Damage-associated molecular patterns in patients with intramural uterine fibroids and infertility
Dubinskaya E.D., Kolesnikova S.N., Gasparov A.S., Alyoshkina E.V.
Objective: To investigate the levels of damage-associated molecular patterns (DAMPs) in fertile and infertile patients with uterine fibroids (UF).
Materials and methods: This single-center prospective study included 90 women. The main group comprised 60 patients with intramural UF, with 30 infertile (study group) and 30 fertile patients (comparison group). The control group consisted of 30 healthy women without uterine fibroids. The serum levels of DAMPs, including tumor necrosis factor alpha (TNF-α), protein S100, interleukin-10, glutathione, uric acid, low-density lipoprotein cholesterol (LDL-C), and fibrinogen, were measured.
Results: The data obtained indicated a significant increase in glutathione, uric acid, and LDL-C in the presence of UF, regardless of fertility, compared to the control group. More pronounced changes in parameters (increased protein levels of S100, uric acid, and LDL-C, as well as decreased glutathione levels) were observed in the presence of intramural UF and infertility compared to fertile and healthy patients. The proposed DAMPs markers (glutathione, LDL-C, S100 protein, and uric acid) can be used as diagnostic and prognostic markers of infertility in the presence of intramural UF. When threshold values are reached (glutathione less than 413 µmol/l; S100 protein more than 0.172 µg/l; uric acid more than 280.5 µmol/l; LDL-C more than 3.78 mmol/l), the relative risk of infertility increases.
Conclusion: This study significantly enhances our understanding of the pathogenesis of infertility in UF and confirms the presence of systemic metabolic disorders (oxidative-inflammatory stress) as a cofactor of infertility.
Authors' contributions: Dubinskaya E.D., Kolesnikova S.N., Gasparov A.S. – conception and design of the study, editing of the manuscript; Kolesnikova S.N., Alyoshkina E.V. – material collection and processing, drafting of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the Patrice Lumumba Peoples' Friendship University of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Dubinskaya E.D., Kolesnikova S.N., Gasparov A.S., Alyoshkina E.V. Damage-associated molecular patterns in patients with intramural uterine fibroids and infertility.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (5): 108-117 (in Russian)
https://dx.doi.org/10.18565/aig.2024.48
Keywords
Today, it is widely accepted that inflammation plays a significant role in the development of various proliferative gynecological diseases, such as adenomyosis, uterine fibroids (UF), and endometriosis-associated infertility [1, 2].
Numerous studies have provided convincing evidence that chronic active immune inflammation is closely associated with UF formation [3, 4]. When the uterus is exposed to insults, such as intrauterine infections, intrauterine interventions, and menstruation, a chronic inflammatory immune profile can lead to immune system activation, resulting in proliferation and fibrosis that contribute to the development of fibroids [5, 6]. Studies have also reported elevated levels of proinflammatory mediators, cytokines, and profibrotic substances in the UF [1, 7].
In recent years, there has been increased focus on the role of inflammation in reproductive medicine. Inflammation plays a crucial role in various reproductive processes, including ovulation, menstruation, placentation, and pregnancy [8]. Dysregulation of inflammation in terms of its degree or duration is directly linked to the pathophysiology of infertility. Chronic low-grade systemic inflammation is believed to be associated with numerous diseases related to infertility, such as polycystic ovarian syndrome, endometriosis-associated infertility, and primary ovarian failure [9–11].
The exact mechanisms by which chronic aseptic inflammation affects fertility are not fully understood. Some hypotheses suggest that it may affect folliculogenesis through oxidative stress [12] or affect endometrial receptivity [13].
Chronic aseptic inflammation is characterized by accumulation and elevated levels of endogenous molecules released from damaged or dead cells, known as damage-associated molecular patterns (DAMPs). These DAMPs can serve as valuable biomarkers for assessing inflammation severity. Examples of DAMPs include extracellular proteins such as biglycan and tenascin C, as well as intracellular proteins like high mobility group box 1 (HMGB1), histones, S100 proteins, heat shock proteins (HSPs), and plasma proteins such as fibrinogen, Gc-globulin, and serum amyloid A [14].
Considering the existing literature, we found it interesting to investigate the systemic levels of DAMPs (tumor necrosis factor alpha (TNF-α), S100 protein, interleukin-10, glutathione, uric acid, cholesterol-low-density lipoprotein (LDL-C), and fibrinogen) as markers of systemic aseptic inflammation in fertile women with UF, patients with UF and infertility, and a control group of healthy women.
This study aimed to examine damage-associated molecular patterns in both fertile and infertile patients with uterine fibroids.
Materials and methods
In accordance with the objective, the study included 90 patients of reproductive age with UF (FIGO types 3–6).
This single-center prospective study included 90 women. During the study, patients were divided into two groups. The main group comprised 60 patients with intramural UF, with 30 infertile (study group) and 30 fertile patients (comparison group). The control group was comprised of 30 healthy women without fibroids.
Group designations: UF+B – study group, with infertility; UF-B – comparison group, without infertility.
The criteria for inclusion in the study group were age 20-35 years, UF (FIGO types 3–6), absence of pregnancy and more than 1 year of regular sexual activity; regular menstrual cycle; no hormonal medication in the last 6 months; history of failed in vitro fertilization attempts; normal ovarian reserve (anti-Müllerian hormone (AMH) level³1.2 ng/ml); no history of surgery for UF; informed consent to participate in the study.
Inclusion criteria for the comparison group were age 20–35 years; UF (FIGO types 3–6); regular menstrual cycle, women seeking routine and clinical monitoring and choice of contraception within 12 months of delivery, diagnosed with UF during pregnancy and/or before pregnancy, and informed consent to participate in the study.
The exclusion criteria from the study group were as follows: submucous UF (FIGO type 0–2) and subserous UF (FIGO type 7), large fibroids and uterus (more than 12 weeks of gestation) [15]; acute pelvic inflammatory disease, female infertility of tubal origin verified at the time of the study, female infertility of cervical origin, female infertility associated with male factors, female infertility associated with lack of ovulation, low ovarian reserve (AMH≤1.2 ng/ml), ovarian tumors, history of surgical treatment for UF, common forms of endometriosis confirmed by laparoscopy, oncological diseases, hyperprolactinemia associated with macro- or microadenoma of the pituitary gland (according to MRI), and underweight (body mass index <19.9 kg/m2).
The exclusion criteria for the comparison group were submucous UF (FIGO type 0–2), subserous UF (FIGO type 7), large fibroids, and uterus (more than 12 weeks of pregnancy) [15].
The inclusion criterion for the control group was the absence of UF on physical and ultrasound examination; this group included women seeking routine annual examination and choice of contraception.
The location of fibroids was determined using the FIGO classification [16], and the characteristics of the influence of large and small nodules on fertility (according to the clinical recommendations of the Ministry of Health of the Russian Federation for UF from 2020, nodules >12 weeks of pregnancy should be considered large [15]. Ultrasound diagnosis was performed using a transvaginal volumetric sensor on a Voluson E 10 Expert device. This study included an analysis of the sociodemographic and clinical anamnestic data of the patients in the study groups.
Blood samples were taken from all patients in the 1st phase of the menstrual cycle, after the end of bleeding, to exclude any bias in the results due to physiological inflammatory changes in the reproductive system (menstruation, ovulation, and secretory phase of the cycle). No acute inflammatory diseases or exacerbations of chronic diseases were present at the time of blood sampling. The levels of serum DAMPs (tumor necrosis factor alpha (TNF-α), protein S100, interleukin-10, glutathione, uric acid, low-density lipoprotein (LDL-C), and fibrinogen) were determined. The material for the study were samples of venous blood serum obtained by centrifugation for 20 minutes at a rotation speed of 3500 rpm (Elmi CM-6MT.02 laboratory centrifuge (Latvia)).
The study of TNF-α and Intelekin-10 was performed using enzyme-linked immunosorbent assay. The level of reduced glutathione was determined using high-performance liquid chromatography. This method of laboratory diagnostics makes it possible to detect free glutathione in a sample of biological material and accurately calculate its quantitative content. A chemiluminescence immunoassay was used to detect S100 protein. The enzymatic method was used to detect uric acid, coagulation method was used to detect fibrinogen, and colorimetric method was used to determine LDL-C levels.
Statistical analysis
Statistical analysis was performed using SPSS (version 10.0.7) and Statistica (version 10.0); some calculations were carried out using Excel tables. Differences between the groups were considered statistically significant at p<0.05.
The distribution of continuous variables was tested for normality using the Kolmogorov–Smirnov test. Continuous variables showing normal distribution were expressed as mean (M) and standard deviation (SD) and presented as M (SD); otherwise, the median (Me) with interquartile range (Q1; Q3) was reported. Frequencies and percentages were reported for categorical variables
Statistical significance of the differences in normally distributed continuous variables was assessed using one-way ANOVA, followed by pairwise comparison of groups using Student’s t-test. Levene's test of equality of variances was used to assess the assumption of the homogeneity of variance. Variables not meeting normality assumptions were compared using nonparametric Kruskal–Wallis and Mann–Whitney U tests. For multiple comparisons, Bonferroni correction was used for the three independent groups (p<0.017).
Categorical data were compared using the chi-squared test (χ2) with maximum likelihood correction, two-sided Fisher's exact test, or Z-test with correction for end points (in the case of comparisons of 0% or 100%), which allowed us to determine the independence of the distribution of the indicators in the groups. In the analysis of factors affecting female fertility and the presence of UF, the relative risk with 95% confidence interval (95% CI) was calculated. The relationship between the binary and quantitative characteristics was evaluated by calculating the biserial correlation coefficient.
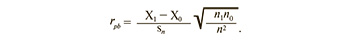
Results
The mean age of the patients was 31.8 (3.7) years in the study group, 31.4 (3.9) years in the comparison group and 32.3 (3.6) years in the control group (p>0.05).
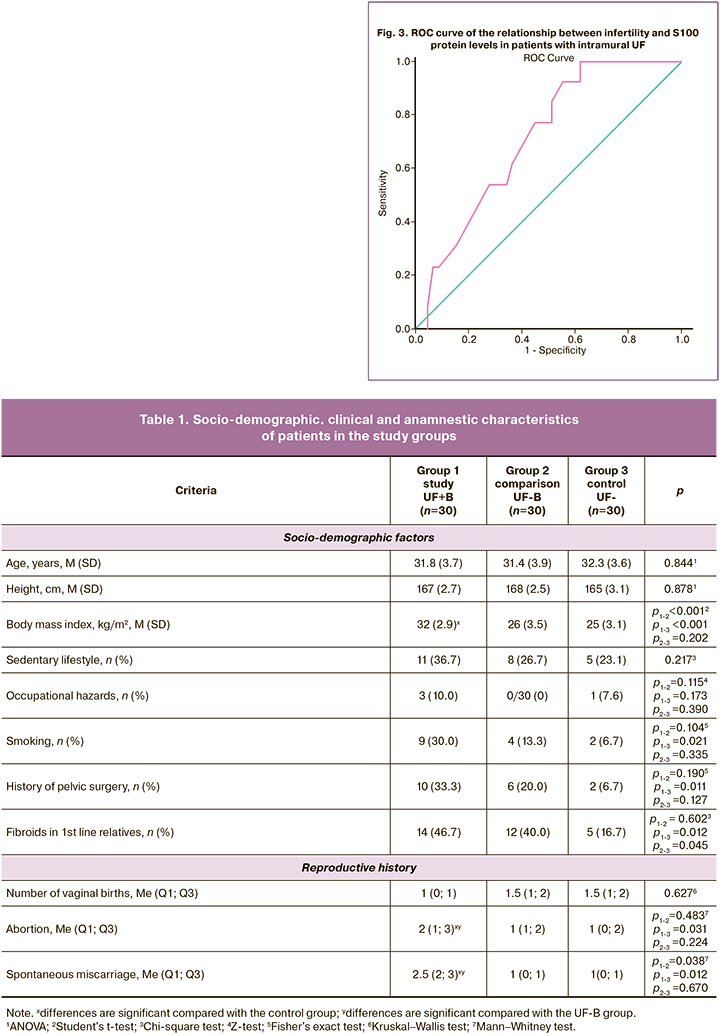
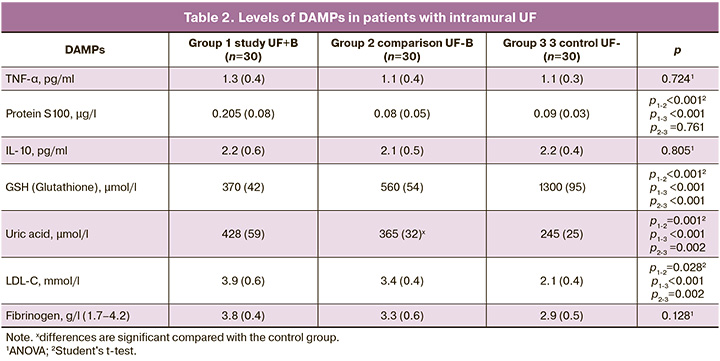
As follows from the data presented (Table 1), in patients with UF and infertility, the history of pelvic surgery, abortions and spontaneous miscarriages was statistically significantly more common than in the group of fertile patients with UF and the control group.
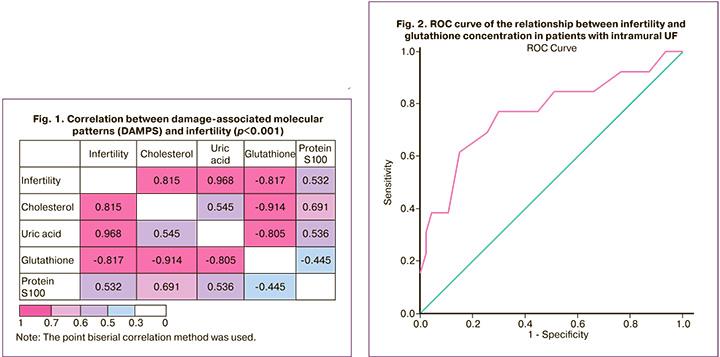
The results of the assessment of damage-associated molecular patterns (DAMPs) in the study groups are presented in Table 2.
The data obtained indicated a statistically significant increase in glutathione, UA, and LDL-C in the presence of UF, regardless of fertility, compared to the control group. More pronounced changes in parameters (increased protein levels of S100, UA, and LDL-C, as well as decreased glutathione levels) were detected in the presence of intramural UF and infertility than in fertile and healthy patients. No statistically significant changes in fibrinogen, TNF-α and interleukin-10 levels were observed at the systemic level.
In the next step of the study, a correlation analysis was performed using the point biserial correlation method, which showed a strong positive correlation between infertility (a categorical variable where 1=infertility, 0=no infertility) and LDL-C (R=0.832, p<0.001), UA (R=0.985, p<0.001), S100 protein (R=0.739, p<0.001), and a strong negative correlation with glutathione (R=-0.821, p<0.001).
The ROC analysis (AUC=0.768±0.083, p=0.003) determined the GSH (glutathione) value of 413.0 µmol/l as the threshold value below which the risk of infertility increases 2.56 times (relative risk (RR] = 2.56, 95% CI 1.43–4.57, p< 0.001). The sensitivity of this factor as a predictor of the development of infertility in women with intramural UF was 76.67% (95% CI 59.07–88.21%), specificity – 70.0% (95% CI 59.07–88.21%), and overall accuracy of 73.33% (95% CI 58.79–84.13%) (Fig. 2).
Using ROC analysis (AUC=0.714±0.070, p=0.019), the S100 protein value of 0.172 μg/L was determined as the threshold value above which the risk of infertility increases 3 times (RR=3, 0.95% CI 1.51–5.98, p<0.001). The sensitivity of this factor as a predictor of the development of infertility in women with intramural UF was 70.0% (95% CI 52.12–83.34%), specificity was 76.67% (95% CI 59.07–88.21%), and overall accuracy was 73.33% (95% CI 58.79–84.13%) (Fig. 3).
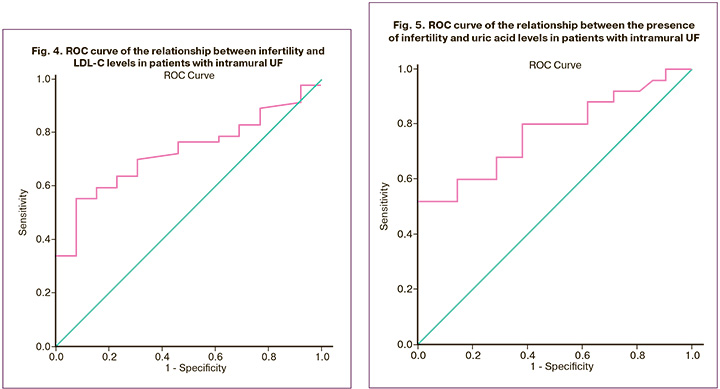
Using ROC analysis (AUC=0.730±0.067, p=0.012) for LDL-C, a threshold value of 3.78 mmol/l was determined, above which the risk of infertility increases by 2.86 times (RR=2, 86, 95% CI 1.42–5.73, p<0.001). The sensitivity of this factor as a predictor of infertility development was 66.67% (95% CI 48.78–80.77%), specificity was 76.67% (95% CI 59.07–88.21%), and overall accuracy was 71.57% (95% CI 58.86–82.92%) (Fig. 4).
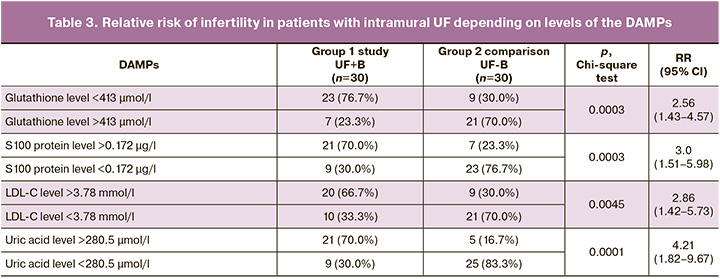
A uric acid level of 280.5 μmol/L obtained using ROC analysis (AUC = 0.772 ± 0.069, p=0.002) can be considered a risk factor for infertility in patients with intramural UF. The uric acid value was determined to be 280.5 µmol/l, above which the risk of infertility increases 4.2 times (RR=4.20, 95% CI 1.82–9.67, p<0.001). The sensitivity of this factor as a predictor of the development of infertility in women with UF was 70.0% (95% CI 52.12–83.34%), specificity – 83.33% (95% CI 66.44–92.66%), and overall accuracy – 76.67% (95% CI 62.69–86.53%) (Fig. 5).
The relative risk of infertility in patients with intramural UF, depending on the content of the DAMPs, is presented in Table 3.
Thus, the study revealed a number of indicators, including DAMPs markers (glutathione, LDL-C, S100 protein, UA), which can be used as diagnostic and prognostic markers of infertility in the presence of intramural UF.
Discussion
This study clearly established statistically significant serum concentrations of damage-associated molecular patterns (DAMPs) as biomarkers of chronic aseptic inflammation in patients with uterine fibroids (UF) and infertility. This was compared with fertile patients with UF and a control group. The study found increased concentrations of S100 protein, uric acid, and LDL-C cholesterol as well as decreased glutathione concentrations. Furthermore, the relative risk of infertility in patients with intramural UF was calculated based on the level of DAMPs.
Traditionally, intramural UF has not been considered a factor in infertility; however, this study suggests that it may contribute to infertility in the absence of other causes. These findings support the hypothesis that a certain level of systemic mild aseptic inflammation combined with UF can lead to reproductive system disorders and infertility.
In 1994, Polly Matzinger proposed the "danger model,” which introduced new mechanisms for activating natural and adaptive immune responses. According to this theory, the innate immune defense system recognizes "danger signals" known as DAMPs and responds by activating the immune system and creating a pro-inflammatory environment. DAMPs are molecules that are typically found in the body but have highly pro-inflammatory properties when detected in abnormal forms, such as extracellular or free forms. They can serve as indicators of tissue damage and systemic inflammation.
Various stressors can trigger the release of DAMPs, including physical (trauma and radiation), chemical (toxins and osmolarity), metabolic (ischemia and reperfusion), infectious (viruses, bacteria, and protozoans), and programmed cell death.
In 2015, most human diseases were hypothesized to be associated with disturbed physiological homeostasis caused by tissue damage, cellular stress, or even minor metabolic disturbances in the extracellular and intracellular environments. This suggests that human diseases can be understood as a response of the innate immune system to damage. The principal model suggests that microbial/non-microbial stress leads to cellular stress or tissue damage, which in turn leads to the production of DAMPs. These DAMPs are recognized by immune cells, leading to the activation of the innate immune system and an inflammatory response, whether caused by infection or sterile conditions. Normally, DAMPs are located intracellularly and are not recognized by the immune system. The present study collected data on the association between changes in damage-associated molecular patterns (DAMPs) and infertility in intramural uterine fibroids (UF). The data revealed an increase in uric acid, S100 protein, and LDL-C cholesterol concentrations, as well as a decrease in glutathione concentration, compared to the control group and fertile patients with UF. We now consider each of these significantly altered indicators.
Glutathione is a vital molecule that plays a key role in maintaining cellular homeostasis and serves as the primary defense against oxidative damage in various diseases. It is often referred to as a "master antioxidant" due to its involvement in antioxidant defense, detoxification, regulation of redox potential, cell signaling, protein function, gene expression, differentiation/proliferation, immune response, and antiviral protection [21]. Glutathione is present in all cellular components and circulates within compartments to counteract reactive oxygen species [23]. Recent studies have revealed that glutathione can function as both an antioxidant and a pro-oxidant, in addition to its role as a cofactor for antioxidant and detoxifying enzymes [24].
The present study confirmed a decrease in serum glutathione concentration among infertile patients with intramural UF. Similar changes in glutathione concentration have been observed in chronic lung diseases [25], Parkinson's disease [26], cardiovascular diseases [27], and other conditions reported in the literature.
The findings of this study were somewhat unexpected, as they revealed a decrease in glutathione concentration in the main group of patients, along with an increase in other indicators. Considering glutathione's potential as a pro-oxidant, one would expect an increase in its concentration. However, given its antioxidant properties, it is possible that the decrease in concentration is the result of excessive long-term consumption.
Another interesting DAMP is the S100 protein, discovered in 1965 by Moore. It was named S100 because it is completely soluble in ammonium sulfate under neutral pH conditions [28]. This calcium-binding protein is found in various organs and tissues and plays a regulatory role in intracellular calcium concentration.
The S100 protein family is often referred to as "alarm bells" [29]. These proteins contribute significantly to inflammation and immune responses, providing protection to the body by triggering immune reactions. Disease conditions characterized by sterile inflammation and an increase in specific S100 protein types include gout, allergies, arthritis, atherosclerosis, autoimmune diseases, and intestinal diseases [30]. The results of the study suggest that infertile patients with uterine fibroids (UF) have higher levels of S100 protein in their blood than healthy and fertile women. This protein likely possesses antioxidant properties, and its increase may indicate mild systemic aseptic inflammation. Previous research has shown that S100 protein plays a vital role in successful implantation, embryo development, and pregnancy. It is worth noting that this protein is found in the amniotic fluid, endometrial tissue, and fetal brain, and alterations in its concentration can result in pregnancy complications [31, 32].
Uric acid is a product of purine catabolism and a DAMP released by ischemic tissues and dying cells. Uric acid is present in normal cells and in biological fluids [33].
Uric acid acts as a pro-oxidant molecule, reducing the availability of nitric oxide, increasing the production of reactive oxygen species, and stimulating chemotaxis [34]. In contrast, it is considered an antioxidant with pronounced protective properties. Thus, DAMPs are a type of two-faced Janus, and according to modern data, their properties likely depend on the specific clinical situation [35]. Fundamental studies have provided data on the antioxidant properties of uric acid only in a hydrophilic environment, but its biologically active pro-inflammatory properties have also been reliably proven [36].
Our data on an increase in the concentration of uric acid during infertility in patients with intramural uterine fibroids (UF) are consistent with the hypothesis of the presence of systemic chronic aseptic inflammation, in which uric acid acts as a pro-oxidant and may be a diagnostic marker of infertility.
Interestingly, the data we discovered during preparation for the publication of this work showed similar changes in the concentration of uric acid in patients with infertility associated with age and body mass index [37, 38]. Our previously published data on the presence of metabolic disorders in infertile patients with intramural UF, as well as the results of this study, are fully consistent with those of previous studies [39].
LDL-C as a marker of chronic inflammation was also not proposed by chance in this study. Lipid metabolism profoundly influences both innate and adaptive immune responses. LDL-C activates the immune system through the activation of Toll-like receptors, triggering pro-inflammatory defense mechanisms [40]. Phospholipids on the surface of LDL-C are recognized by the body as DAMPs [41]. Under conditions of chronic inflammation, the normal proinflammatory environment damages molecules that regulate efferocytosis. Defective efferocytosis causes apoptotic cells to accumulate and undergo secondary necrosis, which, in turn, leads to the production of other DAMPs. These links in pathogenesis have already been studied in detail in atherosclerosis [42].
The literature provides evidence that UF formation and relapses are associated with elevated LDL-C levels [43, 44]. One study demonstrated an association between UF size and lipid profile [45]. Our data are consistent with the literature: fertile patients with UF have significantly higher levels of LDL-C in the blood serum than the control group.
At the same time, more pronounced changes in LDL-C content in infertile patients with UF are noteworthy. Altered lipid metabolism significantly affects the hormonal profile and ovarian and uterine functions, undoubtedly affecting fertility. Colleagues have already presented the results of an analysis indicating that altered LDL-C concentrations are risk factors for infertility [46].
Conclusion
Based on the results of this study, several conclusions can be drawn. First, with intramural UF, changes were identified at the systemic level, characterized by systemic mild chronic aseptic inflammation, which is expressed as a pro-oxidant shift in DAMPs. Second, the group of patients with intramural UF was heterogeneous in terms of the severity of the systemic sterile inflammation. Moreover, the degree of proinflammatory change is strongly associated with symptoms such as infertility. This significantly expands our understanding of the pathogenesis of infertility in UF and verifies the presence of systemic metabolic disorders as a cofactor of infertility. Third, the proposed DAMPs markers (glutathione, LDL-C, S100 protein, and UA) can be used as diagnostic and prognostic markers of infertility in intramural UF. When threshold values are reached (glutathione less than 413 µmol/L, S100 protein > 0.172 µg/L, UA more than 280.5 µmol/L; LDL-C more than 3.78 mmol/L), the relative risk of infertility increases.
References
- Дубинская Е.Д., Колесникова С.Н., Алёшкина Е.В., Гаспаров А.С. Хроническое стерильное воспаление в патогенезе доброкачественных заболеваний миометрия. Вопросы гинекологии, акушерства и перинатологии. 2024; 23(1): 84-93. [Dubinskaya E.D., Kolesnikova S.N., Aleshkina E.V., Gasparov A.S. Chronic sterile inflammation in the pathogenesis of benign myometrial diseases. Gynecology, Obstetrics and Perinatology. 2024; 23(1): 84-93. (in Russian)]. https://dx.doi.org/10.20953/1726-1678-2024-1-84-93.
- Оразов М.P., Радзинский В.E., Носенко Е.Н. Роль воспаления и иммунореактивности в развитии болевого синдрома при аденомиозе. Патологическая физиология и экспериментальная терапия. 2016; 60(1): 40-4. [Orazov M.R., Radzinskiy V.E., Nosenko E.N. The role of inflammatory and immune reactivity in developing pain in adenomyosis. Pathological physiology and experimental therapy. 2016; 60(1): 40-4. (in Russian)].
- Сотникова Н.Ю., Малышкина Д.А., Воронин Д.Н. Особенности продукции цитокинов, регулирующих активность натуральных киллеров, у женщин с лейомиомой матки. Иммунология. 2023; 44(2): 202-8. [Sotnikova N.Yu., Malyshkina D.A., Voronin D.N. The type of cytokines production that regulate NK activity in women with uterine leiomyoma. Immunologiya. 2023; 44(2): 202-8. (in Russian)].
- Li F., Wang J., Liu W. Search for key genes, key signaling pathways, and immune cell infiltration in uterine fibroids by bioinformatics analysis. Medicine (Baltimore). 2023; 102(20): e33815. https://dx.doi.org/10.1097/MD.0000000000033815.
- Шрамко С.В., Зорина В.Н., Баженова Л.Г., Зорина Р.М., Рябичева Т.Г., Чевычалова Е.В., Зорин Н.А. Регуляторно-транспортные белки и цитокины в крови больных с заболеваниями матки. Акушерство и гинекология. 2016; 5: 104-8. [Shramko S.V., Zorina V.N., Bazhenova L.G., Zorina R.M., Ryabicheva T.G., Chevychalova E.V., Zorin N.A. Regulatory transport proteins and cytokines in the blood of patients with proliferative diseases of the uterus. Obstetrics and Gynecology. 2016; (5): 104-8. (in Russian)]. http://dx.doi.org/10.18565/aig.2016.5.104-108.
- Yang Q., Ciebiera M., Bariani M.V., Ali M., Elkafas H., Boyer T.G. et al. Comprehensive review of uterine fibroids: developmental origin, pathogenesis, and treatment. Endocr. Rev. 2022; 43(4): 678-719. https://dx.doi.org/10.1210/endrev/bnab039.
- Duan Y., Peng Y., Shi X., Zhao Y., Liu K., Zhou R. et al. Correlation between platelet-lymphocyte ratio and neutrophil-lymphocyte ratio in patients with uterine leiomyoma: A cross-sectional study. J. Oncol. 2022; 2022: 3257887. https://dx.doi.org/10.1155/2022/3257887.
- Fabozzi G., Verdone G., Allori M., Cimadomo D., Tatone C., Stuppia L. et al. Personalized nutrition in the management of female infertility: new insights on chronic low-grade inflammation. Nutrients. 2022; 14(9): 1918. https://dx.doi.org/10.3390/nu14091918.
- Rudnicka E., Suchta K., Grymowicz M., Calik-Ksepka A., Smolarczyk K., Duszewska A.M. et al. Chronic low grade inflammation in pathogenesis of PCOS. Int. J. Mol. Sci. 2021; 22(7): 3789. https://dx.doi.org/10.3390/ijms22073789.
- Ehsani M., Mohammadnia-Afrouzi M., Mirzakhani M., Esmaeilzadeh S., Shahbazi M. Female unexplained infertility: a disease with imbalanced adaptive immunity. J. Hum. Reprod. Sci. 2019; 12(4): 274-82. https://dx.doi.org/ 10.4103/jhrs.JHRS_30_19.
- Dull A.-M., Moga M.A., Dimienescu O.G., Sechel G., Burtea V., Anastasiu C.V. Therapeutic approaches of resveratrol on endometriosis via anti-inflammatory and anti-angiogenic pathways. Molecules. 2019; 24(4): 667. https://dx.doi.org/ 10.3390/molecules24040667.
- Yang Z., Tang Z., Cao X., Xie Q., Hu C., Zhong Z. et al. Controlling chronic low-grade inflammation to improve follicle development and survival. Am. J. Reprod. Immunol. 2020; 84(2): e13265. https://dx.doi.org/10.1111/aji.13265.
- Pirtea P., Cicinelli E., De Nola R., de Ziegler D., Ayoubi J.M. Endometrial causes of recurrent pregnancy losses: Endometriosis, adenomyosis, and chronic endometritis. Fertil. Steril. 2021; 115(3): 546-60. https://dx.doi.org/10.1016/j.fertnstert.2020.12.010.
- Koncz G., Jenei V., Tóth M., Váradi E., Kardos B., Bácsi A. et al. Damage-mediated macrophage polarization in sterile inflammation. Front. Immunol. 2023; 14: 1169560. https://dx.doi.org/10.3389/fimmu.2023.1169560.
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Миома матки. 2020. [Ministry of Health of the Russian Federation. Clinical guidelines. Uterine fibroids. 2020. (in Russian)].
- Munro M., Critchley H., Broder M., Fraser I. The FIGO classification system ("PALM-COEIN") for causes of abnormal uterine bleeding in non-gravid women in the reproductive years, including guidelines for clinical investigation. Int. J. Gynaecol. Obstet. 2011; 113 (1): 3-13. https://dx.doi.org/10.1016/j.ijgo.2010.11.011.
- Matzinger P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994; 12: 991-1045. https://dx.doi.org/10.1146/annurev.iy.12.040194.005015.
- Caballero-Herrero M., Jumilla E., Buitrago-Ruiz M., Valero-Navarro G., Cuevas S. Role of damage-associated molecular patterns (DAMPS) in the postoperative Period after colorectal surgery. Int. J. Mol. Sci. 2023; 24(4): 3862. https://dx.doi.org/10.3390/ijms24043862.
- Murao A., Aziz M., Wang H., Brenner M., Wang P. Release mechanisms of major DAMPs. Apoptosis. 2021; 26(3-4): 152-62. https://dx.doi.org/10.1007/s10495-021-01663-3.
- Land W.G. The role of damage-associated molecular patterns in human diseases: Part I - Promoting inflammation and immunity. Sultan Qaboos Univ. Med. J. 2015; 15(1): e9-e21.
- Labarrere C.A., Kassab G.S. Glutathione: A Samsonian life-sustaining small molecule that protects against oxidative stress, ageing and damaging inflammation. Front. Nutr. 2022; 9: 1007816. https://dx.doi.org/10.3389/fnut.2022.1007816.
- Meister A., Anderson M.E. Glutathione. Annu. Rev. Biochem. 1983; 52: 711-60. https://dx.doi.org/10.1146/annurev.bi.52.070183.003431.
- Marí M., de Gregorio E., de Dios C., Roca-Agujetas V., Cucarull B., Tutusaus A. et al. Mitochondrial glutathione: recent insights and role in disease. Antioxidants (Basel). 2020; 9(10): 909. https://dx.doi.org/10.3390/antiox9100909.
- Averill-Bates D.A. The antioxidant glutathione. Vitam. Horm. 2023; 121: 109-41. https://dx.doi.org/10.1016/bs.vh.2022.09.002.
- Zuo L., Wijegunawardana D. Redox role of ROS and inflammation in pulmonary diseases. Adv. Exp. Med. Biol. 2021; 1304: 187-204. https://dx.doi.org/10.1007/978-3-030-68748-9_11.
- Bjørklund G., Peana M., Maes M., Dadar M., Severin B. The glutathione system in Parkinson's disease and its progression. Neurosci. Biobehav. Rev. 2021; 120: 470-8. https://dx.doi.org/10.1016/j.neubiorev.2020.10.004.
- Sobha S.P, Kesavarao K.E. Contribution of glutathione-S-transferases polymorphism and risk of coronary artery diseases: a meta-analysis. Curr. Aging Sci. 2022; 15(3): 282-92. https://dx.doi.org/10.2174/1874609815666220304193925.
- Moore B.W. A soluble protein characteristic of the nervous system. Biochem. Biophys. Res. Commun. 1965; 19: 739-44. https://dx.doi.org/10.1016/0006-291x(65)90320-7.
- Allgöwer C., Kretz A.L., von Karstedt S., Wittau M., Henne-Bruns D., Lemke J. Friend or foe: S100 proteins in cancer. Cancers (Basel). 2020; 12(8): 2037. https://dx.doi.org/10.3390/cancers12082037.
- Singh P., Ali S.A. Multifunctional role of S100 protein family in the immune system: an update. Cells. 2022; 11(15): 2274. https://dx.doi.org/10.3390/cells11152274.
- Verma R., Verma P., Budhwar S., Singh K. S100 proteins: An emerging cynosure in pregnancy & adverse reproductive outcome. Indian J. Med. Res. 2018; 148(Suppl): S100-S106. https://dx.doi.org/10.4103/ijmr.IJMR_494_18.
- Sadigh A.R., Mihanfar A., Fattahi A., Latifi Z., Akbarzadeh M., Hajipour H. et al. S100 protein family and embryo implantation. J. Cell. Biochem. 2019; 120(12): 19229-44. https://dx.doi.org/10.1002/jcb.29261.
- Alberts A., Klingberg A., Hoffmeister L., Wessig A.K., Brand K., Pich A. et al. Binding of macrophage receptor MARCO, LDL, and LDLR to disease-associated crystalline structures. Front. Immunol. 2020; 11: 596103. https://dx.doi.org/10.3389/fimmu.2020.596103.
- Braga T.T., Forni M.F., Correa-Costa M., Ramos R.N., Barbuto J.A., Branco P. et al. Soluble uric acid activates the NLRP3 inflammasome. Sci. Rep. 2017; 7: 39884. https://dx.doi.org/10.1038/srep39884.
- Chhana A., Pool B., Wei Y., Choi A., Gao R., Munro J. et al. Human cartilage homogenates influence the crystallization of monosodium urate and inflammatory response to monosodium urate crystals: a potential link between osteoarthritis and gout. Arthritis Rheumatol. 2019; 71(12): 2090-9. https://dx.doi.org/10.1002/art.41038.
- Sautin Y.Y., Johnson R.J. Uric acid: the oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids. 2008; 27(6): 608-19. https://dx.doi.org/10.1080/15257770802138558.
- Luo C., Cheng H., He X., Tan X., Huang X. Association between serum uric acid and female infertility: a cross-sectional study of National Health and Nutrition Examination Survey (NHANES) 2013-2018. BMC Womens Health. 2023; 23(1): 224. https://dx.doi.org/10.1186/s12905-023-02376-2.
- Liang J., Chen X., Huang J., Nie W., Yang Q., Huang Q., Deng K. Implications of serum uric acid for female infertility: results from the national health and nutrition examination survey, 2013-2020. BMC Womens Health. 2023; 23(1): 103. https://dx.doi.org/10.1186/s12905-023-02234-1.
- Дубинская Е.Д., Колесникова С.Н., Алёшкина Е.В., Гаспаров А.С., Башкирова Е.С., Леффад М.Л. Дополнительные факторы инфертильности при интрамуральной миоме матки. Акушерство и гинекология. 2023; 5: 75-82. [Dubinskaya E.D., Kolesnikova S.N., Alyoshkina E.V., Gasparov A.S., Bashkirova E.S., Leffad M.L. Supplementary infertility factors in patients with intramural uterine fibroids. Obstetrics and Gynecology. 2023; (5): 75-82 (in Russian)]. https://dx.doi.org/10.18565/aig.2023.28.
- Jukema R., Ahmed T., Tardif J. Does low-density lipoprotein cholesterol induce inflammation? If so, does it matter? Current insights and future perspectives for novel therapies. BMC Med. 2019; 17(1): 197. https://dx.doi.org/10.1186/s12916-019-1433-3.
- Dzobo K., Kraaijenhof J., Stroes E., Nurmohamed N., Kroon J. Lipoprotein(a): An underestimated inflammatory mastermind. Atherosclerosis. 2022; 349: 101-9. https://dx.doi.org/10.1016/j.atherosclerosis.2022.04.004.
- Yurdagul A. Jr., Doran A.C., Cai B., Fredman G., Tabas I.A. Mechanisms and consequences of defective efferocytosis in atherosclerosis. Front. Cardiovasc. Med. 2018; 4: 86. https://dx.doi.org/10.3389/fcvm.2017.00086.
- Sharami S., Fallah Arzpeyma S., Shakiba M., Montazeri S., Milani F., Kazemi S. et al. Relationship of uterine fibroids with lipid profile, anthropometric characteristics, subcutaneous and preperitoneal fat thickness. Arch. Iran Med. 2019; 22(12): 716-21.
- Tonoyan N.M., Chagovets V.V., Starodubtseva N.L., Tokareva A.O., Chingin K., Kozachenko I.F. et al. Alterations in lipid profile upon uterine fibroids and its recurrence. Sci. Rep. 2021; 11(1): 11447. https://dx.doi.org/10.1038/s41598-021-89859-0.
- Turkey B.N., Rubayae B.J.A. Possible association between lipid profile and uterine fibroid size. Int. J. Reprod. Contracept. Obstet. Gynecol. 2023; 12(7): 1969-74. https://dx.doi.org/10.18203/2320-1770.ijrcog20231905.
- Zhu X., Hong X., Wu J., Zhao F., Wang W., Huang L. et al. The association between circulating lipids and female infertility risk: a univariable and multivariable Mendelian randomization analysis. Nutrients. 2023; 15(14): 3130. https://dx.doi.org/10.3390/nu15143130.
Received 06.03.2024
Accepted 25.04.2024
About the Authors
Ekaterina D. Dubinskaya, Dr. Med. Sci., Professor at the Department of Obstetrics, Gynecology and Perinatology, Patrice Lumumba Peoples’ Friendship University of Russia,8 Miklukho-Maklaya str., Moscow, 117198, Russia, +7(903)117-55-58, eka-dubinskaya@yandex.ru, https://orcid.org/0000-0002-8311-0381
Svetlana N. Kolesnikova, PhD, Associate Professor at the Department of Obstetrics, Gynecology and Pediatrics, Reaviz MMU, 2-2 Krasnobogatyrskaya str., Moscow,
107564, Russia, +7(916)500-10-99, ksnmed@mail.ru, https://orcid.org/0000-0001-9575-02741
Alexander S. Gasparov, Dr. Med. Sci., Professor, Professor at the Department of Obstetrics, Gynecology and Perinatology, Patrice Lumumba Peoples’ Friendship University
of Russia, 8 Miklukho-Maklaya str., Moscow, 117198, Russia, +7(985)776-77-78, 13513522@mail.ru, https://orcid.org/0000-0001-6301-1880
Elizaveta V. Alyoshkina, Teaching Assistant at the Department of Obstetrics, Gynecology and Reproductive Medicine, Faculty of Postgraduate Education, Patrice Lumumba Peoples’ Friendship University of Russia, 8 Miklukho-Maklaya str., Moscow, 117198, +7(926)768-44-27, alyoshkina.ev@yandex.ru, https://orcid.org/0000-0001-5339-1285



