The role of plasma extracellular vesicles as predictors of gestational diabetes mellitus in the first trimester of pregnancy
Objective: To investigate the composition and concentration of plasma extracellular vesicles (ECVs) in the first trimester of pregnancy, and assess their potential as an early predictor of gestational diabetes mellitus (GDM).Khodzhaeva Z.S., Abramova M.E., Muminova K.M., Gorina K.A., Frolova E.R., Goryunov K.V., Silachev D.N., Shevtsova Yu.A.
Materials and methods: The prospective study enrolled 45 pregnant women aged 24 to 42 years managed at the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. The patients were divided into two groups categorized by pregnancy outcome. Group I (study group) included pregnant women with GDM (n=20), Group II (control group) included pregnant women with normoglycemia (n=25). Group I inclusion criteria were single pregnancy and GDM confirmed by oral glucose tolerance test (OGTT). The inclusion criteria for Group II were singleton pregnancy with normal OGTT results. All patients gave their informed consent to participate in the study. The criteria for not including patients were multiple pregnancies, chromosomal abnormalities, type 1 and 2 diabetes mellitus, autoimmune diseases, cancer, and congenital fetal malformations. Venous blood samples were taken at 11–14 weeks of pregnancy. Extracellular vesicles were isolated from plasma by centrifugation. The linear size and the number of extracellular vesicles were measured by nanoparticle tracking analysis (NTA).
Results: NTA showed that the mean size of the extracellular vesicles was almost identical [92 (85, 103) nm in the study group and 92 (84, 101) nm in the control group]. However, the concentration of ECVs was significantly higher in patients who subsequently developed GDM. The likelihood of developing GDM based on ECV concentration was evaluated using receiver operating characteristics (ROC) analysis. The area under the ROC curve was 0.813±0.080 with 95% CI: 0.657–0.970. The resulting model was statistically significant (p=0.003). The optimal cut-off value of ECV concentration corresponding to the highest value of the Youden’s index was 3.224×1011 parts/ml. The development of GDM was predicted at ECVs concentrations above or equal to this value. The sensitivity and specificity of the model were 80.0% and 66.7%, respectively.
Conclusion: The study findings suggest new possibilities for early prediction of GDM by studying the concentration of extracellular vesicles.
Keywords
Gestational diabetes mellitus (GDM) occurs in almost 13% of pregnant women [1], i.e., more than one in seven pregnancies is affected by gestational diabetes mellitus [2]. GDM remains a significant health and social issue associated with the consequences for maternal and child health. The average prevalence of GDM varies by climatic and geographic region of residence and ranges from 12.9% in the Middle East, approximately 11% in Southeast Asia, the Western Pacific, South, and Central America, to 5.8% in the European region [3]. According to the Federal Diabetes Register, the prevalence of GDM in Russia is 8–9% [4]. GDM prevalence rates show wide variations due to ethnicity and ethnic heterogeneity among the different populations tested, which are further exacerbated by the different screening and diagnostic criteria used [3].
The development of GDM involves numerous molecular genetic factors [5]. GDM is associated with risk for complications during pregnancy [6], and an increased long-term risk of type 2 diabetes mellitus and cardiovascular disease after GDM. The risk of type 2 diabetes mellitus (type 2 DM) is 8 times higher in children exposed to intrauterine hyperglycemia, suggesting that inheritance of acquired characteristics, now called epigenetics, provides a close molecular link in programming the future health of the child [7]. Thus, hyperglycemia during pregnancy contributes to fetal epigenetic changes and is associated with an increased risk of chronic disease in adulthood.
Screening and diagnostic criteria for the diagnosis of GDM remain a topic of debate in the literature [8, 9]. The gold standard is considered the oral glucose tolerance test (OGTT) at 24–28 weeks of gestation, which in some cases leads to a delayed diagnosis of GDM and adverse perinatal outcomes. The development of applied molecular technologies aimed at finding early predictors of diseases, including GDM, contributes not only to the prevention of maternal and fetal complications, but also to the reduction of immediate and long-term risks to their health.
The efficiency of early diagnosis of GDM can be improved by using additional biomarkers, such as extracellular vesicles (ECVs) [10]. ECV is a generic term recognized by the International Society for Extracellular Vesicles (ISEV) and includes a whole family of nanoparticles. ECVs can be classified into exosomes (EXOs), microvesicles (MVs) or ectosomes, apoptotic bodies (ABs), and retrovirus-like particles/microvesicles [11, 12].
ECVs are involved locally at the cell niche level and systemically at the organism level in intercellular communication (cross-signaling information exchange) in the form of large biomolecules such as RNA and enzyme proteins [13, 14]. During pregnancy, the placental syncytiotrophoblast layer secretes small ECVs into the maternal bloodstream as early as 6 weeks of pregnancy [15]. They are secreted by most cells and are found in various body fluids, including blood, urine, breast milk, amniotic fluid, cerebrospinal fluid, semen, ascitic fluid, bile, and saliva. Exosomes carry a wide range of biologically active molecules, including proteins, lipids, and nucleic acids, which can be delivered to other cells to facilitate intercellular interactions [16, 17]. Studies have shown the involvement of circulating exosomes in normal and complicated pregnancies (including GDM) [18]. Liu et al. showed that ECVs derived from plasma of pregnant women with GDM significantly increased the release of inflammatory cytokines from endothelial cells [19], which may be related to the inflammatory process in GDM. In addition, umbilical vein endothelial cells (UVECs) also released ECVs in vitro. A study [20] showed that UVEC ECVs released in normal pregnancy could reverse the phenotype of GDM, whereas ECVs from UVECs in pregnancies complicated by GDM carried factors that induced endothelial cell dysfunction in normal pregnancy. Although the mechanisms underlying maternal metabolic adaptation in healthy pregnancy and in GDM remain poorly understood, ECVs may represent a novel mechanism of maternal glucose homeostasis regulation during pregnancy. For example, ECVs isolated from healthy pregnant women have been found to promote islet glucose-stimulated insulin secretion and peripheral insulin resistance in non-pregnant mice, while ECVs from women with GDM do not stimulate insulin secretion and cause increased insulin resistance [21]. The placenta produces hundreds of microRNAs that are released into the maternal circulation encapsulated in ECVs [22]. Some of these microRNAs are unique to the placenta and are co-regulated in clusters (e.g., cluster chromosome 14 and cluster chromosome 19) depending on gestational age [23].
ECVs are stable containers containing various biomolecules that are protected from degradation by blood enzymes within their structure. Besides, the cytoplasmic membrane of cellular origin protects the contents of the vesicles from degradation by extracellular enzymes [24]. Given the relative stability of the contents of ECVs, they may be the preferred source of biomarkers. We suggest that the efficiency of early detection of GDM can be improved by using additional biomarkers such as ECVs.
The present study aimed to investigate the composition and concentration of plasma extracellular vesicles (ECVs) during the first trimester of pregnancy, and assess their potential as an early predictor of gestational diabetes mellitus.
Materials and methods
This prospective study enrolled 45 pregnant women aged 24 to 42 years managed at the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Based on the extended first-trimester combined screening results, the participants had an uncomplicated obstetric history and a low risk of pregnancy complications. They underwent OGTT with 75 g of glucose at 24–28 weeks of gestation. The diagnosis of GDM was established based on the IADPSG criteria [25]. Based on the test results, the patients were divided into two groups categorized by pregnancy outcome. Group I (study group) included pregnant women with GDM (n=20), Group II (control group) included pregnant women with normoglycemia (n=25). Baseline clinical, anamnestic and laboratory data incuded somatic and obstetric gynecological history, pregnancy course, and fetometric ultrasound measurements.
All participants provided signed informed consent to take part in the study.
Exclusion criteria were multiple pregnancies, chromosomal abnormalities, type 1 and 2 diabetes, autoimmune diseases, cancer, and congenital fetal malformations.
The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P (Ref. 11.11.2019).
ECVs isolation
Blood samples (10 ml) were taken at 11–14 weeks of gestation using EDTA (BD Vacutainer) tubes. The samples were centrifuged at 2000 g for 10 min at +4ºC. The obtained plasma was divided into 3 ml aliquots and placed in cryoprobes for storage at -80°C. The 2.8 ml of thawed plasma was then perfused with PBS (phosphate buffered saline) to 40 ml and centrifuged at 2000 g for 30 min at +4°C. The resulting supernatant was then poured into centrifuge cups and centrifuged at 12000 g for 45 min at + 4°C. The resulting supernatant was centrifuged again at 108000 g for 120 min at + 4°C. The precipitate was dissolved in commercial PBS (Gibco) (100 µl) and stored at -80°C. The Biobank of the V.I. Kulakov NMRC for OG&P participated in the collection and storage of biological materials.
Analysis of nanoparticle trajectory
Particle size distribution and concentrations of isolated vesicles were measured by nanoparticle tracking analysis (NTA) using the Nanosight LM10 HS instrument (NanoSightLtd. Amesbury, UK) as previously described (https://www.mdpi.com/2073-4409/8/3/258). All measurements were carried out according to the recommendations of ASTM E2834-12. Briefly, the samples were diluted with PBS to a final concentration of about 1.5×108 particles/mL. Each Brownian particle motion video was recorded for 1 minute at room temperature with passive temperature readout and the following camera settings (calibration: 166 nm/pixel; blur: automatic; detection threshold: 8, minimum track length: automatic, minimum expected particle size: 30 nm, temperature: 24.7°C, viscosity: 0.90 Pa*s) optimized for ECV (12–18 videos per sample). Videos were processed using Nanoparticle Tracking Analysis software version 2.3 build 0033 (NanoSight Ltd., Amesbury, UK).
Statistical analysis
Statistical analysis was performed using StatTech v. 2.4.7 (Stattech Ltd., Russia). The normality of the distribution was tested by the Shapiro–Wilk test. Quantitative variables showing normal distribution were expressed as means (M), standard deviation (SD), and 95% confidence interval (95% CI) and presented as M (SD); otherwise the median (Me) with interquartile range (Q1; Q3) was reported. Between-group differences in continuous variables showing normal distribution and equality of variance were assessed by Student’s t-test. Comparison of rates was performed using the Fisher's exact test (with expected counts less than 10) for 2×2 contingency tables. Analysis of diagnostic accuracy was based on the receiver operating characteristic (ROC). The optimal cut point was determined by the highest value of Youden's index. Differences between groups were considered statistically significant at p<0.05 for all analyzes.
Results
Clinical and anamnestic characteristics of the study participants
As mentioned above, the patients were divided into 2 groups (with GDM and normal glucose tolerance). There were no statistically significant differences in age, gestational age at the time of inclusion in the study, body mass, height, and BMI. All women were nonsmokers, had singleton pregnancies, and had no infectious-inflammatory and other obstetric complications except GDM (group I). Clinical characteristics of the patients are presented in Table 1.
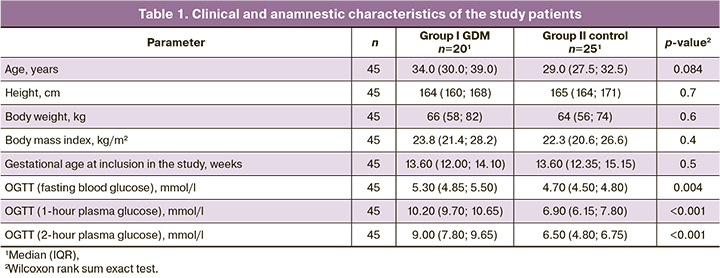
Characterization and quantification of ECVs
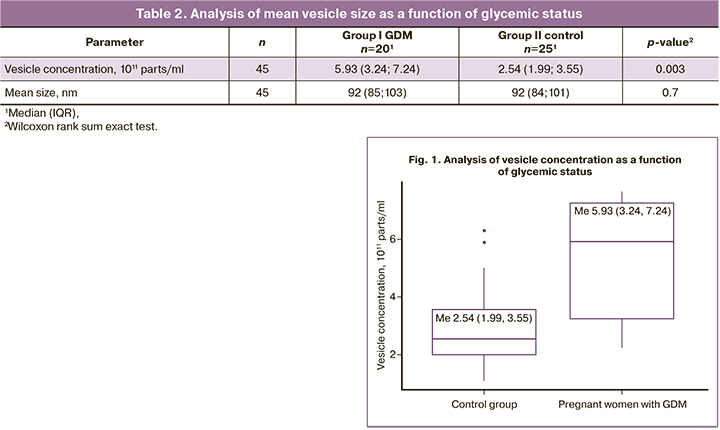
To evaluate differences in ECVs obtained from maternal plasma in the first trimester between study groups (GDM and comparison group), the size distribution of ECVs obtained by centrifugation was analyzed. NTA analysis did not show significant differences in mean vesicle sizes between the groups [92 (84, 101) nm for controls and 92 (85, 103) for patients with GDM (Table 2)]. However, ECV concentrations were significantly higher in patients who subsequently developed GDM compared with normoglycemic pregnant women (Table 2, Figs. 1, 2).
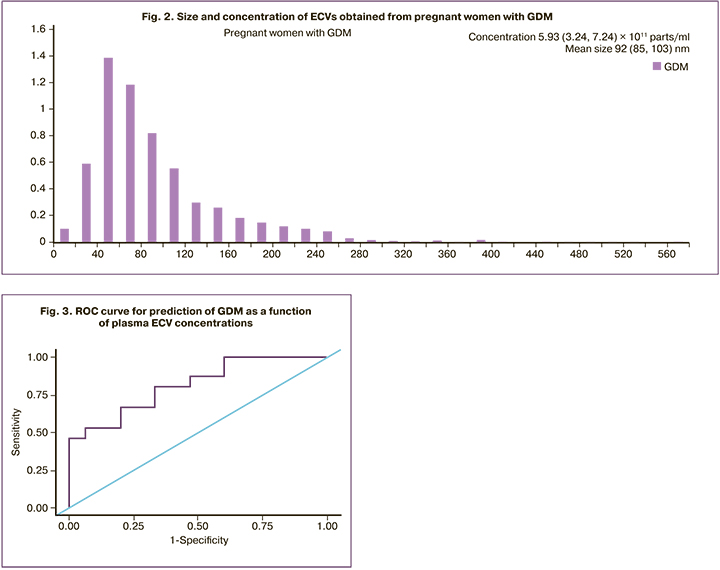
The diagnostic accuracy of the ECV concentration to predict GDM was evaluated by receiving operating curve (ROC) analysis (Fig. 3). The area under the ROC curve was 0.813±0.080 with 95% CI: 0.657–0.970. The resulting model was statistically significant (p=0.003). The optimal cut point determined by the highest value of Youden's index was 3.224×1011 parts/ml. The development of GDM was predicted at a concentration of ECVs greater than or equal to this value. The sensitivity and specificity of the model were 80.0 and 66.7%, respectively.
Discussion
Our study aimed to elucidate the ability of ECVs in predicting GDM. The data showed that women who developed GDM at 11–14 weeks of gestation had significantly higher concentrations of ECV than normoglycemic women.
It should be noted that we, like most researchers, were guided in our work by the recommendations developed by ISEV [26]. There is often confusion in the literature regarding nomenclature in reporting extracellular vesicle research. According to ISEV guidelines, exosomes and ECVs should not be equalized [12]. In turn, it is worth noting that exosomes, being the smallest nanoparticles among ECVs (30–100 nm) generated along the classical endosome-multivisceral body (MVB) pathway, are also formed as a rather heterogeneous population. H. Zhang, et al. described a separate population of non-membranous exosomes called exomers (≈35 nm) [27]. Moreover, a team of other authors later showed that the subpopulation of exosomes called exomers differs from "classical" exosomes in their proteomic composition and includes proteins of response to hypoxia, microtubules, coagulation participants and a number of others, which also functionally distinguishes them from "classical" exosomes [28]. ECVs that are larger than exosomes are mostly derived from the plasma membrane and reach sizes ranging from hundreds of nm to several μm. Among them, the most studied are apoptotic corpuscles [29] and ectosomes/microvesicles [30].
There is a common belief that most of the available extraction kits isolate exactly exosomes, which allows a faster focus on the smallest population of ECVs compared to other populations. However, the process of biogenesis and release of large ECVs (microvesicles and apoptotic bodies) derived from the plasma membrane involves both mechanical and biochemical processes. In this regard, the biogenesis of large ECVs, microvesicles in particular, is very sensitive to microenvironmental conditions. This factor explains, at least in part, the considerable variability in the data obtained on both the biogenesis and the functioning of large ECVs. The exception here is apoptotic corpuscles associated with programmed cell death [31].
Based on the above-mentioned data, in this study we used the term ECVs, thereby generalizing and postulating the fact that we obtain a mixed population of ECVs consisting mainly of exosomes with an admixture of microvesicles using differential centrifugation without a gradient, as evidenced by the histogram of nanoparticle size distribution by nanoparticle trajectory analysis (NTA) (Figure 2).
We chose the method of differential centrifugation not accidentally, but because of its high productivity, relative ease of execution and effective integration into the clinic [32]. In fact, the literature suggests that high-speed centrifugation for more than 4 h significantly reduces the purity of ECVs and leads to their mechanical damage [33], but we use shorter centrifugation times and perform all operations according to the protocol developed to obtain ECVs from blood plasma, adapted to our technical capabilities [34]. Visual NTA data and electronic micrographs suggest that we obtain a homogeneous population of ECVs. Regarding the stability of ECVs and the storage conditions of biological fluids, we were guided by ISEV-approved literature data. In particular, it has recently been shown that the most optimal condition for long-term storage of the obtained ECVs is -80°C [35] with the avoidance of freezing/thawing cycles.
Our data are consistent with the results of nanoparticle trajectory analysis obtained in a similar way by ultracentrifugation [36]. Furthermore, a study by Salomon et al. showed that pregnant women who develop GDM show elevated concentrations of total and placental ECV compared to normoglycemic pregnant women [34]. This increase in circulating ECVs associated with GDM continues throughout pregnancy, with the number of circulating ECVs increasing approximately 2-fold during pregnancy compared with healthy pregnant women [37].
Importantly, ECVs can be isolated from various biological fluids (plasma, urine, and saliva) using various techniques, including ultracentifugation. They are very stable components of the cell secretome and can protect their biological contents from degradation. Between 10 and 14 weeks of gestation, the characteristics of ECV distribution and their total number can be used to identify asymptomatic women who are diagnosed with GDM at a later gestational age. This allows early treatment and prevention of not only the specific antepartum and perinatal complications, but also the transformation of GDM into other forms of diabetes after delivery for the pregnant woman and for the fetus to alleviate the burden of perinatal programming associated with intrauterine hyperglycemia.
However, despite the optimistic and promising results, further research is needed. Prediction of GDM in early pregnancy based on the number of ECVs is not highly specific, since ECVs are an important component of the secretome of various cells, providing an integrated transfer of biologically active molecules and horizontal transfer of genetic information. Therefore, the study of the ECV content, in particular, the analysis of the microRNA profile, seems very promising. There is evidence that ECVs with increased content of certain microRNAs can be used as biomarkers for diagnostics of various diseases [38]. Moreover, the GDM-specific microRNA profile analysis that we presently perform will allow for predicting the disease development and serving as a monitoring tool during pregnancy, including the treatment effectiveness.
Conclusion
GDM has become a global public health problem and its early diagnosis remains a challenge because none of the existing biomarkers has high specificity. Future, more extensive studies are needed to assess the prognostic value of ECV. We isolated ECVs from plasma using a method that can be used in a clinical setting. Our results provide insight into the potential of ECVs as early biomarkers to predict GDM in the first trimester of pregnancy. Further study of the profile of specific microRNAs will determine the role of ECVs in the early prediction of GDM.
References
- Melchior H., Kurch-Bek D., Mund M. The prevalence of gestational diabetes. Dtsch. Arztebl. Int. 2017; 114(24): 412-8. https://dx.doi.org/10.3238/arztebl.2017.0412.
- Hod M., Kapur A., Sacks D.A., Hadar E., Agarwal M., Di Renzo G.C. et al. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: A pragmatic guide for diagnosis, management, and care. Int. J. Gynaecol. Obstet. 2015; 131(Suppl. 3): S173-211. https://dx.doi.org/10.1016/S0020-7292(15)30033-3.
- Zhu Y., Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: A global perspective. Curr. Diab. Rep. 2016; 16(1): 7. https://dx.doi.org/10.1007/s11892-015-0699-x.
- Дедов И.И., Шестакова М.В., Викулова О.К., Железнякова А.В., Исаков М.А. Сахарный диабет в Российской Федерации: распространенность, заболеваемость, смертность, параметры углеводного обмена и структура сахароснижающей терапии по данным Федерального регистра сахарного диабета, статус 2017 г. Сахарный диабет. 2018; 21(3): 144-59. [Dedov I.I., Shestakova M.V., Vikulova O.K., Zheleznyakova A.V., Isakov M.A. Diabetes mellitus in Russian Federation: prevalence, morbidity, mortality, parameters of glycaemic control and structure of glucose lowering therapy according to the Federal Diabetes Register, Status 2017. Diabetes mellitus. 2018; 21(3): 144-59.(in Russian)]. https://dx.doi.org/10.14341/DM9686.
- Ходжаева З.С., Снеткова Н.В., Клименченко Н.И., Абрамова М.Е., Дегтярева Е.И., Донников А.Е. Клинико-молекулярно-генетические детерминанты формирования гестационного сахарного диабета. Акушерство и гинекология. 2019; 4: 18-26. [Khodzhaeva Z.S., Snetkova N.V., Klimenchenko N.I., Abramova M.E., Degtyareva E.I., Donnikov A.E. Clinical and molecular genetic determinants of the development of gestational diabetes mellitus. Obstetrics and Gynecology. 2019; 4: 18-26. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.4.18-24.
- Ходжаева З.С., Снеткова Н.В., Муминова К.Т., Горина К.А., Абрамова М.Е, Есаян Р.М. Особенности течения беременности у женщин с гестационным сахарным диабетом. Акушерство и гинекология. 2020; 7: 47-52. [Khodzhaeva Z.S., Snetkova N.V., Muminova K.T., Gorina K.A., Abramova M.E., Esayan R.M. Clinical characteristics of pregnancy in women with gestational diabetes mellitus. Obstetrics and Gynecology. 2020; 7: 47-52. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.7.47-52.
- Mirghani Dirar A., Doupis J. Gestational diabetes from A to Z. World J. Diabetes. 2017; 8(12): 489-511. https://dx.doi.org/10.4239/wjd.v8.i12.489.
- Brink H.S., van der Lely A.J., van der Linden J. The potential role of biomarkers in predicting gestational diabetes. Endocr. Connect. 2016; 5(5): R26-34. https://dx.doi.org/10.1530/EC-16-0033.
- Абрамова М.Е., Ходжаева З.С., Горина К.А., Муминова К.Т., Горюнов К.В., Рагозин А.К., Силачев Д.Н. Гестационный сахарный диабет: скрининг и диагностические критерии в ранние сроки беременности. Акушерство и гинекология. 2021; 5: 25-32. [Abramova M.E., Khodzhaeva Z.S., Gorina K.A., Muminova K.T., Goryunov K.V., Ragozin A.K., Silachev D.N. Gestational diabetes mellitus: screening and diagnostic criteria in early pregnancy. Obstetrics and Gynecology. 2021; 5: 25-32. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.5.25-32.
- van der Pol E., Böing A., Harrison P., Sturk A., Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 2012; 64(3): 676-705. https://dx.doi.org/10.1124/pr.112.005983.
- Akers J., Gonda D., Kim R., Carter B., Chen C. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neurooncol. 2013; 113(1): 1-11. https://dx.doi.org/10.1007/s11060-013-1084-8.
- Witwer K.W., Théry C. Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. J. Extracell. Vesicles. 2019; 8(1): 1648167. https://dx.doi.org/10.1080/20013078.2019.1648167.
- Mir B., Goettsch C. Extracellular vesicles as delivery vehicles of specific cellular cargo. Cells. 2020; 9(7): 1601. https://dx.doi.org/10.3390/cells9071601.
- Colombo M., Raposo G., Théry C. Biogenesis, secretion and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014; 30: 255-89. https://dx.doi.org/10.1146/annurev-cellbio-101512-122326.
- Sarker S., Scholz-Romero K., Perez A., Illanes S.E., Mitchell M.D., Rice G.E., Salomon C. Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy. J. Transl. Med. 2014; 12: 204. https://dx.doi.org/10.1186/1479-5876-12-204.
- Truong G., Guanzon D., Kinhal V., Elfeky O., Lai A., Longo S. et al. Oxygen tension regulates the miRNA profile and bioactivity of exosomes released from extravillous trophoblast cells - Liquid biopsies for monitoring complications of pregnancy. PLoS One. 2017; 12(3): e0174514. https://dx.doi.org/10.1371/journal.pone.0174514.
- Jayabalan N., Lai A., Nair S., Guanzon D., Scholz-Romero K., Palma C. et al. Quantitative proteomics by SWATH-MS suggest an association between circulating exosomes and maternal metabolic changes in gestational diabetes mellitus. Proteomics. 2019; 19(1-2): e1800164. https://dx.doi.org/10.1002/pmic.201800164.
- Salomon C., Yee S., Mitchell M., Rice G. The possible role of extravillous trophoblast-derived exosomes on the uterine spiral arterial remodeling under both normal and pathological conditions. Biomed. Res. Int. 2014; 2014: 693157. https://dx.doi.org/10.1155/2014/693157.
- Liu J., Wang S.Z., Wang Q.L., Du J.G., Wang B.B. Gestational diabetes mellitus is associated with changes in the concentration and Bioactivity of Placental Exosomes in the Maternal Circulation across gestation. Eur. Rev. Med. Pharmacol. Sci. 2018; 22: 2036-43. https://dx.doi.org/10.26355/eurrev_201804_14733.
- Sáez T., Salsoso R., Leiva A., Toledo F., deVos P., Faas M. et al. Human umbilical vein endothelium-derived exosomes play a role in foetoplacental endothelial dysfunction in gestational diabetes mellitus. Biochim. Biophys. Acta. Mol. Basis Dis. 2018; 1864(2): 499-508. https://dx.doi.org/10.1016/j.bbadis.2017.11.010.
- James-Allan L.B., Rosario F.J., Barner K., Lai A., Guanzon D., McIntyre H.D. et al. Regulation of glucose homeostasis by small extracellular vesicles in normal pregnancy and in gestational diabetes. FASEB J. 2020; 34(4): 5724-39. https://dx.doi.org/10.1096/fj.201902522RR.
- Poirier C., Desgagné V., Guérin R., Bouchard L. MicroRNAs in pregnancy and gestational diabetes mellitus: emerging role in maternal metabolic regulation. Curr. Diab. Rep. 2017; 17(5): 35. https://dx.doi.org/10.1007/s11892-017-0856-5.
- Salomon C., Torres M.J., Kobayashi M., Scholz-Romero K., Sobrevia L., Dobierzewska A. et al. A gestational profile of placental exosomes in maternal plasma and their effects on endothelial cell migration. PLoS One. 2014; 9(6): e98667. https://dx.doi.org/10.1371/journal.pone.0098667.
- Lai R.C., Yeo R.W.Y., Tan K.H., Lim S.K. Exosomes for drug delivery – a novel application for the mesenchymal stem cell. Biotechnol. Adv. 2013; 31(5):543-51. https://dx.doi.org/10.1016/j.biotechadv.2012.08.008.
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010; 33(3): 676-82. https://dx.doi.org/10.2337/dc09-1848.
- Thery C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018; 7(1): 1535750. https://dx.doi.org/10.1080/20013078.2018.1535750.
- Zhang H., Freitas D., Kim H.S., Fabijanic K., Li Z., Chen H. et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 2018; 20(3): 332-43. https://dx.doi.org/10.1038/s41556-018-0040-4.
- Lee S.S., Won J.H., Lim G.J., Han J., Lee J.Y., Cho K.O., Bae Y.K. A novel population of extracellular vesicles smaller than exosomes promotes cell proliferation. Cell Commun. Signal. 2019; 17(1): 95. https://dx.doi.org/10.1186/s12964-019-0401-z.
- Tixeira R., Caruso S., Paone S., Baxter A.A., Atkin-Smith G.K., Hulett M.D., Poon I.K. Defining the morphologic features and products of cell disassembly during apoptosis. Apoptosis. 2017; 22(3): 475-7. https://dx.doi.org/10.1007/s10495-017-1345-7.
- Surman M., Hoja-Łukowicz D., Szwed S., Kędracka-Krok S., Jankowska U., Kurtyka M. et al. An insight into the proteome of uveal melanoma-derived ectosomes reveals the presence of potentially useful biomarkers. Int. J. Mol. Sci. 2019; 20(15): 3789. https://dx.doi.org/10.3390/ijms20153789.
- Ciardiello C., Migliorino R., Leone A., Budillon A. Large extracellular vesicles: Size matters in tumor progression. Cytokine Growth Factor Rev. 2020; 51: 69-74. https://dx.doi.org/10.1016/j.cytogfr.2019.12.007.
- Zhang M., Jin K., Gao L., Zhang Z., Li F., Zhou F., Zhang L. Methods and technologies for exosome isolationand characterization. Small Methods. 2018; 2: 1800021. https://dx.doi.org/10.1002/SMTD.201800021.
- Zaborowski M.P., Balaj L., Breakefield X.O., Lai C.P. Extracellular vesicles: composition, biological relevance, and methods of study. Bioscience. 2015; 65(8): 783-97. https://dx.doi.org/10.1093/biosci/biv084.
- Salomon C., Scholz-Romero K., Sarker S., Sweeney E., Kobayashi M., Correa P. et al. Gestational diabetes mellitus is associated with changes in the concentration and bioactivity ofplacenta-derived exosomes in maternal circulation across gestation. Diabetes. 2016; 65(3): 598-609.
- Yuan F., Li Y.M., Wang Z. Preserving extracellular vesicles for biomedical applications: consideration of storage stability before and after isolation. Drug Deliv. 2021; 28(1):1501-9. https://dx.doi.org/10.1080/10717544.2021.1951896.
- Arias M., Monteiro L.J., Acuña-Gallardo S., Varas-Godoy M., Rice G.E., Monckeberg M. et al. Extracellular vesicle concentration in maternal plasma as an early marker of gestational diabetes. Rev. Med. Chil. 2019; 147(12): 1503-9. https://dx.doi.org/10.4067/S0034-98872019001201503.
- Nakahara A., Elfeky O., Garvey C., Guanzon D., Longo S.A., Salmon C. Exosome profiles for normal and complicated pregnancies – a longitudinal study. Obstet. Gynecol. 2019; 133: 162. https://dx.doi.org/10.1097/01.AOG.0000558864.31601.aa.
- Kuwabara Y., Ono K., Horie T., Nishi H., Nagao K., Kinoshita M., Watanabe S. Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circ. Cardiovasc. Genet. 2011; 4(4): 446-54. https://dx.doi.org/10.1161/CIRCGENETICS.110.958975.
Received 25.02.2022
Accepted 28.03.2022
About the Authors
Zulfiya S. Khodzhaeva, Dr. Med. Sci., Professor, Deputy Director of Obstetrics Institute, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,+7(916)407-75-67, zkhodjaeva@mail.ru, https://orcid.org/0000-0001-8159-3714,
117997, Russia, Moscow, Akademika Oparina str., 4.
Maria E. Abramova, graduate student at the High Risk Pregnancy Department, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(917)577-12-77, m_abramova@oparina4.ru, https://orcid.org/0000-0001-8689-4700, 117997, Russia, Moscow, Akademika Oparina str., 4.
Kamilla T. Muminova, PhD, Researcher at the High Risk Pregnancy Department, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(916)373-77-07, k_muminova@oparina4.ru, https://orcid.org/0000-0003-2708-4366, 117997, Russia, Moscow, Akademika Oparina str., 4.
Kseniia A. Gorina, PhD, Researcher at the High Risk Pregnancy Department, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(926)649-77-32, k_gorina@oparina4.ru, https://orcid.org/0000-0001-6266-2067, 117997, Russia, Moscow, Akademika Oparina str., 4.
Ekaterina R. Frolova, graduate student at the High Risk Pregnancy Department, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(985)473-85-47, kattirella@gmail.com, https://orcid.org 0000-0003-2817-3504, 117997, Russia, Moscow, Akademika Oparina str., 4.
Kirill V. Goryunov, PhD, Researcher at the Department of Cell Technologies, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(916)410-88-44, k_gorunov@oparina4.ru, https:// orcid.org 0000-0002-8776-7196, 117997, Russia, Moscow, Akademika Oparina str., 4.
Denis N. Silachev, PhD (Bio), Head of the Department of Cell Technologies, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(905)792-13-01, d_silachev@oparina4.ru, https://orcid.org 0000-0003-0581-9755, 117997, Russia, Moscow, Akademika Oparina str., 4.
Yulia A. Shevtsova, Junior Researcher at the Department of Cell Technologies, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(925)673-24-27, yu_shevtsova@oparina4.ru, 117997, Russia, Moscow, Akademika Oparina str., 4.
Authors’ contributions: Khodzhaeva Z.S., Abramova M.E., Muminova K.T., Gorina K.A., Frolova E.R. – conception and design of the study, data collection and analysis, review and translation of the relevant literature, statistical analysis, manuscript preparation; Goryunov K.V., Silachev D.N., Shevtsova Yu.A. – design of the study, laboratory data analysis; Khodzhaeva Z.S. – conception and design of the study, data analysis, and manuscript editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P (Ref. 11.11.2019).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Khodzhaeva Z.S., Abramova M.E., Muminova K.M., Gorina K.A., Frolova E.R., Goryunov K.V., Silachev D.N., Shevtsova Yu.A. The role of plasma extracellular vesicles as predictors of gestational diabetes mellitus in the first trimester of pregnancy.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 4: 76-83 (in Russian)
https://dx.doi.org/10.18565/aig.2022.4.76-83



