На сегодняшний день вопросы этиологии, патогенеза, диагностики и лечения женского бесплодия составляют одну из актуальных проблем гинекологии. Распространенность этого заболевания в Российской Федерации за период с 2011 по 2021 гг. выросла на треть и составила 735,9 человек на 100 тыс. женщин [1].
Современным методом лечения бесплодия являются вспомогательные репродуктивные технологии (ВРТ), развитие которых способствовало значительному прогрессу в репродуктологии. Однако, несмотря на это, эффективность экстракорпорального оплодотворения (ЭКО) остается в пределах 22–35%, что может быть связано с неудовлетворительным качеством и количеством получаемых ооцитов [2] и определяется многими факторами – возрастом женщины, показателями овариального резерва, а также причинами бесплодия, экстрагенитальным анамнезом и морбидностью.
Известно, что в развитии ооцитов важную роль играет микроокружение, которое создается, в том числе, при участии эндотелиальных клеток, продуцирующих множество биологически активных веществ. Среди них можно выделить молекулы, участвующие в реализации воспалительной реакции и различные факторы роста (таблица).
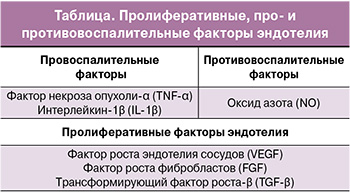
Было показано повышение уровня провоспалительных цитокинов и изменение активности факторов роста в сыворотке крови пациенток, проходящих ЭКО, что может являться основным предрасполагающим фактором неудачных попыток процедуры [3]. Поэтому изучение роли эндотелия и выделяемых им факторов в созревании ооцитов у женщин, участвующих в программах ВРТ, позволит выработать новые схемы терапии и будет способствовать персонификации лечения бесплодия.
Целью настоящего обзора является оценка роли пролиферативных, про- и противовоспалительных факторов эндотелия в созревании ооцитов при лечении бесплодия методами ВРТ.
Роль про- и противовоспалительных факторов эндотелия в формировании ооцитов
Известно, что умеренный воспалительный процесс необходим для успешного фолликулогенеза, созревания ооцитов и овуляции. Однако чрезмерное нефизиологичное воздействие провоспалительных факторов способно привести к бесплодию, что наблюдается при разных патологиях женской репродуктивной системы. Эндотелий сосудов вырабатывает биологически активные вещества, которые влияют на формирование и течение воспалительной реакции.
Провоспалительные цитокины – фактор некроза опухоли α (tumor necrosis factor-alpha, TNF-α) и интерлейкин-1β (IL-1β) могут локально синтезироваться в яичнике и участвовать в паракринной/аутокринной регуляции созревания, пролиферации и апоптозе фолликулярных клеток у млекопитающих.
В in vitro исследовании Paulino L.R.F.M. et al. (2018) показано, что TNF-α, добавленный в среду с вторичными фолликулами крупного рогатого скота, способствует увеличению их диаметра и скорости образования антрального отдела [4]. Данные ультраструктурного анализа свидетельствуют о том, что фолликулы, культивируемые совместно с TNF-α, имеют наилучшую сохранность гранулезных клеток относительно показателей фолликулов, к которым добавляли TNF-α и IL-1β или только IL-1β. В то же время культивирование преантральных фолликулов совместно с TNF-α (10 нг/мл) приводит к снижению их выживаемости и увеличивает количество апоптотических клеток в ткани яичника [5].
Обнаружено, что IL-1β способствует улучшению качества ооцитов у крупного рогатого скота вследствие повышенной экспрессии генов, отвечающих за рост кумулюсных клеток [6]. Однако IL-1β ингибирует индуцированное гонадотропинами созревание ядер ооцитов [7]. Расхождение в данных, вероятно, связано с тем, что в зависимости от стадии развития фолликула TNF-α и IL-1β влияют на клетки яичника по-разному с учетом уровня экспрессии соответствующих рецепторов.
Интенсивный синтез цитокинов в различных компартментах яичника может быть результатом воспалительного процесса, характерного для эндометриоза, и влиять на фертильность. Обнаружено, что при перитонеальном эндометриозе у крыс было повышено содержание TNF-α в перитонеальной жидкости, которое сопровождалось сверхэкспрессией мРНК и непосредственно самого фактора транскрипции Egr1 в фолликулярной жидкости [8]. Последний способен препятствовать наступлению овуляции, очевидно, за счет ингибирования пролиферации и активации апоптоза в гранулезных клетках через сигнальный путь NF-κB, приводя к атрезии фолликулов [9].
Было показано, что у пациенток с эндометриозом, проходящих процедуру ЭКО/интрацитоплазматической инъекции сперматозоида (ИКСИ), уровни провоспалительных IL-1β и IL-18 были значительно выше, чем в группе с бесплодием, связанным с трубным или мужским фактором (5010 пг/мл против 2738 пг/мл соответственно, p<0,05). Кроме того, у женщин с названной патологией в гранулезных клетках была обнаружена высокая экспрессия инфламмасомы NLRP3 как на уровне белка, так и на уровне мРНК [10]. Следует отметить, что повышенная концентрация IL-1β в фолликулярной жидкости способна стимулировать овуляцию путем активации киназ, регулируемых внеклеточными сигналами, ERK1/2 (extracellular signal-regulated kinase) и CCAAT-энхансер-связывающего белка β (C/EBPβ), чего, однако, не происходит у женщин с эндометриозом. В исследовании Lin X. et al. (2022) выявлено, что у пациенток с данным заболеванием в гранулезных клетках уменьшается активность гистон-метилтрансферазы EZH2, что ингибирует каскад событий, ведущих к овуляции, путем повышения экспрессии рецепторов IL-1R2, которые служат «ловушкой» для IL-1β [11].
Вялотекущее хроническое воспаление характерно для синдрома поликистозных яичников (СПКЯ); системный воспалительный ответ возникает на высвобождение лейкоцитов и цитокинов из яичников в кровоток. Было показано, что у пациентов с данным синдромом наблюдаются более высокие, по сравнению со здоровыми женщинами, уровни циркулирующих лимфоцитов, нейтрофилов, эозинофилов, моноцитов и Т-хелперов, которые задействованы в аутоиммунных процессах; в то время как число регуляторных Т-клеток снижается. В периферической крови женщин с СПКЯ отмечают увеличение следующих маркеров воспаления: С-реактивного белка, интерлейкинов (IL-6, IL-17A, IL-17F, IL-18, IL-23), TNF-α, α-1 кислого гликопротеина, моноцитарного хемоаттрактантного белка-1. Количество противовоспалительных факторов – IL-10, IL-17E, IL-27, IL-35 и IL-37, трансформирующего фактора роста-β, оментина-1, секретируемого frizzled-родственного белка 5 (SFRP5) – снижается [12].
У пациенток с синдромом гиперстимуляции яичников (СГЯ), проходящих лечение бесплодия с использованием методов ВРТ, в фолликулярной жидкости статистически значимо повышен уровень провоспалительных цитокинов IL-17, IL-12, IL-18 и IL-23 относительно контрольной группы [13, 14]. Синдром сопровождается лейкоцитозом и высокими концентрациями IL-6, TNF-α, фибриногена, D-димеров [15]. Однако в исследовании Alhilali M.J. et al. (2020) отмечено, что при СГЯ внутрифолликулярная концентрация TNF-α в день забора ооцитов отрицательно коррелирует с риском формирования заболевания среднего и тяжелого течения [16]. Очевидно, что различные уровни TNF-α наблюдаются в зависимости от стадии развития фолликула и способны инициировать либо апоптотические процессы, либо развитие ооцита.
В исследовании Lamaita R.M. et al. (2012) показано, что у пациенток с хронической ановуляцией, подвергшихся процедуре ИКСИ, наблюдается значительно более высокая активация фолликулярных макрофагов/нейтрофилов, сопровождающаяся повышенной концентрацией С-реактивного белка в крови и фолликулярной жидкости, по сравнению с женщинами с нормальной овуляцией [17]. Высокий уровень С-реактивного белка в крови ассоциирован со временем развития СГЯ. Выявлено, что данный параметр был значительно выше при раннем (медиана 21; межквартильный размах 8–33 мг/л), чем при позднем (медиана 6; межквартильный размах 3–9 мг/л, р=0,001) СГЯ [18].
Следует отметить, что уровень С-реактивного белка также может рассматриваться в качестве прогностического маркера исхода ЭКО. В клиническом исследовании Herzberger E.H. et al. (2016) отмечено, что у пациентов с количеством С-реактивного белка ≥0,5 мг/дл в день забора яйцеклетки были эмбрионы низкого качества [19].
NO также относится к синтезируемым эндотелием противовоспалительным факторам и, продуцируемый eNOS в небольших концентрациях, ингибирует экспрессию молекул адгезии, синтез цитокинов и хемокинов, а также адгезию и миграцию лейкоцитов. При этом активация iNOS и избыточное образование NO, наоборот, оказывают провоспалительный эффект.
Воспалительный процесс, характерный для СПКЯ, сопровождается уменьшением количества NO. В клиническом исследовании с участием 62 женщин с ожирением и бесплодием, вызванным СПКЯ, выявлено повышение уровня матриксной металлопротеиназы 9 (ММП-9) в венозной крови. При этом количество ММП-9 положительно коррелировало с продолжительностью бесплодия (r=0,253; p=0,047) и отрицательно – с уровнями NO (r=-0,259; p=0,042) [20].
Пролиферативные факторы эндотелия и созревание ооцитов
Известно, что способность к формированию и количество новых сосудов в фолликулах напрямую влияют на созревание ооцитов и последующую успешность имплантации эмбриона. Во время фолликулогенеза расширение сосудистой системы яичников обусловлено повышенной потребностью в питательных веществах и кислороде. У женщин, вовлеченных в процедуру ЭКО и имеющих низкую степень перифолликулярной васкуляризации, частота наступления беременности заметно снижается по сравнению с пациентками с адекватной васкуляризацией, проходящими лечение бесплодия методами ВРТ (13,7% против 34%) [21].
Фактор роста эндотелия сосудов (VEGF) секретируется в эндотелиоцитах гранулезными, тека-клетками и играет значительную роль в созревании ооцитов и эмбриогенезе (рисунок) [22].
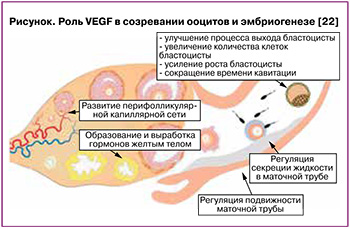
Высокий уровень VEGF в фолликулярной жидкости положительно коррелирует с большой частотой наступления оплодотворения и качеством эмбрионов [22]. В in vitro исследовании Trau H.A. et al. (2016) показано, что после введения хорионического гонадотропина в рамках лечения бесплодия методами ВРТ в гранулезных клетках овуляторных фолликулов через 18–34 ч наблюдается появление эндотелиоцитов. VEGF-A усиливал пролиферацию эндотелиальных клеток микрососудов яичников, миграцию и образование структур, напоминающих капиллярные отростки. При этом передача сигнала через рецепторы VEGFR1 способствует перемещению эндотелиоцитов, пролиферации и образованию нескольких длинных отростков, в то время как стимуляция VEGFR2 ассоциирована с миграцией эндотелиальных клеток и образованием множества коротких отростков [23].
Несмотря на то что высокий уровень VEGF указывает на улучшение васкуляризации и положительно влияет на развитие фолликулов, в случае аномального ангиогенеза он отрицательно коррелирует с исходами беременности при проведении ЭКО. Например, в исследовании случай-контроль с участием 134 женщин было показано, что у пациенток с рецидивирующей неудачей имплантации уровень VEGF-A в крови был значительно выше по сравнению со здоровой контрольной группой (362,9 против 171,6 пг/мл, p<0,0001) [24]. Хотя у пациенток с данной патологией экспрессия VEGF-A, VEGF-C и плацентарного фактора роста (placental growth factor, PLGF) в эндометрии (железистый эпителий, люминальный эпителий и строма) была снижена относительно показателей фертильных женщин [25].
Довольно частой причиной бесплодия является эндометриоз. В исследовании Zhang L. et al. (2016) было показано, что у женщин с этим заболеванием отмечается повышенная экспрессия VEGF, рецептора эстрогена α и β-катенина в очагах эндометриоза по сравнению с нормальным эндометрием. Авторы полагают, что 17β-эстрадиол способствует увеличению образования β-катенина и активации сигнального пути Wnt/β-катенин, что усиливает транскрипцию гена VEGF. Все это приводит к стимуляции ангиогенеза и патологическим изменениям эндометрия [26]. Обнаружено, что нарушение регуляции экспрессии рецепторов VEGF (снижение уровня VEGFR-1 и повышение VEGFR-2) ассоциировано с развитием заболевания [27].
Для женщин с СПКЯ характерны повышенная васкуляризация яичников и чрезмерно активный ангиогенез как следствие увеличения уровня VEGF. Кроме того, четко показана взаимосвязь между различными вариантами VEGF и возможностью развития синдрома [28]. В метаанализе Huang L., Wang L. (2020), включающем результаты 29 исследований случай-контроль по 11 различным полиморфизмам, было выявлено, что наличие пяти вариантов VEGF в крови – rs699947, rs833061, rs1570360, rs3025020, rs3025039 – ассоциировано с высоким риском формирования СПКЯ [29].
Факторы роста фибробластов (FGF) представляют собой группу полипептидов от 17 до 34 кДа, которые синтезируются в фибробластах, хондроцитах, эндотелиальных, гладкомышечных, тучных клетках и активно участвуют в процессах ангиогенеза и эмбриональном развитии, контролируя клеточную пролиферацию, миграцию, дифференцировку и апоптоз. Было показано, что в репродуктивной системе млекопитающих FGF-10 экспрессируется в преантральных фолликулах. В тканях плода белок был обнаружен в 50% образцов ооцитов и в 30% образцов гранулезных клеток. В ткани яичников девочек и женщин FGF-10 (мРНК и белковый продукт) присутствовал в ооцитах и гранулезных клетках всех проб [30]. В недавнем in vitro исследовании Kanke T. et al. (2022) было показано, что белки семейства FGF, которые продуцируются в фолликулах, участвуют в регуляции развития и функционирования кумулюсных клеток. После обработки кумулюс-ооцитарных комплексов соединением NVP-BGJ398 (ингибитор FGF) значительно повышалась экспрессия генов Ptgs2 и Ptx3, которые отвечают за экспансию кумулюсных клеток [31].
Выявлено, что одной из причин повторной неудачи имплантации при проведении ЭКО является недостаточная, по сравнению с фертильными женщинами, экспрессия FGF-1 в эндометриальных железистых эпителиальных и стромальных клетках, а также эндотелиоцитах, полученных в лютеиновую фазу [32]. Гетерозиготная нонсенс-мутация Arg622X в гене рецептора FGFR1 вызывает гипогонадотропный гипогонадизм [33]. Уровень основного фактора роста фибробластов bFGF в сыворотке крови у пациенток, перенесших контролируемую гиперстимуляцию яичников, разнится в зависимости от причин бесплодия: у женщин с СПКЯ исследуемый показатель составил 4,8±2,3 пг/мл, у пациенток с эндометриозом – 5,9±3,0 пг/мл, у женщин с мужским фактором бесплодия – 7,4±4,5 пг/мл [34].
Избыток FGF приводит к увеличению массы яичников вследствие образования новых кровеносных сосудов в строме и теке, что является ключевым признаком СПКЯ. Выявлено, что в сыворотке крови у женщин с СПКЯ количество FGF значительно превышает аналогичный показатель в контрольной группе как в день введения хорионического гонадотропина, так и в день забора ооцитов. Уровень FGF прямо коррелировал с количеством ранее введенного фолликулостимулирующего гормона, а концентрация этого фактора в фолликулярной жидкости имела обратную зависимость с процентом собранных зрелых ооцитов [35].
Обнаружено, что количество мРНК и белка FGF-2 повышено в эктопическом эндометрии. Активация FGF-2 нарушает сигнальный путь SPRYs/DUSP6/ERK в железистых эпителиальных клетках эндометрия, что способствует развитию эндометриоза [36]. На мышиной модели эндометриоза in vivo было показано, что ингибитор FGFR1 – соединение AZD4547 (25 мг/кг), которое вводили, начиная со дня индукции эндометриоза или через 2 недели (когда поражения уже были установлены) в течение 20 дней, уменьшало размер эндометриоидного поражения, не нарушая эстральный цикл [37].
В исследовании Filant J. et al. (2014) показано, что у самок мышей с делецией FGFR2 ухудшается фертильность – количество пометов и детенышей у животных этой группы было статистически значимо меньше по сравнению с контрольными самками. Авторы считают, что причиной наблюдаемой аномалии является ухудшение проницаемости сосудов и ангиогенеза, которые имеют решающее значение для успешной имплантации, децидуализации и плацентации: на 5,5 гестационный день у мышей с генотипом Fgfr2d/d места имплантации аккумулировали заметно меньше красителя по сравнению с животными без делеции [38].
Трансформирующий фактор роста β (transforming growth factor-β, TGF-β) играет важную роль в передаче сигналов от клетки к клетке и контролирует индукцию пролиферации, апоптоз, миграцию, адгезию, продукцию белков внеклеточного матрикса, организацию цитоскелета. Млекопитающие экспрессируют три из пяти изоформ TGF-β: TGF-β1, TGF-β2 и TGF-β3. Изменение регуляции передачи сигналов TGF-β вызывает нарушения процессов васкулогенеза, ангиогенеза, лимфангиогенеза. В репродуктивной системе человека передача сигналов посредством TGF-β ассоциирована с фолликулогенезом, овуляцией, формированием ооцитов и децидуализацией, имплантацией, беременностью и развитием матки [39].
Была оценена взаимосвязь между TGF-β1 и его рецептором, растворимым эндоглином (sENG), в сыворотке женщин с СПКЯ во время контролируемой стимуляции яичников. Обнаружено, что исходный уровень, а также концентрация TGF-β1 в дни введения хорионического гонадотропина и забора ооцитов были больше по сравнению с контрольной группой женщин без патологии. Следует отметить, что одновременно в сыворотке было понижено количество sENG [40]. В недавнем исследовании Alvandian F.
et al. (2022) выявлено, что при СПКЯ изменяется синтез членов суперсемейства TGF-β, которое включает в себя костный морфогенетический белок 15 (bone morphogenetic protein 15, BMP15), антимюллеров гормон, фактор дифференцировки роста 9 (growth differentiation factor-9, GDF9) и соответствующие им рецепторы BMPR1A (bone morphogenetic protein receptor type 1A), BMPR1B и BMPR2. Профиль экспрессии мРНК этих факторов в гранулезных и кумулюсных клетках у пациенток после стимуляции яичников при проведении ЭКО/ИКСИ показал, что количество антимюллерова гормона и BMPR1A было значительно повышено относительно контрольной группы. Напротив, экспрессия GDF9, BMP15, BMPR1B и BMPR2 была снижена [41].
В исследовании Fang L. et al. (2020) была продемонстрирована взаимосвязь между экспрессией TGF-β1 и VEGF. В фолликулярной жидкости женщин с СГЯ уровни этих белков положительно коррелировали и были повышены относительно контрольной группы. Кроме того, TGF-β1 стимулирует экспрессию и секрецию VEGF в иммортализованных и первичных лютеинизированных клетках гранулезы фолликулов человека. Авторами было показано, что TGF-β1-индуцированная экспрессия и секреция VEGF обусловлены активацией транскрипционных факторов SMAD2/3, ERK1/2, а также сигнального пути p38 митоген-активируемой протеинкиназы (mitogen-activated protein kinase, MAPK) [42].
Увеличение активности TGF-β1 ассоциировано со снижением функционирования иммунных клеток в брюшине, с увеличением выживаемости, прикрепления, инвазии и пролиферации эктопических клеток эндометрия. Уровень этого белка существенно повышен в перитонеальной жидкости, сыворотке, эктопированном эндометрии и брюшине у пациентов с эндометриозом по сравнению с женщинами без патологии, а у мышей с отсутствием TGF-β1 наблюдается снижение роста очагов эндометриоза по сравнению с животными дикого типа [43].
Заключение
Таким образом, процессы фолликулогенеза, созревания ооцитов и овуляции контролируются рядом про- и противовоспалительных цитокинов, а также пролиферативными факторами, синтезируемыми эндотелиальными клетками. Нарушение гомеостаза вследствие эндотелиальной дисфункции и изменения количества и/или активности этих биологически активных веществ ассоциировано с патологиями репродуктивной системы: СПКЯ, СГЯ, эндометриозом, рецидивирующими неудачами имплантации. Следует отметить, что индуцированная стимуляция овуляции при лечении бесплодия методами ВРТ также может способствовать нарушению функционирования эндотелия из-за высокой гормональной нагрузки. Это может быть сопряжено с низкой эффективностью терапии.
Поэтому определение роли пролиферативных, про- и противовоспалительных эндотелиальных факторов актуально для практикующих врачей с целью повышения успешности и персонификации лечения женского бесплодия методами ВРТ.



