Quantitative analysis of piwiRNAs in the culture medium of euploid and aneuploid blastocysts as an additional method of selecting a high-quality embryo for transfer to the uterine cavity in assisted reproductive technology programs
Timofeeva A.V., Fedorov I.S., Savostina G.V., Ekimov A.N., Perminova S.G.
Objective: To identify piwiRNAs in the culture medium of a blastocyst associated with cell ploidy and the implanting ability of an embryo after transfer into the uterine cavity.
Materials and methods: The study included 73 couples whose culture medium of 93 embryos was analyzed; among them there were 53 euploid blastocysts (according to the results of PGT-A) with implantation (27 embryos) and without implantation (26 embryos) after transfer of cryopreserved embryos into the uterine cavity in ART programs using cyclic hormone therapy; 40 blastocysts were aneuploid. piwiRNAs were isolated from 20 µl of the culture medium with the miRNeasy Serum/Plasma Kit (Qiagen) and analyzed using deep sequencing on the NextSeq 500/550 platform (Illumina, USA). The obtained data were subsequently validated by quantitative polymerase chain reaction in real time with the miScript II RT Kit and miScript SYBR Green PCR Kit (Qiagen, Hilden, Germany).
Results: Two logistic regression models were built after quantification of hsa_piR_020497 and hsa_piR_020829, or hsa_piR_016677 and hsa_piR_020829, which have 93% and 100% specificity, respectively, in identifying euploid blastocysts with high implantation potential. The miRanda algorithm was used to analyze potential target genes of piwiRNAs associated with aneuploidy, which are involved in the formation of the spindle apparatus, the development and functioning of kinetochore, and cytokinesis.
Conclusion: A noninvasive method of selecting euploid embryo with a high implantation potential for transfer into the uterine cavity in ART programs was developed. At this stage of the study, this method is additional but not alternative to the PGT-A method.
Authors’ contributions: Timofeeva A.V. – obtaining experimental data, writing the article; Fedorov I.S. – obtaining experimental data and their statistical processing; Savostina G.V. – collecting samples of the blastocyst culture medium, creating the clinical database of patients, writing the article; Ekimov A.N. – preimplantation genetic testing of embryos for aneuploidy; Perminova S.G. – editing the article.
Conflicts of interest: Authors declare lack of the possible conflicts of interest.
Funding: The study was carried out with the financial support of the State Assignment “Solving the problem of infertility under the current conditions, by creating a clinical diagnostic model of infertile marriage and using innovative technologies in assisted reproduction programs”, registration number 22-A21.
Ethical Approval: The study was approved by the Ethical Review Board of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
Patient Consent for Publication: The patients provided an informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Timofeeva A.V., Fedorov I.S., Savostina G.V., Ekimov A.N., Perminova S.G. Quantitative analysis of piwiRNAs in the culture medium of euploid and aneuploid blastocysts as an additional method of selecting a high-quality embryo for transfer to the uterine cavity in
assisted reproductive technology programs. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (11): 115-130 (in Russian)
https://dx.doi.org/10.18565/aig.2023.180
Keywords
The positive outcome of assisted reproductive technology (ART) programs depends on many factors but the key determinants are a high-quality embryo, receptive endometrium and a complex cross-talk between them [1, 2]. However, the transfer of a good-quality embryo into the uterine cavity in the appropriate phase of the menstrual cycle does not lead to pregnancy in all cases [3]. Chromosomal abnormalities in embryos play a significant role in the etiology of implantation failures and early pregnancy losses. More than half of cases of early pregnancy losses are known to be associated with aneuploid embryos [4]. The frequency of embryos with chromosomal abnormalities has a stable correlation with the age of a woman. For example, the rate of aneuploid embryos in women aged 26–30 years is 20–27%, while the rate of chromosomal abnormalities in women aged 45 years is 95.5% of embryos [5, 6]. Given the current trend towards later childbearing, aneuploid embryo formation is critically important, and the possibility of identifying embryos with chromosomal abnormalities at the preimplantation stage is especially relevant.
The visual methods which are used in clinical practice for the assessment of embryo quality do not reflect the chromosomal status and do not exclude the transfer of an aneuploid embryo into the uterine cavity [7]. About 44.5% of aneuploid blastocysts have a normal morphological structure [8]. The only method for assessing the ploidy of embryos used in clinical practice nowadays is preimplantation genetic testing for aneuploidy (PGT-A). The probability of the transfer of aneuploid embryo into the uterine cavity was reduced after the introduction of PGT-A; however, about 50% of euploid embryos are not implanted. The effect of PGT-A on the outcomes of ART programs in various groups of patients is subject of discussion [9–13]. In addition to the undeniable advantages, PGT-A has a number of disadvantages, including invasiveness, high cost and complexity, as well as the presence of false results, which are explained by the inability to determine accurately the percentage of mosaic cells in the embryo using the analysis of only a few trophectoderm cells [14].
One of the most promising areas in reproduction is studying the role of post-transcriptional regulators of expression of protein-coding genes in embryogenesis and the possibility of using them as biomarkers of embryo quality. Much attention in this group is given to small non-coding RNAs [15–17], and the most numerous class among them in humans is piwiRNA (8438265 types of molecules according to piRBase v.2.0) in comparison with microRNA (2600 varieties of molecules according to miRBase v.22). A characteristic feature of piwiRNAs is the regulation of the stability of the cell genome by suppressing the activity of mobile genetic elements, namely transposons [18, 19]. The existing mechanisms for controlling the degree of expression and mobilization of transposons are subtly regulated, especially in germ line cells, since complete suppression of transposon expression prevents the normal development of gametes and embryos [20].
To date, the researchers have identified characteristics of microRNA expression in the culture media of embryos at different stages of embryogenesis, depending on the ploidy of their cells, the ability to implant and the outcomes of ART programs [21, 22]. However, the scientists have not studied the role of piwiRNA as a marker of the ploidy of embryo cells for selecting the highest quality blastocyst and its transfer into the uterine cavity, except for our previous studies on the prognostic significance of this class of molecules in assessing the implantation potential of the embryo at the stage of morula and blastocyst without taking into account the karyotype of their cells [23, 24].
Therefore, the aim of this study was to analyze the content of piwiRNAs in the culture medium of a blastocyst with a characteristic karyotype according to PGT-A data for the identification of molecules associated with the ploidy of embryo cells and optimization of selective embryo transfer in the ART programs.
Materials and methods
A total of 343 couples participated in the study. They presented to the National Medical Research Centre for Obstetrics, Gynecology and Perinatology, Moscow for IVF/ICSI+PGT-A (in vitro fertilization with intracytoplasmic sperm injection and subsequent PGT-A testing) in the period from 2018 to 2021. The analysis of 400 cycles of IVF/ICSI+PGT-A and 309 cryoprotocols was carried out. PGT-A was performed on 1115 embryos out of 2046 embryos on day 5 of cultivation.
There were the following inclusion criteria: the absence of pregnancy for one year or more, the normal karyotype of the spouses, indications for PGT-A, such as the older reproductive age of the woman (35 years or more), recurrent pregnancy loss (the history of two or more spontaneous abortions up to 22 weeks), repeated unsuccessful attempts of ART (three or more unsuccessful attempts of selective fresh and frozen-thawed embryo transfer) or severe pathozoospermia in the partner (the concentration of sperm is less than 5 million/ml, the number of progressively motile spermatozoa (a+b) is 19% or less, the number of morphologically normal spermatozoa is 1% or less according to the results of the spermogram).
The non-inclusion criteria were all patient’s conditions which are contraindications to ART and pregnancy in accordance with the Order of the Ministry of Health of the Russian Federation dated July 31, 2020 No. 803n.
All couples that presented to the Centre for the IVF/ICSI + PGT-A program underwent a full clinical and laboratory examination.
The patients underwent hormonal stimulation of the ovaries according to the standard protocols with a gonadotropin-releasing hormone antagonist (antGnRH) or with a gonadotropin-releasing hormone agonist (aGnRH). Transvaginal puncture of the ovaries was performed in aseptic conditions under intravenous anesthesia through 35-36 hours after the introduction of the ovulation trigger. IVF or ICSI was performed depending on the parameters of the spermogram and the history of the couple.
All embryos were cultured individually in droplets in multi-gas incubators. The quality of the obtained embryos was assessed on day 5 of cultivation on the basis of morphological criteria according to the Gardner grading system (ESHRE 2011 modified D. Gardner classification). Samples of culture media of embryos suitable for PGT-A were transferred to Eppendorf tubes and cryopreserved. The embryos of good and excellent quality underwent biopsy of trophectoderm cells followed by cryopreservation of the embryo. The obtained trophectoderm cells were transferred to Eppendorf tubes containing a lysis buffer. Subsequent PGT-A analysis was performed by next-generation sequencing (NGS). PGT-A-detected euploid embryos were transferred to the uterine cavity as part of a cryopreserved embryo transfer on cyclic hormonal therapy or in the natural menstrual cycle when endometrial thickness was greater than 7 mm. On day 10 after embryo transfer to the uterus, a human chorionic hormone gonadotropin (hCG) β-subunit blood test was performed. A transvaginal ultrasound scan was performed 21 days after the transfer to diagnose a clinical pregnancy in case of a positive result of a blood test for β-hCG. Further monitoring and pregnancy management were individually performed by the patient’s doctor.
After the results of PGT-A and the outcomes of ART programs, 73 couples were selected whose culture medium of 93 embryos was analyzed. These embryos were divided into the following groups:
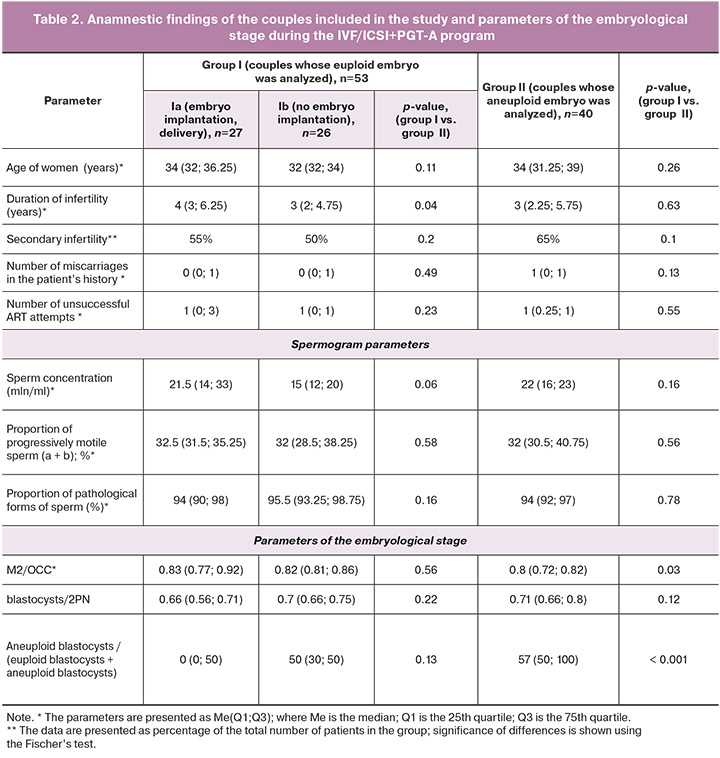
- group I (n=53) included euploid embryos and was divided into two subgroups: group Ia (n=27) consisted of embryos, the transfer of which led to implantation and subsequently to childbirth, group Ib (n=26) included embryos that were not implanted after the transfer into the uterine cavity;
- group II (n=40) included aneuploid embryos (numerical or structural chromosome abnormalities on one chromosome – 65% of cases, two chromosomes – 30% of cases, three chromosomes – 5% of cases; numerical anomalies prevailed among chromosomal disorders (53%); structural changes of chromosomes were detected in 33% of cases; combined changes were much less common (14%); aneuploidy was detected on 3–7, 10–19, 21, 22, X chromosomes.
Culture media (n=3) without embryo cultivation in them for 5 days were used as a control group.
RNA isolation from culture media samples
RNAs were isolated from the collected 96 samples of culture media by the column technique using the miRNeasy Serum/Plasma Kit (Qiagen).
Deep sequencing of small non-coding RNAs
Six of the fourteen microliters of column eluate miRNeasy Serum/Plasma Kit (Qiagen) containing RNAs from the culture medium were used to synthesize cDNA Library NEBNext® Multiplex Small RNA Library Prep Set for Illumina (Set11, New England Biolab), amplified over 30 cycles during polymerase chain reaction (PCR), purified in 6% polyacrylamide gel and sequenced on the NextSeq 500/550 (Illumina) platform. The sequences in the range from 16 to 50 bp were mapped to human databases GRCh38.p15, miRBase v21 and piRNABase using the Bowtie algorithm [25]. The differential expression of piwiRNA was analyzed using the DESeq2 software package [26].
Reverse transcription and quantitative polymerase chain reaction in real time
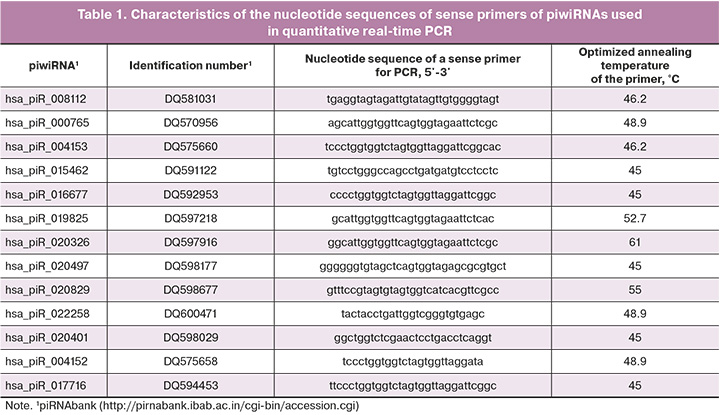
Five of the fourteen microliters of column eluate miRNeasy Serum/Plasma Kit (Qiagen) containing RNAs from the culture medium were used to synthesize cDNA with the help of miScript II RT Kit (Qiagen) following the protocol of the manufacturer. Quantitative real-time polymerase chain reaction (PCR) was performed using the miScript SYBR Green PCR Kit (Qiagen, Hilden, Germany) and sense primers specific to certain mRNAs and piwiRNAs (Table 1). The PCR program included the following: (1) 15 min at 95°C and (2) 40 cycles: 94°C for 15 s, optimized annealing temperature (45–61.6°C) for 30 s and 70°C for 30 s; (3) heating of the reaction mixture from 65 to 95 °C step 0.1°C to plot the melting curve of the PCR product in the thermal cycler StepOnePlus (Applied Biosystems). The relative expression of piwiRNAs in the culture medium was determined with the help of ∆∆Ct method using hsa_piR_022258 as a reference RNA and medium without embryo cultivation as a control sample.
Statistical analysis
For statistical processing of the results, we used scripts written in the R language [27] and the RStudio program [28]. The compliance of the analyzed parameters with the normal distribution was evaluated using the Shapiro–Wilk test. Statistical analysis was carried out using the Mann–Whitney U-test with a pairwise comparison when normal distribution could not be applied. The parameters which have a distribution other than normal are presented in the format Me (Q1; Q3). The significance level p was assumed to be 0.05. If the p-value was less than 0.001, it was indicated as p<0.001. Absolute values and percentage of the total number in the group were used to describe categorical binary data, and the Fisher's test was used for comparative analysis of the samples.
The logistic regression method was chosen as a tool for developing probabilistic models of the occurrence of an event. In order to assess the quality of the models, the method of ROC analysis (Receiver operating characteristic) was used, which is a graphical representation and shows the dependence of true positive examples on false negative ones. Cut-off points (threshold values) which demonstrated the most adequate separation of one group from another were found for the proposed mathematical and statistical models.
Logistic regression models (linear regression with logit transformation) were developed using the RStudio program through step-by-step inclusion and exclusion of piwiRNA predictors of receptive endometrium depending on their contribution to the model. The predictive ability of the model was evaluated using ROC analysis by the AUC (Area Under Curve) value, statistical significance, level of specificity and sensitivity.
Results
The study included 73 couples who were selected in such a way as to minimize the influence of interfering factors on the results of ART programs. The evaluation of clinical and laboratory data of the couples in the study groups revealed no statistically significant differences except for a longer period of infertility in group Ia compared to group Ib (Table 2). The couples who had a euploid embryo but had no implantation were characterized by a lower concentration of spermatozoa in comparison with the couples whose pregnancy ended with term delivery after the transfer of the blastocyst (p=0.06). The couples of group II who had an aneuploid embryo were statistically significantly different from the couples of group I who had a euploid embryo and demonstrated a reduced number of mature oocytes (M2) relative to the total number of oocyte-cumulus complexes (OCC) (p=0.03) and an increased number of aneuploid blastocysts relative to the total number of blastocysts (p<0.001). It should be noted that group II showed a trend of an increase in the proportion of aneuploid blastocysts with a decrease in the percentage of mature oocytes and these findings are consistent with the literature data on the occurrence of aneuploidies mainly due to oogenesis disorders [29].
The analysis of the characteristics of the menstrual cycle, ovarian reserve and gynecological history in the groups revealed no statistically significant differences.
In order to identify small non-coding RNAs associated with embryo aneuploidy, deep sequencing of RNAs isolated from 20 samples of blastocyst culture medium was performed: 8 euploid embryos and 12 aneuploid embryos according to PGT-A (seq(22)x1; seq(22)x1; seq(22)x3; seq(7p)x3,(22)x3; seq(5)x1, (7)x1,6; seq(7)x1, (14)x1; seq(12)x3, (16)x3, (21)x3; seq(7,22)x1, (16)x3; seq(18)x1; seq(19)x3; seq(14)x3; seq(12)x3,(22)x1). Due to the important role of piwiRNA in the regulation of the stability of the human cell genome, this type of small non-coding RNA was the subject of this study. A total of 133 piwiRNA types with 10 or more reads were identified.
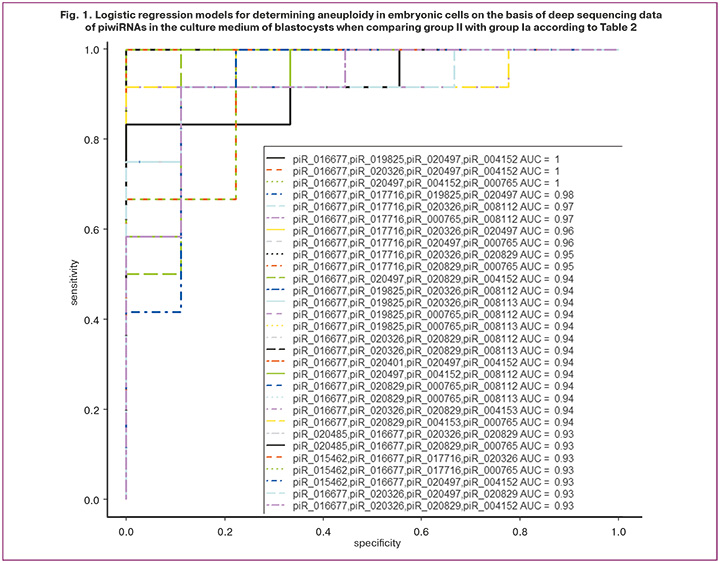
Optimal combinations of RNA markers of aneuploid embryo were found in the RStudio program via gradual inclusion and exclusion of each identified piwiRNA molecule depending on their contribution to the construction of logistic regression models (Fig. 1), where the dependent variable (response variable) was embryo ploidy (0 refers to a euploid embryo transferred to the uterine cavity with the subsequent development of pregnancy and childbirth; 1 refers to an aneuploid embryo that cannot be transferred to the uterine cavity). Twelve piwiRNAs were identified; their various combinations in binary logistic regression models (p<0.001) with high sensitivity (83–100%) and specificity (78–100%) diagnosed the euploid embryo.
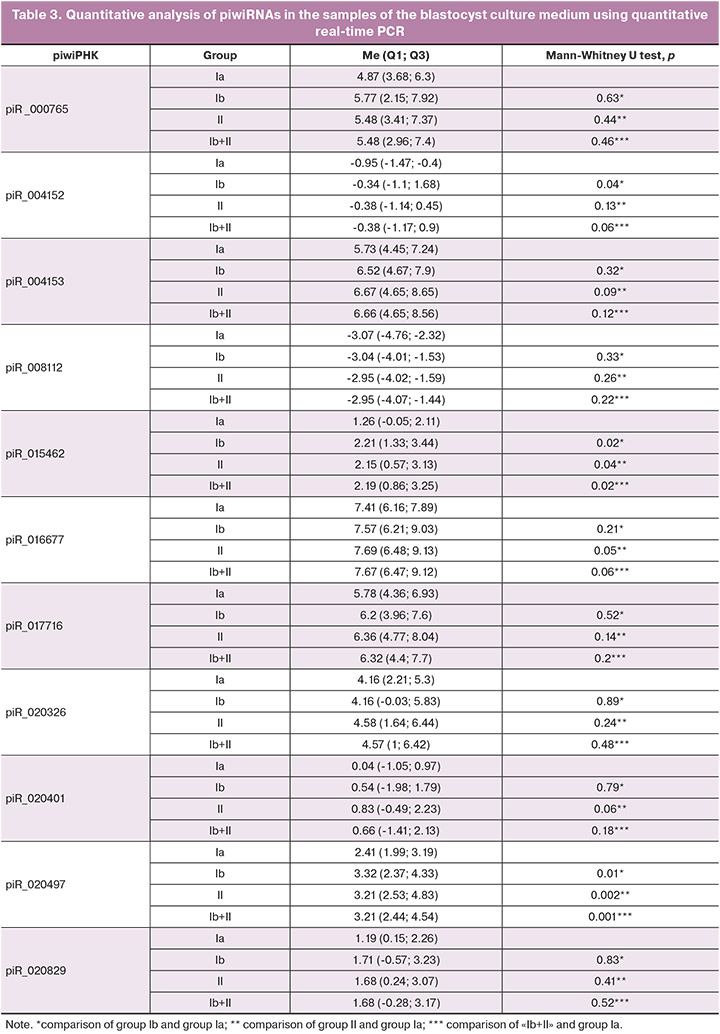
The sequencing data were validated using quantitative real-time PCR on the entire sample of 93 samples listed in Table 2 and three control samples of the medium without embryo cultivation in it. A box diagram of the piwiRNA content in each of the study groups was constructed in the RStudio program based on the values of «-ΔΔСt» for each piwiRNA listed in Table 1 (Fig. 2).
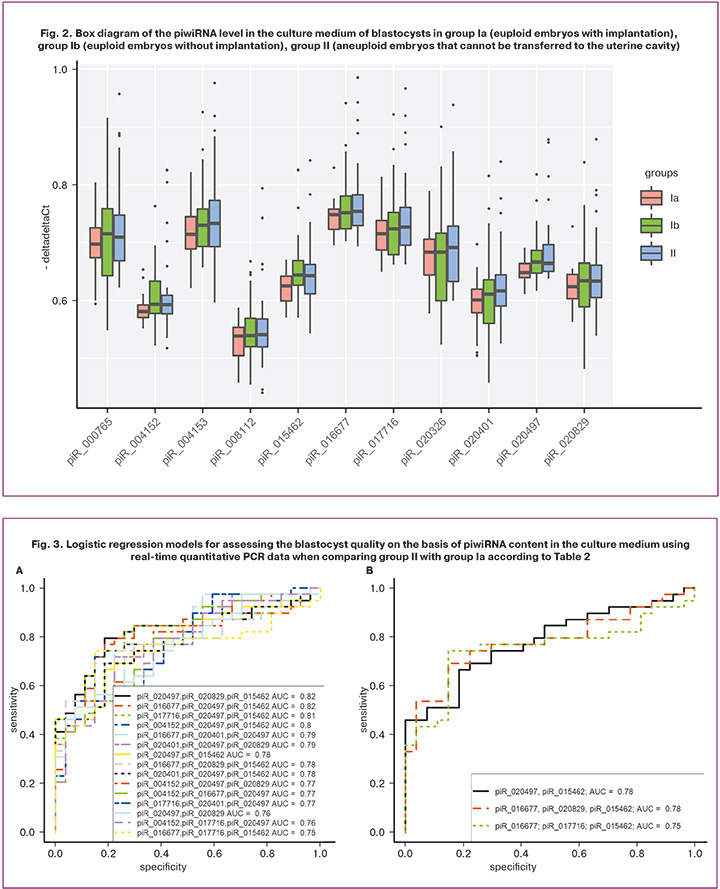
The box diagrams presented in Figure 2 show the values of the logarithm along the ordinate axis based on the 2-fold variation of piwiRNA in the culture medium of the blastocysts. A statistically significant increase in the level of piR_004152, piR_015462, piR_020497 was found in the culture medium of euploid embryos with a negative result of the ART program in comparison with the group of euploid embryos with implantation (Table 3, comparison of groups Ia and Ib). A statistically significant increase in the level of piR_015462, piR_016677, and piR_020497 was noted in the culture medium of aneuploid embryos in comparison with the group of euploid embryos with implantation (Table 3, comparison of groups Ia and II). It should be noted that the group of euploid blastocysts capable of implanting into endometrial tissue was statistically significantly different from any other blastocysts (euploid embryos without implantation potential and aneuploid blastocysts that cannot be transferred to the uterine cavity) in levels of piR_015462 and piR_020497 in their culture medium which were reduced (Table 3, comparison of groups Ia and Ib+II). It is worth noting that we have previously identified the relationship between the level of piR_020497 in the follicular fluid and the quality of an oocyte capable of fertilization and the formation of a blastocyst with a high implantation potential [30].
In order to verify the significance of piwiRNAs listed in Table 3 in terms of assessing the quality of the blastocyst (euploid set of chromosomes in blastomeres and high implantation potential), logistic regression models were constructed. Optimal combinations of piwiRNAs associated with the embryo quality were found in the RStudio program via gradual inclusion and exclusion of each molecule depending on their contribution to the construction of logistic regression models (Fig. 3), where the dependent variable (response variable) was blastocyst quality (0 refers to a euploid embryo transferred to the uterine cavity with the subsequent development of pregnancy and childbirth; 1 refers to an aneuploid embryo that cannot be transferred to the uterine cavity).
Figure 3A shows all possible combinations of piwiRNAs involved in the identification of a euploid embryo with a high implantation potential. The models presented in Figure 3B were selected from the above combinations, where all independent variables were statistically significant. The parameters of the models in Figure 3B are shown in Table 4. Formulas 1, 2 and 3 describing the models of Figure 3B are presented below.
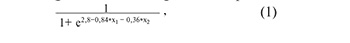
where х1 is the value of -∆∆Ct for piR_020497, х2 is the value of –∆∆Ct for piR_015462;
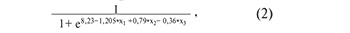
where х1 is the value of -∆∆Ct for piR_016677, х2 is the value of –∆∆Ct for piR_020829, х3 is the value of –∆∆Ct for piR_015462;
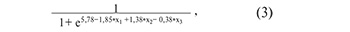
where х1 is the value of -∆∆Ct for piR_016677, х2 is the value of –∆∆Ct for piR_017716, х3 is the value of –∆∆Ct for piR_015462.
The models of Figure 3B had high specificity (81–85%), which means that they had high diagnostic value for detecting euploid blastocysts with high implantation potential. Lower sensitivity values of the constructed models (67–74%) may be due to the lack of information about the implantation potential of the aneuploid embryo because of contraindications to its transfer into the uterine cavity.
Since the euploid embryo does not always have a high implantation potential, it was decided to build logistic regression models when comparing group Ia with the combined groups Ib+II to increase the probability of selecting the highest-quality blastocyst for its transfer into the uterine cavity taking into account two factors, namely the ploidy of the embryonic cells and its implantation potential. Optimal combinations of piwiRNAs were found in the RStudio program via gradual inclusion and exclusion of each molecule depending on their contribution to the construction of logistic regression models (Fig. 4), where the dependent variable (response variable) was blastocyst quality (0 refers to a euploid embryo transferred to the uterine cavity with the subsequent development of pregnancy and childbirth; 1 refers to a poor quality embryo: aneuploid embryo that cannot be transferred to the uterine cavity, and a euploid embryo without implantation after its transfer to the uterine cavity).
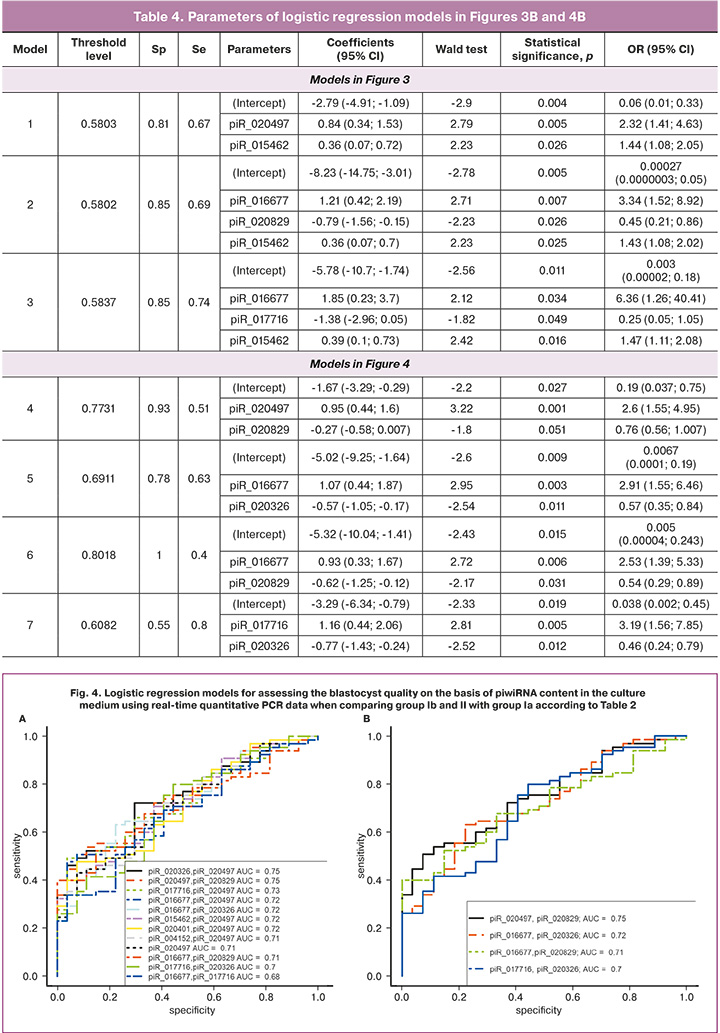
Figure 4A shows all possible combinations of piwiRNAs involved in the identification of a high quality blastocyst. The models presented in Figure 4B were selected from the above combinations, where all independent variables were statistically significant. The parameters of the models in Figure 4B are shown in Table 4. Formulas 4, 5, 6 and 7 describing the models of Figure 4B are presented below.
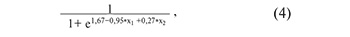
where х1 is the value of -∆∆Ct for piR_020497, х2 is the value of –∆∆Ct for piR_020829;
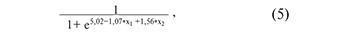
where х1 is the value of -∆∆Ct for piR_016677, х2 is the value of –∆∆Ct for piR_020326;
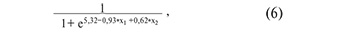
where х1 is the value of -∆∆Ct for piR_016677, х2 is the value of –∆∆Ct for piR_020829;
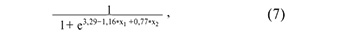
where х1 is the value of -∆∆Ct for piR_017716, х2 is the value of –∆∆Ct for piR_020326.
Models 4 and 6 in Figure 4B have the highest diagnostic significance in terms of identifying a high-quality embryo (euploid with high implantation potential) due to the highest specificity (93% and 100%, respectively) in comparison with models 5 and 7 in Figure 4B. The lower sensitivity of models 4 and 6 (51% and 40%, respectively) in comparison with their specificity can be explained by the lack of information about the implantation potential of the aneuploid embryo due to contraindications to its transfer into the uterine cavity and the lack of information about the receptivity of the endometrium at the time of transfer of the euploid embryo into the uterine cavity.
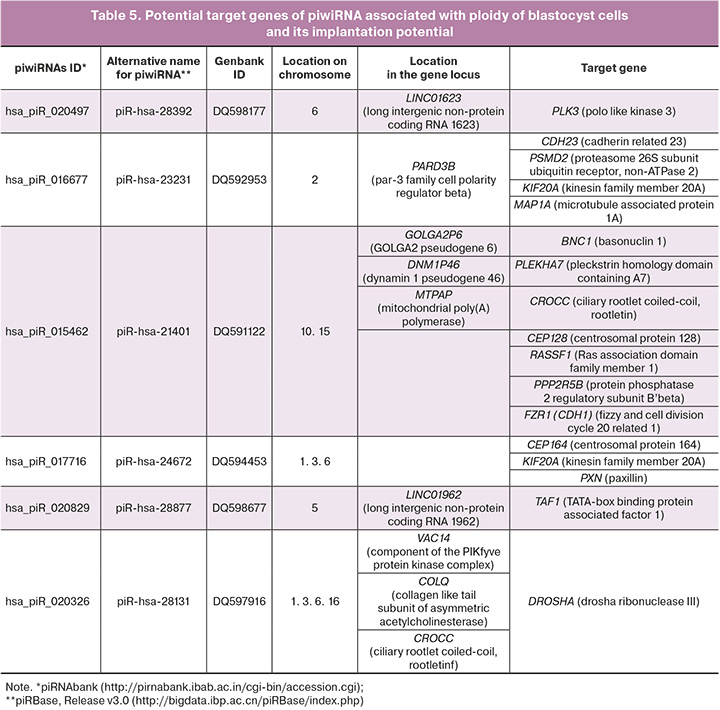
In order to analyze the functional significance of piwiRNAs included in the models in Figures 3B and 4B, potential target genes of piwiRNAs were identified using the GRCh38 database containing sequences of protein-coding transcripts (https://www.ncbi.nlm.nih.gov/genome/guide/human /) and the miRanda algorithm with the following parameters: alignment score sc ≥ 170 and binding energy en ≤ -20.0 kcal mol-1, as described in our recent study [24]. Tens of thousands of target genes were identified and some of them were found to be involved in the regulation of the formation of the spindle apparatus, the development and functioning of kinetochore, and cytokinesis; aneuploidy is known to be caused by the impairment of this regulation (Table 5).
Discussion
In the present study, logistic regression models were obtained to identify euploid blastocysts with high implantation potential on the basis of the piwiRNA level in the embryo culture medium on day 5 after fertilization. The method of deep sequencing of small non-coding RNAs was used with subsequent validation of the data obtained by quantitative real-time PCR. As a result, quantitative assessment of the combination of piR_020497 and piR_020829 or piR_016677 and piR_020829 had the greatest specificity (93% and 100%, respectively), as well as diagnostic value in the selection of the highest quality blastocyst for transfer into the uterine cavity taking into account two factors, namely the ploidy of the embryonic cells and implantation potential of the embryo. In order to apply the developed models in clinical practice, it is necessary to validate them on numerous test samples with confirmation of the ploidy of embryonic cells using the PGT-A method. Moreover, it is also necessary to assess the receptivity of the endometrium on the day of cryopreserved embryo transfer to improve the accuracy of the developed models. In case of obtaining evidence of the reliability and effectiveness of the developed models, it will be possible not to use the invasive PGT-A method for conducting a screening test to assess the quality of all embryos obtained from a couple.
One of the functions of piwiRNA is to regulate the stability of the cell genome by interacting in the nucleus with a retrotransposon transcript as part of the RISC complex (RNA-induced silencing complex), which contacts with histone deacetylase and methyltransferase and/or DNA methyltransferase; they block further transcription of the retrotransposon, thereby preventing its activity and integration into various parts of the genome [17]. Krawetz S.A. et al. detected piwiRNAs of spermatozoa which had a potential affinity for binding to certain transposons [31]. We identified two molecules among piwiRNAs in the culture medium of the blastocyst, namely hsa_piR_020829 and hsa_piR_017716, which were expressed in spermatozoa according to this study; binding sites with retrotransposons L1 (long dispersed nuclear repeats) and MER (moderately repeated dispersed sequences) were identified for hsa_piR_020829.
DNA sequences of certain classes of piwiRNAs are located inside protein-coding genes, and functionally active piwiRNA molecules are mainly produced from 3’-untranslated regions (3’-UTR) of mRNA during their translation [17]. In addition, 3’-UTR mRNAs producing piwiRNAs, as a rule, contain sequences of transposons whose activity is controlled by piwiRNA data at the post-transcriptional level; the post-meiotic stages of spermatogenesis demonstrate this process [32]. The coordinated processes of mRNA translation and simultaneous formation of functional piwiRNAs aimed at reducing the activity of transposons are a manifestation of fine regulation of gametogenesis which further affects the quality of the developing embryo. Some piwiRNAs identified in this study as markers of euploid blastocyst with high implantation potential are located at the loci of protein-coding genes, namely: DNA sequence hsa_piR_016677 is located in the PARD3B gene, hsa_piR_015462 is located in the DNM1P46 and MTPAP genes, hsa_piR_020326 is located in the VAC14, COLQ and CROCC genes.
Besides the suppressive activity of piwiRNAs towards transposons, they are also known to have a regulatory effect on signaling pathways in the cell through various molecular mechanisms [17]. For example, pachytene piwiRNAs direct the MIWI protein to the target mRNA either for interaction with CAF1 deadenylase CAF 1, which performs deadenylation and subsequent degradation of mRNA [33], or for direct cleavage of mRNA [34, 35]. These mechanisms for removing a huge number of mRNA targets, for example, work at the stage of late spermatids before the beginning of their morphological transformation into spermatozoa. Though it might seem paradoxical, piwiRNAs can both destabilize and stabilize the target mRNA by activating its translation. For example, a recent study showed that pachytene piwiRNAs in combination with MIWI, translation initiation factor 3 subunit F (eIF3F) and the RNA-binding protein HuR activate the translation of a number of spermiogenic mRNAs in round spermatids, thereby determining a certain stage of gametogenesis [36]. The potential piwiRNA target genes, which are markers of a euploid blastocyst with a high implantation potential, were identified using the miRanda algorithm. It should be noted that the protein products of these target genes are involved in the formation of the spindle apparatus, the development and functioning of kinetochore, and cytokinesis; their impairments lead to cell aneuploidy.
Thus, for example, Polo-like kinase 3 (PLK3) which is the target gene hsa_piR_020497 is localized in the region of the nucleus, centrosome and furrow of cell division; it participates in the regulation of the cell cycle by activating CDC25A and CDC25C, inducing G1/S and G2/M transitions, respectively, and in cytokinesis [37]. Phylogenetic analysis showed a high conservativeness of the kinase and protein-binding domains in PLK1–3, therefore, the structural similarity of these kinases determines the same functional activity. The involvement of PLK1 in centrosome separation has been proven by maintaining the activity of NEK2A protein kinase that phosphorylates the linker of two centrosomes – rutletin (CROCC, target gene hsa_piR_015462). In the case of mutations in the CROCC gene, severe chromosomal instability and impaired chromosome segregation occur [38]. The participation of PLK1–3 in cytokinesis occurs due to its interaction with various substrates, including the kinesin family protein (KIF20A, the target gene hsa_piR_016677 and hsa_piR_017716); these substrates ensure the movement of PLK1-3 from centrosomes and kinetochores to the middle zone of the spindle apparatus due to the catalysis of rapid spatial remodeling of microtubules necessary for intracellular transport proteins and organelles [39]. In addition, a number of subunits of the main phosphatase of microtubule-associated proteins PP2A (PPP2R1A, PPP2R2D, PPP2R3B, PPP2R5B and PPP2R5D) also interact with PLK1 and are involved in maintaining genomic stability [40]. Inhibition of PPP2R5B (target gene hsa_piR_015462) has been demonstrated to affect the cohesion of sister chromatids in cells with an increased level of PLK1 expression. SET/TAF1 (target gene hsa_piR_020829) is a P2 inhibitor and it is localized in centromeres supporting the activity of the Aurora B kinase, which phosphorylates various components of the kinetochore to correct the improper connection of the kinetochore with microtubules. The timely release of SET from the kinetochore protein complex during metaphase ensures stabilization of the kinetochore compound with microtubules under the action of phosphatase activity of PP2A [41].
The centrosome is the site of concentration of the proteasome and associated regulatory proteins [42]. In eukaryotic cells, 80–90% of proteins are degraded by the 26S proteasome [43] which consists of two complexes, namely core 20S and regulatory 19S, where PSMD2 (target gene hsa_piR_016677) encodes one of the subunits [44]. It should be noted that Polo-like kinases undergo ubiquitin-dependent proteolysis in the proteasome [37]; thus, in the case of PSMD2 and PLK3 concentration regulation under the action of hsa_piR_016677 and piR_020497, respectively, one can see a complex system of control over the formation of spindle apparatus for the correct segregation of chromosomes. Another important regulator of the cell cycle using ubiquitin-mediated proteolysis is the anaphase-inducing complex/cyclosome APC/C activated by Cdc20 or Cdh1 (target gene hsa_piR_015462). During oogenesis, APC/CCdh1 ensures the maintenance of prolonged arrest of primordial follicles at the prophase I stage of meiosis and blocking their entry into metaphase I by degradation of cyclin B1 [45]. At the anaphase I stage, APC/CCdh1 provides phosphorylation of centromere-bound chromosome Sgol2 (shugoshin-like protein 2) under the action of Aurora B/C kinase which leads to dissociation of Sgol2 and chromosome divergence [46]. In case of deletion of Apc and Cdh1 genes in the oocyte, Sgol2 remains bound to chromosomes in anaphase I, which does not lead to their divergence and causes the occurrence of aneuploidy after the first meiotic division.
The piwiRNAs which we identified as ones associated with aneuploidy of blastocyst cells potentially regulate the level of centriolar proteins that play an important role in oogenesis and spermatogenesis. CEP128 (target gene hsa_piR_015462), CEP164 (target gene hsa_piR_017716) and basonuclin 1 (BNC1, target gene hsa_piR_015462) are key proteins of the appendage of the maternal centriole of male germ cells participating in the organization of centriolar microtubules and the formation of axonema; in the case of defects in the genes encoding them, disorders of spermatogenesis and fertilization occur due to a decrease in concentration and morphology of spermatozoa [47–50]. The role of BNC1 in reproduction has been demonstrated not only in terms of regulation of spermatogenesis, but also oogenesis: fertilization occurred in the case of inhibition of the expression of the BNC1 gene in the oocyte, but the development of the embryo stopped at the two-cell stage [51].
At the preimplantation stage of embryogenesis, starting from the 8-cell stage, blastomeres undergo polarization and compactification due to the reorganization of microtubules, actin and intermediate filaments; their precise and timely regulation determines the formation of an embryoblast and a trophoblast of a blastocyst capable of implantation [52]. During cell migration, the growing ends of the microtubules anchor in the focal adhesion sites, and this leads to destabilization of the adhesive contact and detachment of the cell from the substrate. The main role in this process belongs to the focal adhesion protein paxillin (PXN, target gene hsa_piR_017716) via interaction with α-tubulin [53]. There was a significant increase in the expression of PXN in the area of focal contact formation after compaction and blastulation of the embryo to form the adhesive properties of the blastocyst and ensure implantation [54]. The adhesion protein CDH23 (target gene hsa_piR_016677) is a regulator of the microtubule network supporting the polymerization of the end of microtubules by interacting with the CAMSAP3 protein [55] and ensures the stability of adhesive intercellular contact when interacting with the PLEKHA7 protein (target gene hsa_piR_015462) [56]. It is worth noting that PLEKHA7 participates not only in the stabilization of the main transmembrane components of the adhesive intercellular contact, but it also interacts with the enzyme complex of microRNA processing (DROSHA and DGCR8), providing regulation of miR-24, miR-30a, miR30-b and let7-g, which suppress the expression of SNAIL, MYC and cell growth-inducing proteins cyclin D1 directly at the sites of intercellular contact [57]. It should be noted that the potential target gene of the hsa_piR_020326 molecule, identified as one of the markers of aneuploidy of embryo cells, is the DROSHA enzyme, which emphasizes the complex relationship of different types of small non-coding RNAs (piwiRNAs and microRNAs) and protein-coding RNAs; thus, imbalance of them causes one or another pathology.
An important role in spermatogenesis is played by the MAP1A protein (target gene hsa_piR_016677) which is associated with the microtubules of Sertoli cells of the epitheliospermatogenic layer of seminal tubules [58]; it stabilizes the microtubular structure of the directed transport of differentiating gametes from the outer basal part of the tubule wall containing spermatogonia to the inner adluminal part containing spermatocytes, spermatids and spermatozoa. Any impairment of the epithelial cycle of spermatogenesis, including the disorders caused by changes in the expression level of MAP1A, will lead to a decrease in male fertility. One should also note the key role of the RASSF1A protein (target gene hsa_piR_015462) in stabilizing microtubules through inhibitory action on HDAC6 deacetylase. This way, it promotes acetylation of microtubules, a stable, long-lived structure necessary for directed vesicle transport, the formation of the centrosome and Golgi apparatus, stabilization of focal adhesion sites and intercellular contacts [59].
Conclusion
Thus, the analysis of the literature data on the function of potential target genes of a certain set of piwiRNA molecules identified in this study as markers of embryo ploidy and its implantation potential allows us to conclude that the quality of the embryo depends on a well-coordinated gametogenesis. The constancy of the number of chromosomes at each cell division depends on the correct formation of the spindle apparatus which provides chromosome segregation equal for both daughter cells, where piwiRNAs act as one of the key regulatory molecules. In this regard, the present study proposes a non-invasive method for identifying a euploid embryo with a high implantation potential based on a quantitative assessment of piwiRNA in the culture medium. This method demonstrates a high specificity (93-100%) in the selection of an embryo for transfer into the uterine cavity. The technique developed in this study needs to be tested on several test samples for implementation into clinical practice. In order to confirm the relationship between the imbalance in the expression level of piwiRNAs and their target genes experimentally, it is necessary to conduct a transcriptomic study of the protein-coding genes of the embryo genome in groups of aneuploid blastocysts and euploid blastocysts of poor quality that cannot be transferred to the uterine cavity.
References
- Lensen S., Lantsberg D., Gardner D.K., Sophian A.D., Wandafiana N., Kamath M.S. The role of timing in frozen embryo transfer. Fertil. Steril. 2022; 118(5):832-8. https://dx.doi.org/10.1016/j.fertnstert.2022.08.009.
- Governini L., Luongo F.P., Haxhiu A., Piomboni P., Luddi A. Main actors behind the endometrial receptivity and successful implantation. Tissue Cell. 2021; 73: 101656. https://dx.doi.org/10.1016/j.tice.2021.101656.
- Donaghay M., Lessey B.A. Uterine receptivity: alterations associated with benign gynecological disease. Semin. Reprod. Med. 2007; 25(6): 461-75. https://dx.doi.org/10.1055/s-2007-991044.
- Fesahat F., Montazeri F., Hoseini S.M. Preimplantation genetic testing in assisted reproduction technology. J. Gynecol. Obstet. Hum. Reprod. 2020; 49(5): 101723. https://dx.doi.org/10.1016/j.jogoh.2020.101723.
- Franasiak J.M., Forman E.J., Hong K.H., Werner M.D., Upham K.M., Treff N.R., Scott R.T.J. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil. Steril. 2014; 101(3): 656-63.e1. https://dx.doi.org/10.1016/j.fertnstert.2013.11.004.
- Ubaldi F.M., Cimadomo D., Capalbo A., Vaiarelli, A., Buffo L., Trabucco E. et al. Preimplantation genetic diagnosis for aneuploidy testing in women older than 44 years: a multicenter experience. Fertil. Steril. 2017; 107(5): 1173-80. https://dx.doi.org/10.1016/j.fertnstert.2017.03.007.
- Fragouli E., Alfarawati S., Spath K., Wells D. Morphological and cytogenetic assessment of cleavage and blastocyst stage embryos. Mol. Hum. Reprod. 2014, 20(2): 117-26. https://dx.doi.org/10.1093/molehr/gat073.
- Minasi M.G., Fiorentino F., Ruberti A., Biricik A., Cursio E.,Cotroneo E. et al. Genetic diseases and aneuploidies can be detected with a single blastocyst biopsy: a successful clinical approach. Hum. Reprod. 2017; 32(8): 1770–77. https://dx.doi.org/10.1093/humrep/dex215.
- Teh W.T., McBain J., Rogers P. What is the contribution of embryo-endometrial asynchrony to implantation failure? J. Assist. Reprod. Genet. 2016; 33(11): 1419-30. https://dx.doi.org/10.1007/s10815-016-0773-6.
- Sato T., Sugiura-Ogasawara M., Ozawa F., Yamamoto T., Kato T., Kurahashi H. et al. Preimplantation genetic testing for aneuploidy: a comparison of live birth rates in patients with recurrent pregnancy loss due to embryonic aneuploidy or recurrent implantation failure. Hum. Reprod. 2020; 35(1): 255. https://dx.doi.org/10.1093/humrep/dez289.
- Greco E., Bono S., Ruberti A., Lobascio A.M., Greco P., Biricik A. et al. Comparative genomic hybridization selection of blastocysts for repeated implantation failure treatment: a pilot study. Biomed Res. Int. 2014; 2014: 457913. https://dx.doi.org/10.1155/2014/457913.
- Tong J., Niu Y., Wan A., Zhang T. Next-Generation Sequencing (NGS) based preimplantation genetic testing for aneuploidy (PGT-A) of trophectoderm biopsy for recurrent implantation failure (RIF) patients: a retrospective study. Reprod. Sci. 2021; 28(7): 1923-9. https://dx.doi.org/10.1007/s43032-021-00519-0.
- Pantou A., Mitrakos A., Kokkali G., Petroutsou K., Tounta G., Lazaros L. et al. The impact of preimplantation genetic testing for aneuploidies (PGT-A) on clinical outcomes in high risk patients. J. Assist. Reprod. Genet. 2022; 39(6): 1341-9. https://dx.doi.org/10.1007/s10815-022-02461-9.
- Gleicher N., Patrizio P., Brivanlou A. Preimplantation genetic testing for aneuploidy - a castle built on sand. Trends Mol. Med. 2021; 27(8): 731-42. https://dx.doi.org/10.1016/j.molmed.2020.11.009.
- McIlwraith E.K., He W., Belsham D.D. Promise and perils of microRNA discovery research: Working towards quality over quantity. Endocrinology. 2023; 164(9): bqad111. https://dx.doi.org/10.1210/endocr/bqad111.
- Xiong Q., Zhang Y. Small RNA modifications: regulatory molecules and potential applications. J. Hematol. Oncol. 2023; 16(1): 64. https://dx.doi.org/10.1186/s13045-023-01466-w.
- Wang X., Ramat A., Simonelig M., Liu M.F. Emerging roles and functional mechanisms of PIWI-interacting RNAs. Nat. Rev. Mol. Cell Biol. 2023; 24(2): 123-41. https://dx.doi.org/10.1038/s41580-022-00528-0.
- Czech B., Munafò M., Ciabrelli F., Eastwood E.L., Fabry M.H., Kneuss E., Hannon G.J. piRNA-Guided genome defense: from biogenesis to silencing. Ann. Rev. Genet. 2018; 52: 131–57. https://dx.doi.org/10.1146/annurev-genet-120417-031441.
- Tóth K.F., Pezic D., Stuwe E., Webster A. The piRNA pathway guards the germline genome against transposable elements. Adv. Exp. Med. Biol. 2016; 886: 51-7. https://dx.doi.org/10.1007/978-94-017-7417-8_4.
- Russell S.J., LaMarre J. Transposons and the PIWI pathway: genome defense in gametes and embryos. Reproduction 2018; 156(4): 111-24. https://dx.doi.org/0.1530/REP-18-0218.
- Rosenbluth E.M., Shelton D.N., Wells L.M., Sparks A.E.T., Van Voorhis B.J. Human embryos secrete microRNAs into culture media--a potential biomarker for implantation. Fertil. Steril. 2014; 101(5): 1493-500. https://dx.doi.org/10.1016/j.fertnstert.2014.01.058.
- Capalbo A., Ubaldi F.M., Cimadomo D., Noli L., Khalaf Y., Farcomeni A. et al. MicroRNAs in spent blastocyst culture medium are derived from trophectoderm cells and can be explored for human embryo reproductive competence assessment. Fertil. Steril. 2016; 105(1): 225-35. https://dx.doi.org/10.1016/j.fertnstert.2015.09.014.
- Timofeeva A.V, Fedorov I.S., Shamina M.A., Chagovets V.V, Makarova N.P., Kalinina E.A. et al. Clinical relevance of secreted small noncoding RNAs in an embryo implantation potential prediction at morula and blastocyst development stages. Life (Basel, Switzerland). 2021; 11(12): 1328. https://dx.doi.org/10.3390/life11121328.
- Timofeeva A., Drapkina Y., Fedorov I., Chagovets V., Makarova N., ShaminaM. et al. Small noncoding RNA signatures for determining the developmental potential of an embryo at the morula stage. Int. J. Mol. Sci. 2020; 21(24): 9399. https://dx.doi.org/10.3390/ijms21249399.
- Langmead B., Trapnell C., Pop M., Salzberg S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009; 10(3): R25. https://dx.doi.org/10.1186/gb-2009-10-3-r25.
- Love M.I., Huber W. Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. 2014; 15(12): 550. https://dx.doi.org/10.1186/s13059-014-0550-8.
- Team R.C. A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.r-project.org (accessed on Mar 10, 2021).
- Team Rs. RStudio: Integrated Development for R. RStudio Available online: http://www.rstudio.com/ (accessed on Mar 23, 2021).
- Martin R.H. Meiotic errors in human oogenesis and spermatogenesis. Reprod. Biomed. Online. 2008; 16(4): 523-31. https://dx.doi.org/10.1016/s1472-6483(10)60459-2.
- Шамина М.А., Тимофеева А.В., Федоров И.С., Калинина Е.А. Оценка уровня экспрессии пивиРНК hsa_piR_020497 в фолликулярной жидкости пациенток с различными исходами программ экстракорпорального оплодотворения. Акушерство и гинекология 2021;11:143-53. [Shamina M.A., Timofeeva A.V., Fedorov I.S., Kalinina E.A. Assessment of the expression level of hsa_pir_020497 piRNA in the follicular fluid of patients with different in vitro fertilization outcomes. Obstetrics and Gynecology. 2021; (11): 134-53 (in Russian)]. https://dx.doi.org/10.18565/aig.2021.11.143-53.
- Krawetz S.A., Kruger A., Lalancette C., Tagett R., Anton E., Draghici S., Diamond M.P. A survey of small RNAs in human sperm. Hum. Reprod. 2011; 26(12):3401-12. https://dx.doi.org/10.1093/humrep/der329.
- Sun Y.H., Wang R.H., Du K., Zhu J., Zheng J., Xie L.H. et al. Coupled protein synthesis and ribosome-guided piRNA processing on mRNAs. Nat. Commun. 2021; 12(1): 5970. https://dx.doi.org/0.1038/s41467-021-26233-8.
- Gou L.T., Dai P., Yang J.H., Xue Y., Hu Y.P., Zhou Y. et al. Pachytene piRNAs instruct massive mRNA elimination during late spermiogenesis. Cell Res. 2015; 25(2):266. https://dx.doi.org/10.1038/cr.2015.14.
- Goh W.S., Falciatori I., Tam O.H., Burgess R., Meikar O., Kotaja N. et al. PiRNA-directed cleavage of meiotic transcripts regulates spermatogenesis. Genes Dev. 2015; 29(10): 1032-44. https://dx.doi.org/10.1101/gad.260455.115.
- Reuter M., Berninger P., Chuma S., Shah H., Hosokawa M., Funaya C. et al. Miwi catalysis is required for piRNA amplification-independent LINE1 transposon silencing. Nature. 2011; 480(7376):264-7.https://dx.doi.org/10.1038/nature10672.
- Dai P., Wang X., Gou L.T., Li Z.T., Wen Z., Chen Z.G. et al. A Translation-activating function of MIWI/piRNA during mouse spermiogenesis. Cell. 2019; 179(7): 1566-81.e16. https://dx.doi.org/10.1016/j.cell.2019.11.022.
- Zitouni S., Nabais C., Jana S.C., Guerrero A., Bettencourt-Dias M. Polo-like kinases: structural variations lead to multiple functions. Nat. Rev. Mol. Cell Biol. 2014; 15(7): 433-52. https://dx.doi.org/10.1038/nrm3819.
- Remo A., Li X., Schiebel E., Pancione M. The Centrosome linker and its role in cancer and genetic disorders. Trends Mol. Med. 2020; 26(4): 380-93. https://dx.doi.org/10.1016/j.molmed.2020.01.011.
- Li X., Shu K., Wang Z., Ding D. Prognostic significance of KIF2A and KIF20A expression in human cancer: a systematic review and meta-analysis. Medicine (Baltimore). 2019; 98(46): e18040. https://dx.doi.org/10.1097/MD.0000000000018040.
- Cunningham C.E., Li S., Vizeacoumar F.S., Bhanumathy K.K., Lee J.S., Parameswaran S. et al. Therapeutic relevance of the protein phosphatase 2A in cancer. Oncotarget. 2016; 7(38): 61544-61. https://dx.doi.org/10.18632/oncotarget.11399.
- Asai Y., Matsumura R., Hasumi Y., Susumu H., Nagata K., Watanabe Y., Terada Y. SET/TAF1 forms a distance-dependent feedback loop with aurora B and Bub1 as a tension sensor at centromeres. Sci. Rep. 2020; 10(1): 15653. https://dx.doi.org/10.1038/s41598-020-71955-2.
- Wigley W.C., Fabunmi R.P., Lee M.G., Marino C.R., Muallem S., DeMartino G.N., Thomas P.J. Dynamic аssociation of proteasomal machinery with the centrosome. J. Cell Biol. 1999; 145(3): 481-90. https://dx.doi.org/10.1083/jcb.145.3.481.
- Lilienbaum A. Relationship between the proteasomal system and autophagy. Int. J. Biochem. Mol. Biol. 2013; 4(1): 1-26.
- Gerhardt C., Lier J.M., Burmühl S., Struchtrup A., Deutschmann K., Vetter M. et al. The transition zone protein Rpgrip1l regulates proteasomal activity at the primary cilium. J. Cell Biol. 2015; 210(1): 115-33. https://dx.doi.org/10.1083/jcb.201408060.
- Holt J.E., Tran S.M.T., Stewart J.L., Minahan K.,García-Higuera I., Moreno S., Jones K.T. The APC/C activator FZR1 coordinates the timing of meiotic resumption during prophase I arrest in mammalian oocytes. Development. 2011;138(5): 905-13. https://dx.doi.org/10.1242/dev.059022.
- Rattani A., Ballesteros Mejia R., Roberts K., Roig M.B., Godwin J., Hopkins M. et al. APC/C(Cdh1) enables removal of shugoshin-2 from the arms of bivalent chromosomes by moderating cyclin-dependent kinase activity. Curr. Biol. 2017; 27(10):1462-76:e5. https://dx.doi.org/10.1016/j.cub.2017.04.023.
- Zhang X., Wang L., Ma Y., Wang Y., Liu H., Liu M. et al. CEP128 is involved in spermatogenesis in humans and mice. Nat. Commun. 2022; 13(1):1395. https://dx.doi.org/10.1038/s41467-022-29109-7.
- Pitaval A., Senger F., Letort G., Gidrol X., Guyon L., Sillibourne J., Théry M. Microtubule stabilization drives 3D centrosome migration to initiate primary ciliogenesis. J. Cell Biol. 2017; 216(11): 3713-28. https://dx.doi.org/10.1083/jcb.201610039.
- Yang Z., Gallicano G.I., Yu Q.C., Fuchs E. An unexpected localization of basonuclin in the centrosome, mitochondria, and acrosome of developing spermatids. J. Cell Biol. 1997;137(3):657-69. https://dx.doi.org/10.1083/jcb.137.3.657.
- Zhang X., Chou W., Haig-Ladewig L., Zeng W., Cao W., Gerton G. BNC1 is required for maintaining mouse spermatogenesis. Genesis. 2012;50(7):517-24. https://dx.doi.org/10.1002/dvg.22014.
- Ma J., Zeng F., Schultz R.M., Tseng H. Basonuclin: a novel mammalian maternal-effect gene. Development. 2006;133(10):2053-62. https://dx.doi.org/10.1242/dev.02371.
- Lim H.Y.G., Plachta N. Cytoskeletal control of early mammalian development. Nat. Rev. Mol. Cell Biol. 2021; 22(8): 548-62. https://dx.doi.org/10.1038/s41580-021-00363-9.
- Herreros L., Rodríguez-Fernandez J.L., Brown M.C., Alonso-Lebrero J.L., Cabañas C. et al. Paxillin localizes to the lymphocyte microtubule organizing center and associates with the microtubule cytoskeleton. J. Biol. Chem. 2000; 275(34): 26436-40. https://dx.doi.org/10.1074/jbc.M003970200.
- Ezoe K., Miki T., Ohata K., Fujiwara N., Yabuuchi A., Kobayashi T., Kato K. Prolactin receptor expression and its role in trophoblast outgrowth in human embryos. Reprod. Biomed. Online. 2021; 42(4): 699-707. https://dx.doi.org/10.1016/j.rbmo.2021.01.006.
- Takahashi S., Mui V.J., Rosenberg S.K.,Homma K., Cheatham M.A., Zheng J. Cadherin 23-C regulates microtubule networks by modifying CAMSAP3’s function. Sci. Rep. 2016; 6: 28706. https://dx.doi.org/10.1038/srep28706.
- Meng W., Mushika Y., Ichii T., Takeichi M. Anchorage of microtubule minus ends to adherens junctions regulates epithelial cell-cell contacts. Cell. 2008; 135(5): 948-59. https://dx.doi.org/10.1016/j.cell.2008.09.040.
- Shah J., Guerrera D., Vasileva E., Sluysmans S., Bertels E., Citi S. PLEKHA7: Cytoskeletal adaptor protein at center stage in junctional organization and signaling. Int. J. Biochem. Cell Biol. 2016; 75: 112-6. https://dx.doi.org/10.1016/j.biocel.2016.04.001.
- Mao B.P., Ge R., Cheng C.Y. Role of microtubule +TIPs and -TIPs in spermatogenesis - Insights from studies of toxicant models. Reprod. Toxicol. 2020; 91: 43-52. https://dx.doi.org/10.1016/j.reprotox.2019.11.006.
- Dubois F., Bergot E., Zalcman G., Levallet G. RASSF1A, puppeteer of cellular homeostasis, fights tumorigenesis, and metastasis-an updated review. Cell Death Dis. 2019; 10(12): 928. https://dx.doi.org/10.1038/s41419-019-2169-x.
Received 31.07.2023
Accepted 14.11.2023
About the Authors
Angelika V. Timofeeva, Ph.D. (Bio), Head of the Laboratory of Applied Transcriptomics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, avtimofeeva28@gmail.com, 117997, Russia, Moscow, Academician Oparin str., 4.Ivan S. Fedorov, Researcher at the Laboratory of Applied Transcriptomics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, is_fedorov@oparina4.ru, 117997, Russia, Moscow, Academician Oparin str., 4.
Guzel V. Savostina, postgraduate student, Scientific and Clinical Department of ART named after F. Paulsen, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, savostina2324@gmail.com, 117997, Russia, Moscow, Academician Oparin str., 4.
Alexey N. Ekimov, Ph.D., Head of the Laboratory of Preimplantation Genetic Screening, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, a_ekimov@oparina4.ru, 117997, Russia, Moscow, Academician Oparin str., 4.
Svetlana G. Perminova, Dr. Med. Sci., Leading Researcher at the Scientific and Clinical Department of ART named after F. Paulsen, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, s_perminova@oparina4.ru, 117997, Russia, Moscow, Academician Oparin str., 4.



