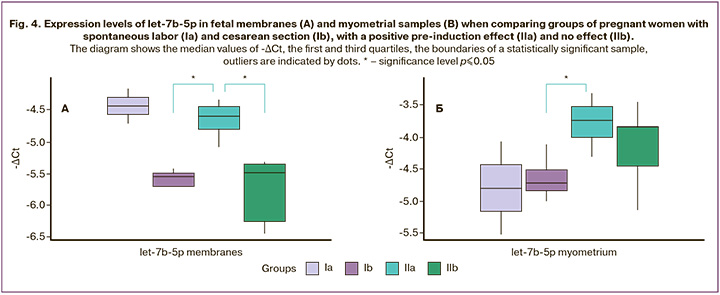
The expression of tissue miRNAs isolated from the fetal membranes and myometrium was compared between the same groups. Pairwise comparison revealed a significantly higher level of let-7b-5p expression in the membranes of group IIa than in groups Ib (p=0.02) and IIb (p=0.01) (Fig. 4A). At the same time, the myometrial expression of let-7b-5p was also significantly higher in group IIa than in group Ib (p=0.05) (Fig. 4B, Table 2).
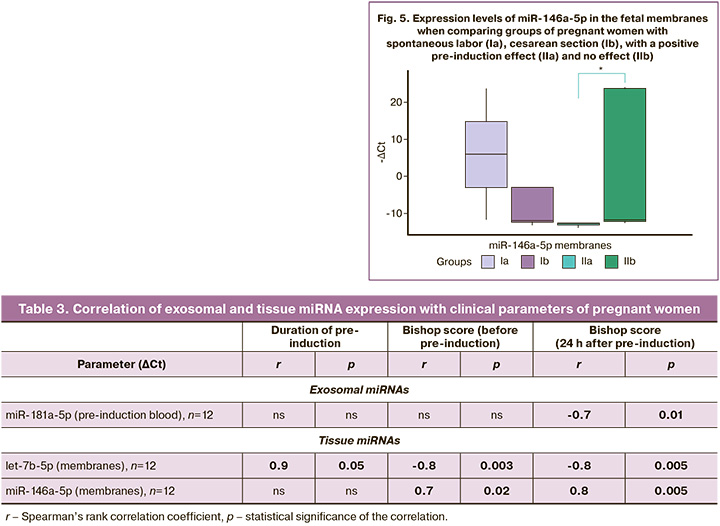
In addition, the expression of miR-146a-5p in the membranes of pregnant women in group IIa was significantly lower than that in group IIb (p=0.01) (Fig. 5).
In general, in pregnant women with a positive effect of pre-induction of labor, the level of let-7b-5p expression in the fetal membranes and myometrium is higher than that in pregnant women with its absence and pregnant women who did not undergo induction.
Considering the identified changes, we assessed the relationship between the expression of exosomal and tissue miRNAs and clinical parameters in groups of pregnant women using nonparametric Spearman rank correlation (Table 3).
The study groups were pooled for the correlation search. The main clinical indicators with which significant correlations were found were the assessment of cervical maturity and start of induction.
The presence of exosomal miR-181a-5p in the blood of pregnant women before induction was associated with an increase in the Bishop score 24 h after induction (r=-0.7; p=0.01). In fetal membranes, increased expression of let-7b-5p was associated with the Bishop score before induction (r=-0.8; p=0.003), with an increase in scores 24 h after induction (r=-0.8; p=0.005) and a decrease in the duration of induction (r=0.9; p=0.05). Low miR-146a-5p expression in membranes also correlated with increased Bishop scores (r=0.7, p=0.02; r=0.8; p=0.005, respectively).
Discussion
miRNAs regulate protein expression at the post-transcriptional level by interacting with target genes. In addition, each miRNA can potentially regulate hundreds of genes, thereby exerting a broad influence on global protein expression.
The coordinated interface between the fetoplacental and maternal systems is a complex multidimensional array of tissues and circulating factors, including the developing fetus, placenta, decidua, and maternal cardiovascular system [17]. The interaction is carried out through extracellular vesicles (exosomes, microvesicles, and apoptotic vesicles), which are secreted by various types of cells, carry a certain cargo (proteins, lipids, mRNA transcripts), have the ability to modulate the function of target cells, and have therapeutic potential [18–21]. Changes in the expression of circulating miRNAs can affect genes located in cells distant from the site and cell type in which they are expressed. Menon R. et al., using deep sequencing, characterized changes in the expression of exosomal miRNA circulating in maternal blood in full-term pregnancy and preterm birth. Highly expressed miRNAs are associated with inflammation and cellular movement during full-term pregnancy. In addition, data have shown that exosomes carry a specific set of miRNAs, which depends on gestational age [22]. We did not find any data in the literature on the expression of exosomal miRNAs during labor induction. In this regard, the expression of exosomal miRNAs was evaluated in maternal blood on the eve of spontaneous labor, before and after labor induction, and in fetal cord blood. It should be emphasized that the criteria for selecting miRNAs for this study were based on the regulation of key genes involved in the mechanisms of inflammatory signaling that contribute to the onset of labor.
The expression level of miR-181a-5p in exosomes before the onset of labor induction was higher in pregnant women with a positive effect, in contrast to pregnant women with no effect of induction. Accumulating evidence supports the involvement of the miR-181 family in the modulation of inflammatory pathways in various cell types [12, 23, 24]. Gao L. et al. conducted a study demonstrating that miR-181a expression in the myometrium decreases in late pregnancy as its pro-inflammatory targets (tumor necrosis factor (TNF-α), c-Fos and estrogen receptor (ERα) and increased estradiol-17β signaling) increase ERα [12]. Our study did not reveal changes in the expression of miR-181a-5p in the myometrium; however, its increased expression in the exosomes of pregnant women may be a reflection of tissue processes. If the repression of miR-181a-5p, mediated by increased estrogen production, initiates a series of pro-inflammatory pathways leading to the onset of labor, then in the exosomes of pregnant women before induction, the opposite is observed: an increase in its expression, resulting in the initiation of inflammatory reactions does not occur. Another proven target of miR-181a-5p is HMGB1, which is part of the endogenous origin of DAMP molecules associated with damage, and induces an inflammatory response [25]. As previously reported, HMGB1 can bind to the TLR4/RAGE receptor of the downstream NFKB pathway to initiate inflammation [26], and miR-181a-5p attenuates the inflammatory response of cells by directly inhibiting HMGB1. Thus, the increased expression of miR-181a-5p may create a certain block the initiation of the inflammatory response. However, it is interesting that in pregnant women with no effect on induction, there is a decrease in its expression in the blood before the onset of induction. Therefore, in this case, nothing should interfere with the initiation of the inflammatory cascade. However, despite this, such pregnant women have difficulty responding to the induction. We suggest that the initiation of inflammatory responses to initiate labor in such pregnant women is most likely not limited to the initiation of the classical inflammatory pathway. Other miRNAs and targets may be involved or inflammation may develop through a non-canonical pathway.
Signals of the inflammatory response leading to full-term delivery come from both the mother [27] and fetus [28, 29]. It should be noted that in both pregnant women with a positive effect from pre-induction and those with no effect, the expression of exosomal miR-92a-3p was significantly increased in the umbilical cord blood of the fetus relative to the blood of pregnant women taken before induction. Several studies have described the role of miR-92a-3p, which mediates inflammatory reactions by promoting the secretion of TNF-α and interleukin-1β by macrophages through the NFKB signaling pathway [30–32]. Interestingly, the expression of miR-181a-5p is reduced in the blood of pregnant women before pre-induction. Therefore, in this case, inflammatory signaling may be mediated by both the mother and fetus. Thus, the success of the induction may depend on the coordinated transmission of inflammatory signals, which is modulated, among other things, by the expression of miR-181a-5p and miR-92a-3p.
During most pregnancies, myometrial quiescence is maintained by elevated circulating progesterone (P4) levels, which act through the nuclear progesterone receptor (PR). This effect of P4–PR signaling is mediated in part by its anti-inflammatory effects, the ability to suppress the expression of genes encoding pro-inflammatory cytokines (interleukins 1, 6, and 8, chemokines), and contraction-associated proteins such as OXTR, GJA1, and PTGS2. Signaling also blocks NF-kB activation by increasing the expression of IκBα, an NF-κB inhibitor. Conversely, increased expression of pro-inflammatory genes is mediated by estradiol-17β and ERα signaling, which reduces PR function, thereby further enhancing the inflammatory response and ultimately leading to parturition [33]. Therefore, miRNAs may be hormonally modulated mediators of gene expression that maintain the balance between anti-inflammatory and pro-inflammatory signaling pathways [33].
In this regard, taking into account changes in miRNA expression in pre-induction exosomes and fetal cord blood, we assessed the profile of tissue miRNAs isolated from the fetal membranes and myometrium of pregnant women at the time of delivery. Changes in the expression of let-7b-5p, which belongs to the let-7 family, were detected in gestational tissues. The latter is considered a major regulator of fundamental cellular processes, including differentiation, pluripotency, proliferation, invasion, and migration, and plays an important role in implantation, placentation, and fetal growth [34]. The analysis revealed a significant increase in the expression level of let-7b-5p in the membranes of pregnant women with a positive response to pre-induction relative to pregnant women who underwent cesarean section without induction and pregnant women with no response. At the same time, in the myometrium, the expression of let-7b-5p was not significantly different between the groups with and without a response. However, it was also increased relative to that in the intact myometrium. Of note, potential targets of let-7b-5p include NF-κB and PTGS2, among others. Interestingly, Chan et al. et al. tissue-specific expression of the let-7 family was previously established in the placenta, choriodecidua, and amnion at the time of delivery, and its target Lin28 was not expressed in fetal membranes. However, the authors did not observe significant differences in expression depending on the onset of labor and delivery, despite the fact that the Lin28/let-7 signaling axis is associated with inflammatory signals. The researchers concluded that tissue inflammation during labor is predominantly mediated by the classical NF-kB signaling pathway [35]. Other authors have shown that increased expression of let-7 inhibits the production of interleukin-6. In this case, NF-kB activates Lin28B, suppressing its expression, which leads to the production of interleukin-6 and subsequent inflammation [36]. Decreased let-7b expression is associated with increased TLR4 regulation, NF-κB activation, and increased PTGS2 expression [37]. Considering our data demonstrating, on the contrary, increased expression of let-7b-5p in the fetal membranes and myometrium, it is likely that let-7b-5p is involved in the hormonal regulation of labor rather than the initiation of the inflammatory response. Our assumption confirms the previously described role of let-7b in the regulation of steroid hormone production [38]. In particular, let-7b can influence estrogen secretion by binding to the Smad2/3 gene in the transforming growth factor (TGF-β) signaling pathway [39]. Furthermore, let-7b expression can be directly induced by estradiol [40]. Moreover, the expression of let-7b-5p in both the myometrium and fetal membranes in pregnant women with a positive effect of pre-induction was significantly different from that in pregnant women who did not receive pre-induction. This may also be an indirect evidence of hormonal regulation mediated by let-7b-5p. In addition, we noticed a significant difference between the groups of pregnant women with spontaneous labor and those with induced expression of let-7b-5p in the fetal membranes and myometrium. This difference was not statistically significant due to the small sample size, although such a trend can be traced.
In the fetal membranes of pregnant women after a positive response to pre-induction, the expression of miR-146a-5p was significantly reduced compared to that in pregnant women with no effect. MiR-146-5p, like other miRNAs, can target a wide range of target genes and thus be involved in the regulation of multiple independent physiological processes. NF-kB and PTGS2 are also considered among its potential targets. It is known that miR-146a-5p is involved in controlling the inflammatory response of cells of the innate immune system, especially monocytes/macrophages. Acting through the NF-kB pathway, miR-146a-5p as a negative regulator can stimulate the release of proinflammatory cytokines [41]. Previously, experiments with tumor cells revealed the regulation of expression along the miR-146a-5p/COX2 axis; low levels of miR-146a-5p are associated with increased levels of COX2 [42]. Cook et al. established the effect of oxytocin on the suppression of miR-146a-5p expression in a culture of primary myocytes, thereby emphasizing that its expression is dependent on the administration of oxytocin [36]. In our study, we did not use oxytocin as a stimulant, and no significant changes in the expression of miR-146a-5p in the myometrium were detected. Therefore, changes in its expression in fetal membranes through the regulation of COX2 may indicate the involvement of miR-146a-5p in the transmission of inflammatory signals from the fetus that stimulate labor.
Considering the obtained data on changes in the expression of the studied miRNAs, we conducted a correlation search using the Spearman nonparametric rank correlation method. The analysis revealed that the expression of miR-181a-5p in the maternal blood before induction and let-7b-5p and miR-146a-5p in the fetal membranes correlated with Bishop scores, confirming their involvement in the initiation of labor.
Limitations of the study
The limitations of our study are related to the small sample size; therefore, there is a need for further study of the signaling molecules that trigger the initiation of labor in an expanded cohort of pregnant women.
Conclusion
The identified differences in the expression of exosomal and tissue miRNAs are due to the highly coordinated regulation of different signaling pathways and modulation of the expression of the corresponding target genes involved in the mechanism of communication between the maternal and fetal compartments.
References
- Saucedo A.M., Cahill A.G. Evidence-based approaches to labor induction. Obstet. Gynecol. Surv. 2023; 78(3): 171-83. https://dx.doi.org/10.1097/OGX.0000000000001110.
- Marconi A.M. Recent advances in the induction of labor. F1000Res. 2019; 8: F1000 Faculty Rev-1829.2019. https://dx.doi.org/10.12688/f1000research.17587.1.
- Российское общество акушеров-гинекологов (РОАГ). Клинические рекомендации "Неудачная попытка стимуляции родов (подготовка шейки матки к родам и родовозбуждение)". 2021. [Russian Society of Obstetricians and Gynecologists. Clinical guidelines "Failed attempt at labor stimulation (cervical preparation and labor induction)". 2021. (in Russian)]. Available at: https://roag-portal.ru/recommendations_obstetrics
- Guarnieri D.J., DiLeone R.J. MicroRNAs: a new class of gene regulators. Ann. Med. 2008; 40(3): 197-208. https://dx.doi.org/10.1080/07853890701771823.
- Tang Y., Ji H., Liu H., Gu W., Li X., Peng T. Identification and functional analysis of microRNA in myometrium tissue from spontaneous preterm labor. Int. J.Clin. Exp. Pathol. 2015; 8(10): 12811-9.
- Williams K.C., Renthal N.E., Gerard R.D., Mendelson C.R. The microRNA (miR)-199a/214 cluster mediates opposing effects of progesterone and estrogen on uterine contractility during pregnancy and labor. Mol. Endocrinol. (Baltimore). 2012; 26(11): 1857-67. https://dx.doi.org/10.1210/me.2012-1199.
- Renthal N.E., Chen C.-C., Williams K.C., Gerard R.D., Prange-Kiel J., Mendelson C.R. miR-200 family and targets, ZEB1 and ZEB2, modulate uterine quiescence and contractility during pregnancy and labor. Proc. Natl. Acad. Sci. USA. 2010; 107(48): 20828-33. https://dx.doi.org/10.1073/pnas.1008301107.
- Sanders A.P., Burris H.H., Just A.C., Motta V., Svensson K., Mercado-Garcia A. et al. microRNA expression in the cervix during pregnancy is associated with length of gestation. Epigenetics. 2015; 10(3): 221-8. https://dx.doi.org/10.1080/15592294.2015.1006498.
- Williams K.C., Renthal N.E., Condon J.C., Gerard R.D., Mendelson C.R. MicroRNA-200a serves a key role in the decline of progesterone receptor function leading to term and preterm labor. Proc. Natl. Acad. Sci. USA. 2012; 109(19): 7529-34. https://dx.doi.org/10.1073/pnas.1200650109.
- Bracken C.P., Gregory P.A., Kolesnikoff N., Bert A.G., Wang J., Shannon M.F. et al. A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res. 2008; 68(19): 7846-54. https://dx.doi.org/10.1158/0008-5472.CAN-08-1942.
- Sun X., Sit A., Feinberg M.W. Role of miR-181 family in regulating vascular inflammation and immunity. Trends Cardiovasc. Med. 2014; 24(3): 105-12. https://dx.doi.org/10.1016/j.tcm.2013.09.002.
- Gao L., Wang G., Liu W.N., Kinser H., Franco H.L., Mendelson C.R. Reciprocal feedback between miR-181a and E2/ERα in myometrium enhances inflammation leading to labor. J. Clin. Endocrinol. Metabo. 2016; 101(10): 3646-56. https://dx.doi.org/10.1210/jc.2016-2078.
- Li H., Zhou J., Wei X., Chen R., Geng J., Zheng R. et al. miR-144 and targets, c-fos and cyclooxygenase-2 (COX2), modulate synthesis of PGE2 in the amnion during pregnancy and labor. Sci. Rep. 2016; 6: 27914. https://dx.doi.org/10.1038/srep27914.
- Kim S.Y., Romero R., Tarca A.L., Bhatti G., Lee J., Chaiworapongsa T. et al. miR-143 regulation of prostaglandin-endoperoxidase synthase 2 in the amnion: implications for human parturition at term. PloS One. 2011; 6(9): e24131. https://dx.doi.org/10.1371/journal.pone.0024131.
- Montenegro D., Romero R., Kim S.S., Tarca A.L., Draghici S., Kusanovic J.P. et al. Expression patterns of microRNAs in the chorioamniotic membranes: a role for microRNAs in human pregnancy and parturition. J. Pathol. 2009; 217(1): 113-21. https://dx.doi.org/10.1002/path.2463.
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25(4): 402-8. https://dx.doi.org/10.1006/meth.2001.1262.
- Ilekis J.V., Tsilou E., Fisher S., Abrahams V.M., Soares M.J., Cross J.C. et al. Placental origins of adverse pregnancy outcomes: potential molecular targets: an Executive Workshop Summary of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Am. J. Obstet. Gynecol. 2016; 215(1, Suppl.): S1-46. https://dx.doi.org/10.1016/j.ajog.2016.03.001.
- Gurung S., Perocheau D., Touramanidou L., Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021; 19(1): 47. https://dx.doi.org/10.1186/s12964-021-00730-1.
- Jadli A.S., Ballasy N., Edalat P., Patel V.B. Inside(sight) of tiny communicator: exosome biogenesis, secretion, and uptake. Mol. Cell. Biochem. 2020; 467(1-2): 77-94. https://dx.doiorg/10.1007/s11010-020-03703-z.
- Simons M., Raposo G. Exosomes--vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 2009; 21(4): 575-81. https://dx.doi.org/10.1016/j.ceb.2009.03.007.
- Robbins P.D., Morelli A.E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014; 14(3): 195-208. https://dx.doi.org/10.1038/nri3622.
- Menon R., Debnath C., Lai A., Guanzon D., Bhatnagar S., Kshetrapal P.K. et al. Circulating exosomal miRNA profile during term and preterm birth pregnancies: a longitudinal study. Endocrinology. 2019; 160(2): 249-75. https://dx.doi.org/10.1210/en.2018-00836.
- Xie W., Li Z., Li M., Xu N., Zhang Y. miR-181a and inflammation: miRNA homeostasis response to inflammatory stimuli in vivo. Biochem. Biophys. Res. Commun. 2013; 430(2): 647-52. https://dx.doi.org/10.1016/j.bbrc.2012.11.097.
- Hutchison E.R., Kawamoto E.M., Taub D.D., Lal A., Abdelmohsen K., Zhang Y. et al. Evidence for miR-181 involvement in neuroinflammatory responses of astrocytes. Glia. 2013; 61(7): 1018-28. https://dx.doi.org/10.1002/glia.22483.
- Wang L., Bi R., Li L., Zhou K., Yin H. lncRNA ANRIL aggravates the chemoresistance of pancreatic cancer cells to gemcitabine by targeting inhibition of miR-181a and targeting HMGB1-induced autophagy. Aging (Albany NY). 2021; 13(15): 19272-81. https://dx.do.org/ 10.18632/aging.203251.
- Han Y., Chen R., Lin Q., Liu Y., Ge W., Cao H. et al. Curcumin improves memory deficits by inhibiting HMGB1-RAGE/TLR4-NF-κB signalling pathway in APPswe/PS1dE9 transgenic mice hippocampus. J. Cell. Mol. Med. 2021; 25(18): 8947-56. https://dx.doi.org/10.1111/jcmm.16855.
- Jin J., Menon R. Placental exosomes: A proxy to understand pregnancy complications. Am. J. Rep. Immunol. 2018; 79(5): e12788. https://dx.doi.org/10.1111/aji.12788.
- Hadley E.E., Sheller-Miller S., Saade G., Salomon C., Mesiano S., Taylor R.N. et al. Amnion epithelial cell-derived exosomes induce inflammatory changes in uterine cells. Am. J. Obstet. Gynecol. 2018; 219(5): 478. e1-478.e21. https://dx.doi.org/10.1016/j.ajog.2018.08.021.
- Menon R., Mesiano S., Taylor R.N. Programmed fetal membrane senescence and exosome-mediated signaling: a mechanism associated with timing of human parturition. Front. Endocrinol. 2017; 8: 196. https://dx.doi.org/10.3389/fendo.2017.00196.
- Lee H., Zhang D., Wu J., Otterbein L.E., Jin Y. Lung epithelial cell-derived microvesicles regulate macrophage migration via MicroRNA-17/221-induced integrin β1 recycling. J. Immunol. 2017; 199(4): 1453-64. https://dx.doi.org/10.4049/jimmunol.1700165.
- Slattery M.L., Mullany L.E., Sakoda L., Samowitz W.S., Wolff R.K., Stevens J.R. et al. The NF-κB signalling pathway in colorectal cancer: associations between dysregulated gene and miRNA expression. J. Cancer Res. Clin. 2018; 144(2): 269-83. https://dx.doi.org/10.1007/s00432-017-2548-6.
- Lee H., Zhang D., Zhu Z., Dela Cruz C.S., Jin Y. Epithelial cell-derived microvesicles activate macrophages and promote inflammation via microvesicle-containing microRNAs. Sci. Rep. 2016; 6: 35250. https://dx.doi.org/10.1038/srep35250.
- Renthal N.E., Williams K.C., Mendelson C.R. MicroRNAs--mediators of myometrial contractility during pregnancy and labour. Nat. Rev. Endocrinol. 2013; 9(7): 391-401. https://dx.doi.org/10.1038/nrendo.2013.96.
- Ali A., Bouma G.J., Anthony R.V., Winger Q.A. The role of LIN28-let-7-ARID3B pathway in placental development. Int. J. Mol. Sci. 2020; 21(10): 3637. https://dx.doi.org/10.3390/ijms21103637.
- Chan H.W., Lappas M., Yee S.W.Y., Vaswani K., Mitchell M.D., Rice G.E. The expression of the let-7 miRNAs and Lin28 signalling pathway in human term gestational tissues. Placenta. 2013; 34(5): 443-8. https://dx.doi.org/10.1016/j.placenta.2013.02.008.
- Cook J.R., MacIntyre D.A., Samara E., Kim S.H., Singh N., Johnson M.R. et al. Exogenous oxytocin modulates human myometrial microRNAs. Am. J. Obstet. Gynecol. 2015; 213(1): 65.e1-65.e9. https://dx.doi.org/10.1016/j.ajog.2015.03.015.
- Teng G., Wang W., Dai Y., Wang S., Chu Y., Li J. Let-7b is involved in the inflammation and immune responses associated with Helicobacter pylori infection by targeting Toll-like receptor 4. PloS One. 2013; 8(2): e56709. https://dx.doi.org/10.1371/journal.pone.0056709.
- Dai T., Kang X., Yang C., Mei S., Wei S., Guo X. et al. Integrative analysis of miRNA-mRNA in ovarian granulosa cells treated with kisspeptin in tan sheep. Animals (Basel). 2022; 12(21): 2989. https://dx.doi.org/10.3390/ani12212989.
- Zhang X.D., Zhang Y.H., Ling Y.H., Liu Y., Cao H.G., Yin Z.J. et al. Characterization and differential expression of microRNAs in the ovaries of pregnant and non-pregnant goats (Capra hircus). BMC Genomics. 2013; 14: 157. https://dx.doi.org/10.1186/1471-2164-14-157.
- Bhat-Nakshatri P., Wang G., Collins N.R., Thomson M.J., Geistlinger T.R., Carroll J.S. et al. Estradiol-regulated microRNAs control estradiol response in breast cancer cells. Nucleic Acids Res. 2009; 37(14): 4850-61. https://dx.doi.org/10.1093/nar/gkp500.
- Taganov K.D., Boldin M.P., Chang K.-J., Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. USA. 2006; 103(33): 12481-6. https://dx.doi.org/10.1073/pnas.0605298103.
- Mohammad N.S., Nazli R., Zafar H., Fatima S. Effects of lipid based multiple micronutrients supplement on the birth outcome of underweight pre-eclamptic women: A randomized clinical trial. Pak. J. Med. Sci. 2022; 38(1): 219-26. https://dx.doi.org/10.12669/pjms.38.1.4396.
Received 15.01.2024
Accepted 24.01.2024
About the Authors
Asiyat R. Gaidarova, PhD Student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, a_gaydarova@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4, https://orcid.org/0000-0003-1415-3318Vladislava A. Gusar, PhD, Senior Researcher at the Laboratory of Applied Transcriptomics of the Department of Systems Biology in Reproduction, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, v_gusar@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4, https://orcid.org/0000-0003-3990-6224
Vitaliy V. Chagovets, PhD, Head of the Laboratory of Metabolomics and Bioinformatics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, v_chagovets@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4, https://orcid.org/oooo-0002-5120-376X
Irina V. Edilberg, PhD Student, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia (Sechenov University),
i_edilberg@oparina4.ru, 119991, Russia, Moscow, Bolshaya Pirogovskaya str., 2-4, https://orcid.org/0000-0003-4194-8730
Natalia E. Kan, Professor, Dr. Med. Sci., Deputy Director for Science, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, kan-med@mail.ru, 117997, Russia, Moscow, Ac. Oparina str., 4, Researcher ID: B-2370-2015, SPIN-код: 5378-8437, Authors ID: 624900, Scopus Author ID: 57008835600, https://orcid.org/0000-0001-5087-5946
Oleg R. Baev, Dr. Med. Sci., Professor, Head of the 1st Maternity Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4; Professor of the Department of Obstetrics, Gynecology, Perinatology and Reproductology, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia ((Sechenov University), 119991, Russia, Moscow, Trubetskaya str., 8-2, o_baev@oparina4.ru, https://orcid.org/0000-0001-8572-1971
Corresponding author: Asiyat R. Gaidarova, a_gaydarova@oparina4.ru



