Неудовлетворительное состояние репродуктивного здоровья населения является одной из главных причин снижения рождаемости. По данным ВОЗ, проблема бесплодия касается от 48 до 186 млн пар в мире. В различных регионах Российской Федерации частота бесплодных браков колеблется от 17,2 до 24% от общего количества. По данным Росстата, в 2020 г. было зарегистрировано 63 тыс. пациентов-женщин с диагнозом «бесплодие» [1].
Для лечения бесплодия на сегодняшний день активно используются вспомогательные репродуктивные технологии (ВРТ). Их неотъемлемой частью является проведение стимуляции суперовуляции. Повышенная гормональная нагрузка, сопровождающая индуцированный цикл, может быть сопряжена с высоким риском развития эндотелиальной дисфункции.
Следует отметить, что одним из важнейших факторов созревания фолликулов яичников и развития ооцитов является нормальное функционирование эндотелия. В исследовании Lee S.H. (2020) было показано, что эндотелиальные клетки-предшественники человека (ЭКПЧ) секретируют основной фактор роста фибробластов (fibroblast growth factor, FGF), фактор роста эндотелия сосудов (vascular endothelial growth factor, VEGF), инсулиноподобный фактор роста-1 (insulin-like growth factor 1, IGF1), интерлейкин-10 (IL-10) и эпидермальный фактор роста (epidermal growth factor, EGF) [2]. Совместное культивирование ЭКПЧ и ооцитов приводит к существенному увеличению скорости созревания последних за счет усиления экспансии кумулюсных клеток и экспрессии генов GDF9 и BMP15, связанных с созреванием яйцеклеток в кумулюсно-ооцитарных комплексах [3]. Кроме того, для обеспечения гомеостаза эндотелиальными клетками выделяется значительное количество соединений, отвечающих за поддержание гемостаза, тонуса сосудов, подавление локального воспаления. При этом ряд веществ, синтезируемых эндотелиоцитами, одновременно выполняют несколько функций. Например, эндотелин-1 (endothelin-1, ET-1) выступает в роли как вазоконстриктора, так и протромбогенного фактора, а оксид азота (NO) является вазодилататором и антитромбогенным агентом (таблица).

В этой связи исследование роли эндотелия в созревании ооцитов у женщин, вступающих в программу реализации репродуктивной функции, актуально, поскольку поможет в прогнозировании и формировании групп риска недостаточного ответа яичников, осуществлении поиска персонализированных подходов к выбору подготовки, времени, схем стимуляции яичников при лечении бесплодия.
Целью настоящего обзора является оценка роли продуцируемых эндотелием вазоактивных, про- и антитромбогенных факторов в созревании ооцитов при лечении бесплодия методами ВРТ.
Вазорегулирующие факторы эндотелия и созревание ооцитов
Участие эндотелия в регуляции сосудистого тонуса заключается в выработке ряда биологически активных веществ. Патологическое увеличение в крови и/или фолликулярной жидкости уровня вазоконстрикторов ET-1 и ангиотензина II, наряду со снижением количества вазодилататоров NO и простациклина, может служить маркером дисфункции эндотелия.
Эндотелины являются местными регуляторами в яичниках, которые участвуют в росте фолликулов, созревании ооцитов, овуляции, образовании желтого тела и лютеолизе. Выявлено, что добавление ET-1 к среде, в которой культивируются незрелые ооциты человека на стадии зародышевого пузырька, способствует их созреванию путем подавления в кумулюсных клетках экспрессии коннексина-26 – белка щелевых контактов, образующего межклеточные мембранные каналы и играющего важную роль в возобновлении мейоза [4].
Следует отметить, что женские половые железы продуцируют ET-1 и эндотелин-2 (ET-2), а также несколько функционально различных рецепторов ETRA (endothelin receptor A) и ETRB (endothelin receptor В), активация которых часто связана с противоположными физиологическими эффектами. Взаимодействие ET-1 или ET-2 с ETRA сопряжено с пролонгированной вазоконстрикцией, в то время как активация ETRB ассоциирована с вазодилатацией посредством индукции сигнальных путей NO [5].
Гонадотропин-опосредованная стимуляция яичников влияет на уровни эндотелинов в плазме, маточный кровоток и толщину эндометрия. Выявлено, что индуцированные циклы связаны с более высокими концентрациями ET-2, большей толщиной эндометрия и меньшим индексом пульсации по сравнению со спонтанными циклами. При этом уровень ET-2 отрицательно коррелирует с концентрацией ET-1 [6].
Нарушение синтеза эндотелина может способствовать развитию патологического паттерна овуляции, характерного для синдрома поликистозных яичников (СПКЯ). Обнаружено, что при СПКЯ в фолликулярных аспиратах, содержащих гранулезно-лютеиновые клетки, экспрессия мРНК ET-1 была повышена в 2,2 раза по сравнению с показателями женщин с нормальной овуляцией, тогда как мРНК ET-2 была снижена в 1,8 раза. Число рецепторов и активность эндотелинпревращающего фермента не имели статистически значимой разницы в двух группах [7]. В исследовании Szymanska M. et al. (2021) также показано, что у женщин с СПКЯ в гранулезо-лютеиновых клетках наблюдается снижение экспрессии ET-2, который играет решающую роль в разрыве фолликула при овуляции, повышая тонус гладкой мускулатуры вокруг периовуляторных фолликулов [8].
Хорошо известно, что в репродуктивной системе человека присутствует локальная ренин-ангиотензиновая система, которая участвует в контроле и регуляции развития фолликулов и ангиогенезе в яичниках, осуществлении плацентарной и маточной функций. Эффекты, оказываемые ангиотензином II, опосредованы его взаимодействием с двумя типами рецепторов (angiotensin II receptor type 1, AT1R, и angiotensin II receptor type 2, AT2R) (рисунок) [9].
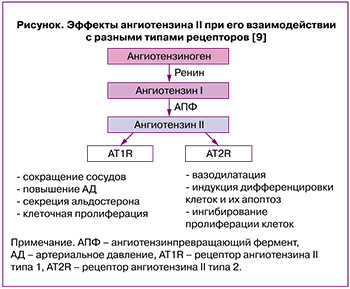
В физиологических условиях ангиотензин II способствует созреванию ооцитов и овуляции [10]. Патологическое функционирование ренин-ангиотензиновой системы в яичниках ассоциировано с бесплодием, СПКЯ, синдромом гиперстимуляции (СГЯ) и раком яичников. Обнаружено, что у женщин с СПКЯ активность ангиотензинпревращающего фермента (АПФ) и ангиотензина II в плазме крови были статистически значимо больше, чем в контрольной группе [11]. В клиническом исследовании с участием 192 пациенток с высоким риском развития СГЯ, подвергшихся процедуре экстракорпорального оплодотворения (ЭКО), было показано, что фолликулярный уровень ангиотензина II значительно выше у женщин с тяжелой формой заболевания по сравнению с легкой/среднетяжелой (281,64 пг/мл против 65,76 пг/мл, p<0,0001) [12]. Следует отметить, что комбинация блокаторов ангиотензиновых рецепторов и ингибитора АПФ у пациенток с СГЯ может предотвратить некоторые симптомы заболевания и поддерживать более высокую частоту наступления беременности после переноса замороженных-размороженных эмбрионов, но не способна полностью устранить СГЯ [13]. Ангиотензин II участвует в образовании сфероидов при раке яичников и метастазировании посредством интенсификации десатурации липидов и подавления стресса эндоплазматического ретикулума внутри сфероида [14].
Одним из факторов, который продуцирует эндотелий, является NO. Образование NO происходит не только в клетках яичников, но и в его сосудах, а также в резидентных или инфильтрирующих макрофагах [15]. В больших сосудах данная молекула подавляет воспалительный процесс, ингибирует тромбообразование, является медиатором вазодилатации, ограничивает ремоделирование сосудистой стенки, тем самым улучшая кровоток. В микроциркуляторном русле NO значим для процесса ангиогенеза [16].
Известно, что NO играет важную роль в физиологии женской репродуктивной системы, контролируя селекцию и развитие фолликулов, в том числе посредством индукции кровотока в растущих фолликулах. NO модулирует образование стероидных гормонов в клетках яичников, участвует в мейотическом созревании [17]. В исследовании Zhao P. et al. (2021) показано, что активация примордиальных фолликулов, рост ооцитов и гранулезных клеток сопряжены с экспрессией эндотелиальной NO-синтазы (eNOS) в прегранулезных клетках [18].
Результаты метаанализа 16 исследований, включающих в общей сложности 895 пациенток с СПКЯ, показывают, что у женщин с данным синдромом наблюдается статистически значимое снижение уровня стабильных продуктов NO – нитритов в сыворотке или плазме [19], что обусловлено эндотелиальной дисфункцией при названной патологии. Krishna M.B. et al. (2017) обнаружено, что низкий уровень нитратов и нитритов в периферической крови у женщин с СПКЯ вызван ухудшением экспрессии eNOS и индуцибельной синтазы оксида азота (iNOS), увеличением образования асимметричного диметиларгинина (ингибитора NOS) и сниженной биодоступностью аргинина [20]. Комбинация кломифена цитрата и донора NO изосорбита мононитрата способствует увеличению частоты овуляции и наступления беременности у пациенток с СПКЯ по сравнению с контрольной группой [21]. Выявлено, что возрастное снижение качества ооцитов обусловлено дефицитом кофакторов NOS тетрагидробиоптерина и цинка с последующим разобщением работы фермента и интенсификацией образования супероксида [22].
В то же время предшественник NO L-аргинин (50–200 мг/кг), вводимый крысам-самкам линии Вистар в течение 9–14 дней, способствовал развитию в яичниках гистологической картины, характерной для СПКЯ [23]. Было обнаружено, что у женщин при проведении процедуры ЭКО/ИКСИ (интрацитоплазматическая инъекция сперматозоида) чрезмерная продукция NO и его повышенный уровень в фолликулярной жидкости ассоциированы с выраженной фрагментацией эмбрионов. Кроме того, концентрация NO в сыворотке была увеличена у пациенток как с трубным, так и перитонеальным бесплодием [24]. Показано, что высокие концентрации NO могут вызывать апоптоз гранулезных клеток, вероятно, вследствие образования пероксинитрита, что является маркером атрезии фолликулов [25].
Известно, что простагландины – это важные медиаторы, регулирующие имплантацию и раннее формирование эмбриона. В исследовании Huang J.C. et al. (2007) было показано, что простагландин I2 (PGI2, или простациклин) способствует развитию эмбрионов in vitro и повышает их способность к внедрению в эндометрий опосредованно через дельта-рецепторы, активируемые пролифератором пероксисом PPARdelta (peroxisome proliferator-activated receptor delta) [26]. У эмбрионов мышей с полным отсутствием рецептора PGI2 названные процессы не стимулировались ни аналогом PGI2 (илопрост), ни лигандом PPARdelta. При этом гетерозиготные особи, несущие нормальный материнский аллель рецептора, лучше развивались и реагировали на илопрост, в отличие от эмбрионов с нормальным отцовским аллелем [27].
Участие про- и антитромбогенных факторов эндотелия в развитии ооцитов
При дисфункции эндотелия отмечается изменение динамического равновесия между протромбогенными и антитромбогенными факторами, что приводит к нарушению регуляции физиологических процессов в женской репродуктивной системе. Более того, стимуляция овуляции при лечении бесплодия методами ВРТ в ряде случаев сопровождается гиперкоагуляцией вследствие гиперэстрогении и может служить причиной тромботических осложнений у женщин [28].
Фактор активации тромбоцитов (platelet-activating factor, PAF) синтезируется многими клетками, в том числе эндотелиоцитами, под контролем фермента ацетилгидролазы. Он является медиатором многих функций лейкоцитов, участвует в реализации воспалительного процесса, агрегации и дегрануляции тромбоцитов; в репродуктивной системе необходим для нормальной имплантации эмбриона и предположительно может являться прогностическим маркером успешной процедуры ЭКО. Было показано, что 15-минутная обработка PAF (10-7 M) эмбрионов мышей на стадии двух клеток перед имплантацией способствовала увеличению числа рожденных детенышей по сравнению с контрольной группой (56/80; 70% против 44/80; 55%) и их веса при рождении (1,31 г против 1,25 г) [29]. В клиническом исследовании Mahdian S. et al. (2021), однако, не было обнаружено статистически значимой взаимосвязи между рецидивирующей неудачей имплантации и уровнем PAF, хотя имелась тенденция к уменьшению количества последнего при бесплодии, вызванном женскими факторами, по сравнению со здоровыми женщинами [30].
Маркером дисфункции эндотелия является фактор фон Виллебранда – крупный адгезивный гликопротеин, который синтезируется эндотелиоцитами и мегакариоцитами. Было показано, что при тяжелом СГЯ у женщин его уровень повышается еще до клинических проявлений заболевания [31].
При оценке количества антител к фактору фон Виллебранда в плазме у женщин с СПКЯ было обнаружено увеличение данного показателя по сравнению с контрольной группой, особенно у пациенток с ановуляцией и гиперандрогенемией [32]. Недавнее исследование Moin A.S.M. et al. (2021) показало, что, помимо фактора фон Виллебранда, у женщин с СПКЯ в крови были повышены уровни следующих маркеров гиперкоагуляции: фибриногена (p<0,01), гамма-цепи фибриногена (p<0,0001), фибронектина (p<0,01), D-димера (p<0,0001), P-селектина (p<0,05) и калликреина (p<0,001) [33]. Более того, система гемостаза у пациенток с СПКЯ находится в заметном дисбалансе в I триместре беременности, что выражается в большей склонности к протромботическому состоянию вследствие увеличения количества фактора фон Виллебранда, VIII и X факторов свертывания по сравнению с показателями при физиологической беременности [34].
Фибринолитическая система и ее ингибиторы необходимы не только для поддержания гемостаза, но и участвуют в фолликулогенезе и овуляции. Увеличение уровня активаторов плазминогена и снижение количества ингибитора активатора плазминогена-1 способствует разрыву фолликула [35]. Кроме того, непосредственно перед овуляцией уровень ингибитора активатора плазминогена-1 увеличивается в той области яичника, где находятся незрелые фолликулы, тогда как тканевого активатора плазминогена – в области локализации преовуляторных фолликулов [36].
Изменение активности фибринолитической системы ассоциировано с различными патологиями репродуктивной системы. Li S. et al. (2021) обнаружено, что у женщин с СГЯ наблюдается статистически значимое увеличение содержания комплексов плазмин-α2-антиплазмин и тканевой активатор плазминогена-ингибитор активатора плазминогена с одновременным уменьшением уровня растворимого тромбомодулина. Концентрация названных маркеров коррелировала с тяжестью заболевания [37]. У пациенток с СПКЯ в плазме крови отмечается увеличение ингибитора активатора плазминогена-1 [33], что может способствовать повышенному тромбообразованию. У женщин с СПКЯ и нормальным уровнем андрогенов в ответ на стимуляцию яичников обнаруживают нарушение кровотока в микроциркуляторном русле, что свидетельствует о ранних дисфункциональных изменениях эндотелия сосудов [38]. В исследовании, включавшем 67 женщин с синдромом привычной потери беременности на ранних сроках, было показано, что преждевременная отслойка хориона и плаценты у них ассоциирована с аллельным вариантом гена ингибитора активатора плазминогена-1, чего не наблюдалось у 53 здоровых первобеременных. При генотипе 4G/4G отслойки встречались в 1,5 раза чаще, чем при генотипе 5G/4G [39].
Заключение
При лечении бесплодия методами ВРТ важнейшими параметрами, определяющими успешность терапии, являются качество и количество получаемых ооцитов. Для нормального развития последних необходимы адекватное формирование и поддержание стабильного кровотока в сосудах яичника, которое определяется эндотелиальными факторами.
Различные патологии женской репродуктивной системы и сопряженное с ними бесплодие часто сопровождаются эндотелиальной дисфункцией. Более того, индукция суперовуляции при лечении бесплодия методами ВРТ также может способствовать срыву адаптационных механизмов и нарушению функциональной активности сосудистого эндотелия вследствие высокой эстроген-гестагеновой нагрузки. Грозным осложнением индуцированного цикла является СГЯ, формирующийся в ответ на увеличение уровня гонадотропинов и характеризующийся генерализованным повреждением эндотелия и выраженной сосудистой проницаемостью.
В этой связи определение маркеров дисфункции эндотелия и оценка его функционирования необходимы врачам-клиницистам для подбора персонализированного лечения. Учет индивидуальных особенностей ответа эндотелия на стимуляцию гонадотропинами для индукции овуляции даст возможность выработать новые рекомендации и стандарты лечении бесплодия методами ВРТ, позволяющие сделать их более безопасными и эффективными.



