Comparative analysis of the effectiveness of programs and perinatal outcomes after frozen-thawed embryo transfer depending on post-transfer support medications
Choosing the optimal protocol for preparing the endometrium for frozen-thawed embryo transfer is the most important issue of personalized selection of therapy for improving the effectiveness of assisted reproductive technology (ART) programs.Bashmakova N.V., Lokshin V.N., Isenova S.Sh., Khramtsova A.Yu., Dankova I.V., Ryabukhin I.V.
Objective: To evaluate the effectiveness of frozen-thawed embryo transfer programs and pregnancy outcomes depending on the type of progestogen used for secretory endometrial transformation and post-transfer support.
Materials and methods: The study included 334 infertile women who had frozen-thawed own embryo transfer with hormone replacement therapy cycle in the Assisted Reproduction Department, Urals Scientific Research Institute for Maternal and Child Care. The first group consisted of 224 patients who took dydrogesterone (30 mg/day orally) for full secretory transformation of the endometrium, the second group included 110 patients who were prescribed micronized vaginal progesterone (600 mg/day).
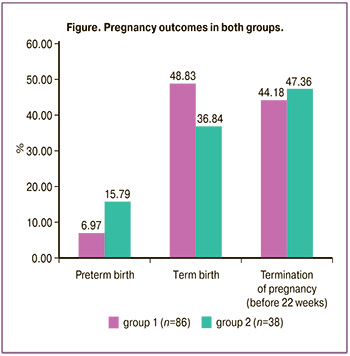
Results: The clinical pregnancy rate in group 1 was 86/224 (38.39%), and it was 38/110 (34.54%) in group 2; however, there was no statistical difference depending on the type of progestogen which was used to support the luteal phase (p=0.468). The term delivery rate in the group of women taking dydrogesterone was 42/86 (48.83%), and it was 14/38 (36.84%) in the comparison group (p=0.216). There was a tendency to a decrease in the preterm birth rate in group 1, namely, 6/86 (6.97%), while it was 6/38 (15.79%) in group 2 (p=0.126). There was no statistically significant difference in the rate of termination of pregnancy before 22 weeks in both groups (p=0.743): the rate of miscarriage in group 1 was 38/86 (44.18%), and it was 18/38 (47.36%) in group 2.
Conclusion: The data obtained in the study showed comparable effectiveness of progestogens in the clinical pregnancy rate and perinatal outcomes in the cycle of preparation for frozen-thawed embryo transfer. It is possible to use both micronized vaginal progesterone and dydrogesterone in cycles of hormone replacement therapy for preparing the endometrium for frozen-thawed embryo transfer.
Authors’ contributions: Bashmakova N.V., Lokshin V.N., Isenova S.Sh. – developing the concept and design of the study, editing; Khramtsova A.Yu., Dankova I.V., Ryabukhin I.V. – collecting and processing the material; Khramtsova A.Yu. – statistical data processing; Bashmakova N.V., Khramtsova A.Yu. – writing the text.
Conflicts of interest: The authors declare that there are no conflicts of interest.
Funding: The study was conducted without sponsorship.
Ethical Approval: The study was approved by the Ethical Review Board of the Urals Scientific Research Institute for Maternal and Child Care, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Bashmakova N.V., Lokshin V.N., Isenova S.Sh., Khramtsova A.Yu., Dankova I.V., Ryabukhin I.V. Comparative analysis of the effectiveness of programs and perinatal outcomes
after frozen-thawed embryo transfer depending on post-transfer support medications.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (7): 103-108 (in Russian)
https://dx.doi.org/10.18565/aig.2023.161
Keywords
The widespread application of advanced cryopreservation techniques has become a breakthrough in assisted reproductive technologies (ART) over the past decade. In frozen-thawed embryo transfer cycle, endometrial receptivity is preserved, ovarian hyperstimulation syndrome is excluded, one embryo can be transferred after preimplantation genetic testing during the implantation window; all these factors contribute to the preservation of fertility and an increase in the number of live births [1, 2]. Perinatal outcomes of cryopreserved embryo transfer have advantages due to the decreased frequency of low birth weight for gestational age, preterm birth, placenta previa, placental abruption and perinatal mortality in comparison with the transfer of fresh embryos [3–5]. However, cryopreserved embryo transfer is accompanied by an increased risk of macrosomia and hypertensive disorders of pregnancy [6, 7]. It still remains unknown whether these differences result from taking hormone replacement therapy in the protocols for preparing the endometrium for the transfer of thawed embryos or from the absence of corpus luteum [8, 9]. The choice of a progestogen for adequate secretory transformation and subsequent post-transfer support is another important issue [10, 11].
The role of progestogens in reducing late pregnancy complications, such as preeclampsia, is currently studied. The data of the previous retrospective studies show a decrease in the frequency of preeclampsia after taking dydrogesterone to support the luteal phase in ART programs [12, 13].
There were some studies that compared the effectiveness of the use of dydrogesterone and micronized vaginal progesterone in the cycles of in vitro fertilization [6, 14–16]. However, there is no evidence base in favor of certain progestogens to support the luteal phase after the transfer of thawed embryos nowadays.
It is necessary to conduct further prospective randomized trials to determine the optimal programs of endometrial preparation with subsequent post-transfer support to improve the outcomes of ART.
The aim of the study is to evaluate the effectiveness of frozen-thawed embryo transfer programs and pregnancy outcomes depending on the type of progestogen used for secretory endometrial transformation and post-transfer support.
Materials and methods
This was a retrospective study of the outcomes of frozen-thawed own embryo transfers with hormone replacement therapy cycle in the period from February 2021 to May 2022 in the Assisted Reproduction Department, Urals Scientific Research Institute for Maternal and Child Care. The study included 334 women diagnosed with female infertility who were prescribed the following hormonal preparations to prepare the endometrium for the transfer of thawed embryos: estrogens (estradiol valerate at a dosage of 6 mg/day from the 2nd–4th day of the menstrual cycle) aimed at achieving proliferative changes in the endometrium, and progestogens for the secretory transformation and decidualization of the endometrium (till the endometrium becomes at least 7 mm thick and up to 12 weeks gestation). All the patients were divided into two groups: the first group consisted of 224 patients who took dydrogesterone (30 mg/day orally) for full secretory transformation of the endometrium, the second group included 110 patients who were prescribed micronized vaginal progesterone (600 mg/day). The preparation of the endometrium did not include methods of surgical, instrumental (cavity irrigation), or biological (intrauterine administration of platelet-enriched plasma) preparation of the uterine cavity and endometrium.
There were the following main outcome indicators: the presence of clinical pregnancy, live birth, obstetric complications (the frequency of miscarriage, preterm birth).
Statistical analysis
Statistical processing of the study results was carried out using Microsoft Excel (2010) application software packages, Stat Soft Statistica 6.0 (Stat Soft, USA), SPSS Statistics version 22.0 (IBM Microsoft, USA). The assessment of the compliance of the sample with the normal distribution was carried out using the Kolmogorov–Smirnov test. If the distribution of the sampling was normal, the data were presented in the form of a mean value (M) and a standard deviation (SD). Qualitative variables were represented as absolute values and relative values in percentage, statistical significance was assessed using the chi-squared test (χ2), if the absolute frequencies were less than 10, then the Yates’ correction was applied. The null hypothesis was rejected at p<0.05. In order to evaluate the final results of each of the clinical outcomes, the effect size was calculated using the odds ratio (OR) with a 95% confidence interval (CI).
Results and discussion
The average age of patients in group 1 was 34.3 (0.63) years, and it was 33.8 (0.75) years in group 2; demographic indicators were comparable in the study groups (p=0.063).
The assessment of the accompanying somatic pathologies revealed that in both groups the endocrine, nutritional and metabolic diseases occupy a leading place: 54/224 in the 1st group (24.10%) and 14/110 (12.72%) in the 2nd group, with a statistically significant difference (p=0.016). The diseases of the gastrointestinal tract occurred in 16/224 patients (7.14%) in group 1, and in 10/110 (9.09%) patients in group 2 (p>0.05). In addition, obesity was the fourth leading somatic pathology in the group of patients who used micronized vaginal progesterone, namely, 8/110 (7.27%) (Table 1).
Most frequently the patients of both groups had the following gynecological diseases: postoperative uterine scar requiring medical care, 42/224 (18.75%) in group 1 versus 18/110 (16.36%) in group 2, p=0.594; polycystic ovary syndrome, 26/224 (11.60%) in group 1 versus 10/110 (9.09%) in group 2, p=0.486; uterine leiomyoma, 20/224 (8.92%) in group 1 versus 4/110 (3.63%) in group 2, p=0.079. There were no statistically significant differences in gynecological pathologies in all groups (p>0.05).
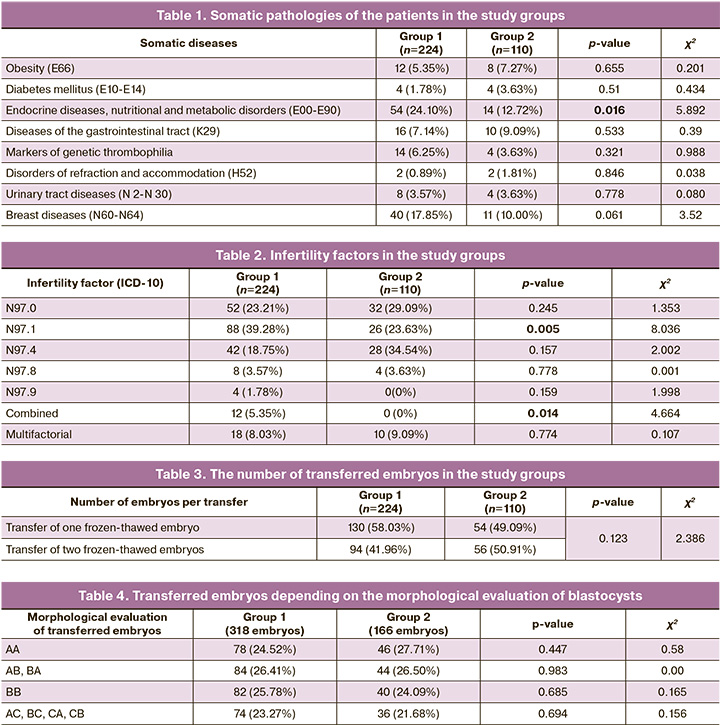
Infertility factors of patients in each group were analyzed in this study (Table 2).
The most common factor of infertility in patients of group 1 who received dydrogesterone to support the luteal phase was tubal factor (N97.1), 88/224 patients (39.28%). The next most frequent factors were anovulatory factor (N97.0), 52/224 patients (23.21%), and male factor (N97.4), 42/224 patients (18.75%). Among the patients who used micronized vaginal progesterone, the most frequent factors were female infertility associated with male factor (N97.4), 28/110 (34.54%), and anovulation (N97.0), 32/110 (29.09%). The patients of group 1 were statistically significantly more likely to have female infertility associated with tubal factor (p=0.005) and combined forms of infertility (p=0.014) than the patients of group 2. Taking into account the statistical significance of differences in infertility factors, we analyzed the embryological stage of frozen-thawed embryo transfer programs in these groups. There were no statistically significant differences in the number of transferred embryos in patients of groups 1 and 2. One embryo was transferred in the group of patients who received dydrogesterone in 130/224 (58.03%) cases, and in the group of patients who used micronized vaginal progesterone in 54/110 (49.09%) cases (p=0.123); two frozen-thawed embryos were transferred to the uterine cavity in the rest of the cases (Table 3).
All transferred embryos were at the blastocyst stage, their cryopreservation took place on the 5th–6th day of embryo development. Preimplantation testing of embryos was not carried out. After embryos were thawed, they were assessed according to the scoring system developed by Gardner. We analyzed embryo transfers using the data on the quality of blastocysts (Table 4). The frequency of transferred embryos of excellent and good quality (AA, AB, BA, BB) in the group of patients who received dydrogesterone was 244/318 (76.73%), and it was 130/166 (78.31%) in the 2nd group of patients (p>0.05). All other embryos were of satisfactory quality: 74/318 (23.27%) cases in group 1; 36/166 (21.68%) cases in group 2 (p=0.694). There were no statistically significant differences in the quality of transferred embryos in all cases.
The outcomes of ART programs were evaluated on the basis of clinical pregnancy rate in the study groups (Table 5).

Conclusion
Our study was aimed at evaluating the effectiveness of ART programs and perinatal outcomes after the transfer of frozen-thawed embryos depending on the progestogen used for secretory endometrial transformation, endometrial decidualization and post-transfer support.
The data obtained in the study showed comparable effectiveness of progestogens in the clinical pregnancy rate and perinatal outcomes in the cycle of preparation for frozen-thawed embryo transfer; however, no statistically significant differences were revealed. The patients who received dydrogesterone were more likely to have preterm birth, but the differences in the rate of live birth and obstetric complications were not statistically significant.
It is possible to use both micronized vaginal progesterone and dydrogesterone in cycles of hormone replacement therapy for preparing the endometrium for frozen-thawed embryo transfer.
It is necessary to conduct further multicenter studies on a large sample of patients to draw conclusions on the possibility of personal use of progestogens in ART programs with frozen-thawed embryo transfer, as well as the assessment of pregnancy complications and perinatal outcomes.
References
- Перминова С.Г., Савостина Г.В., Екимов А.Н., Белова И.С. Роль преимплантационного генетического тестирования эмбрионов на анеуплоидии в исходах программ вспомогательных репродуктивных технологий у различных групп пациентов. Акушерство и гинекология. 2023; 3: 73-82. [Perminova S.G., Savostina G.V., Ekimov A.N., Belova I.S. Role of preimplantation genetic testing of embryos for aneuploidy in assisted reproductive technology outcomes in different groups of patients. Obstetrics and Gynecology. 2023; (3): 73-82. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.288.
- Полумискова А.О., Тевкин С.И., Шишиморова М.С., Джусубалиева Т.М. Переносы медленно растущих эмбрионов: «свежие» морулы/ранние бластоцисты 5-го дня или размороженные бластоцисты 6-го дня развития? Проблемы репродукции. 2022; 28(5): 46 54. [Polumiskova A.O., Tevkin S.I., Shishimorova M.S., Jussubaliyeva T.M. Transfers of slow-growing embryos: «fresh» day 5 morulas/early blastocysts or frozen-thawed day 6 blastocysts? Russian Journal of Human Reproduction. 2022; 28(5): 46 54.(in Russian)]. https://dx.doi.org/10.17116/repro20222805146.
- Ernstad E.G., Wennerholm U.-B., Khatibi A., Petzold M., Bergh C. Neonatal and maternal outcome after frozen embryo transfer: Increased risks in programmed cycles. Am. J. Obstet. Gynecol. 2019; 221(2): 126.e1-126.e18.https://dx.doi.org/10.1016/j.ajog.2019.03.010.
- Sha T., Yin X., Cheng W., Massey I.Y. Pregnancy-related complications and perinatal outcomes resulting from transfer of cryopreserved versus fresh embryos in vitro fertilization: a meta-analysis. Fertil. Steril. 2018; 109(2): 330-342.e9. https://dx.doi.org/10.1016/j.fertnstert.2017.10.019.
- Мелкозёрова О.А., Башмакова Н.В., Данькова И.В., Окулова Е.О. Репродуктивные и перинатальные исходы применения криотехнологий в программах экстракорпорального оплодотворения (обзор литературы). Проблемы репродукции. 2019; 25(3): 82 90. [Melkozerova O.A., Bashmakova N.V., Dankova I.V., Okulova E.O. Reproductive and perinatal outcomes of a frozen embryo transfer in the programs of assisted reproductive technologies (literature review). Russian Journal of Human Reproduction. 2019; 25(3): 82 90. (in Russian)]. https://dx.doi.org/10.17116/repro20192503182.
- Singh B., Reschke L., Segars J., Baker V.L. Frozen-thawed embryo transfer: the potential importance of the corpus luteum in preventing obstetrical complications. Fertil. Steril. 2020; 113(2): 252-7. https://dx.doi.org/10.1016/j.fertnstert.2019.12.007.
- Orvieto R., Kirshenbaum M., Gleicher N. Is embryo cryopreservation causing macrosomia-and what else? Front. Endocrinol. (Lausanne). 2020; 11: 19. https://dx.doi.org/10.3389/fendo.2020.00019.
- Labarta E., Rodríguez C. Progesterone use in assisted reproductive technology. Best Pract. Res. Clin. Obstet. Gynaecol. 2020; 69: 74-84.https://dx.doi.org/10.1016/j.bpobgyn.2020.05.005.
- Rosalik K., Carson S., Pilgrim J., Luizzi J., Levy G., Heitmann R., Pier B. Effects of different frozen embryo transfer regimens on abnormalities of fetal weight: a systematic review and meta-analysis. Hum. Reprod. Update. 2021; 28(1): 1-14. https://dx.doi.org/10.1093/humupd/dmab037.
- Российское общество акушеров-гинекологов (РОАГ), Российская ассоциация репродукции человека (РАРЧ). Клинические рекомендации. Женское бесплодие. 2021. [Russian Society of Obstetricians-Gynecologists, Russian Association of Human Reproduction. Clinical Guidelines. Women's Infertility. 2021. (in Russian)].
- Вспомогательные репродуктивные технологии и искусственная инсеминация. Клинические рекомендации (протокол лечения). 2019. [Assisted reproductive technologies and artificial insemination. Clinical Guidelines (treatment protocol). 2019. (in Russian)].
- Ali A.B., Ahmad M.F., Kwang N.B., Shan L.P., Shafie N.M., Omar M.H. Dydrogesterone support following assisted reproductive technique (ART) reduces the risk of pre-eclampsia. Horm. Mol. Biol. Clin. Investig. 2016; 27(3): 93-6. https://dx.doi.org/10.1515/hmbci-2015-0063.
- Tskhay V., Schindler A., Shestakova M., Klimova O., Narkevich. The role of progestogen supplementation (dydrogesterone) in the prevention of preeclampsia. Gynecol. Endocrinol. 2020; 36(8): 698-701. https://dx.doi.org/10.1080/09513590.2019.1706085.
- Raghupathy R., Szekeres-Bartho J. Progesterone: a unique hormone with immunomodulatory roles in pregnancy. Int. J. Mol. Sci. 2022; 23(3): 1333. https://dx.doi.org/10.3390/ijms23031333.
- Greenbaum S., Athavale A., Klement A.H., Bentov Y. Luteal phase support in fresh and frozen embryo transfers. Front. Reprod. Health. 2022; 4: 919948.https://dx.doi.org/10.3389/frph.2022.919948.
- Griesinger G., Blockeel C., Kahler E., Pexman-Fieth C., Olofsson J.I., Driessen S. et al. Dydrogesterone as an oral alternative to vaginal progesterone for IVF luteal phase support: A systematic review and individual participant data meta-analysis. PLoS One. 2020; 15(11): e0241044. https://dx.doi.org/10.1371/journal.pone.0241044.
- AbdulHussain G., Azizieh F., Makhseed M., Raghupathy R. Effects of progesterone, dydrogesterone and estrogen on the production of Th1/Th2/Th17 cytokines by lymphocytes from women with recurrent spontaneous miscarriage. J. Reprod. Immunol. 2020; 140: 103132. https://dx.doi.org/10.1016/j.jri.2020.103132. .
Received 23.06.2023
Accepted 10.07.2023
About the Authors
Nadezda V. Bashmakova, Dr. Med. Sci., Professor, Honored Doctor of the Russian Federation, Head of department of ART, Chief obstetrician-gynecologist of theUral Federal District, Chief Researcher of Urals Scientific Research Institute for Maternal and Child Care, Ministry of Health of Russia, +7(343)371-87-68,
BashmakovaNV@niiomm.ru, SPIN РИНЦ: 9604-0089, Scopus AuthorID: 270915, 620028, Russia, Yekaterinburg, Repin str., 1.
Vyacheslav N. Lokshin, Dr. Med. Sci., Professor, Academician of the National Academy of Sciences of the Republic of Kazakhstan, President of the Kazakhstan Association of Reproductive Medicine, Rector of the International Academy of Reproductology, President of the Association of International Pharmaceutical Manufacturers, PERSONA International Clinical Center of Reproductology LLP, +77273827777, 32a Utepova str., Almaty, Republic of Kazakhstan.
Sayle Sh. Isenova, Dr. Med. Sci., Professor, Head of the Department of Obstetrics and Gynecology, NAO "Kazakh National University named after S.D. Asfendiyarov",
Almaty, Republic of Kazakhstan, 87051727500, isienova10@mail.ru, https://orcid.org/0000-0003-1869-746X
Alexandra Yu. Khramtsova, PhD, obstetrician-gynecologist, researcher, Department of ART, Urals Scientific Research Institute for Maternal and Child Care,
Ministry of Health of Russia, +7(912)68-26-726, aleksaxr@mail.ru, SPIN РИНЦ: 2467-5326, Scopus AuthorID: 57214320389, https://orcid.org/0000-0002-4304-3516,
620028, Russia, Yekaterinburg, Repin str., 1.
Irina V. Dankova, PhD, Researcher at the Department of Women's Reproductive Function Preservation, Urals Scientific Research Institute for Maternal and Child Care, Ministry of Health of Russia, +7(343)371-08-78, https://orcid.org/0000-0002-7893-4722, 620028, Russia, Yekaterinburg, Repin str., 1.
Igor V. Ryabukhin, Senior Embryologist, Department of Assisted Reproductive Technologies, Urals Scientific Research Institute for Maternal and Child Care,
Ministry of Health of Russia, +7(343)232-55-12, 620028, Russia, Yekaterinburg, Repin str., 1.



