Interleukin gene polymorphism and risk of uterine fibroids
Relevance: Uterine fibroids are common tumors of the female reproductive organs which affect 15–45% of women. Pathogenesis of uterine fibroids is characterized by impaired apoptosis and immune processes due to cytokine disbalance, namely interleukins. Interleukin gene polymorphism can affect their expression and as a result it plays an important role in pathophysiology of uterine fibroids.Altukhova O.B., Radzinsky V.E., Sirotina S.S., Churnosov M.I., Efremova O.A., Batlutskaya I.V., Orlova V.S.
Objective: To study the correlation between interleukin gene polymorphism and uterine fibroids.
Materials and methods: The study included 492 patients. The main group consisted of 109 patients suffering from uterine fibroids. Six polymorphic loci of interleukin genes were selected for the study, namely A/C IL10 rs1800872, C/G IL6 rs1800795, T/C IL5 rs2069812, C/T IL4 rs2243250, Т/С IL1β rs16944, С/Т IL1α rs1800587. The analysis was performed using polymerase chain reaction (PCR) on a CFX-96 Real-Time System thermal cycler. The odds ratio (OR) and 95% confidence interval (CI) were used to calculate the associations. The association of combinations of the gene alleles and genotypes with the formation of uterine fibroids was evaluated on the basis of the APSampler software (https://sourceforge.net/projects/apsampler /).
Results: Molecular genetic markers A/C IL10 rs1800872, T/C IL5 rs2069812, S/T IL4 rs2243250, T/C IL1ß rs16944, S/T IL1α rs1800587 play a significant role in the development of uterine fibroids. Such genotype as TT rs1800587 IL1a (OR=5.70, p=0.017), as well as a combination of polymorphic variants of T rs2243250 IL4, T rs1800587 IL1a and T rs16944 IL1ß (OR=2.60, pperm=0.012), T rs1800587 IL1a, C rs2069812 IL5 and T rs16944 IL1ß (OR=2.60, pperm=0.012), T rs1800587 IL1a, C rs2069812 IL5 and T rs16944 IL1ß (OR=2.71, pperm=0.009), T rs1800587 IL1a, C rs1800872 IL10 and T rs16944 IL1ß (OR=2.73, pperm=0.003) should be considered risk factors for the development of uterine fibroids.
Conclusion: Polymorphic gene loci A/C IL10 rs1800872, T/C IL5 rs2069812, S/T IL4rs 2243250, T/C IL1ß rs16944, S/T IL1α rs1800587 are associated with the development of uterine fibroids. We identified combinations of allelic variants of polymorphic loci of interleukin genes associated with an increased risk of uterine fibroids. These combinations indicate the importance of inter-locus interactions of candidate genes in the development of this pathology. In practical medicine they can be used to identify risk groups and to carry out preventive measures.
Keywords
Uterine fibroids are benign, well-delimited encapsulated tumors that can be found in the uterine myometrium. As these benign tumors occur frequently (from 15 to 45% in different populations), uterine fibroids result in dysmenorrhea, menorrhagia, anemia, urinary incontinence, recurrent miscarriages, premature birth, infertility, dysfunction of adjacent organs, and dyshormonal diseases of the mammary glands [1, 2]. One of the main causes of hysterectomy in many countries, as well as in Russia, is uterine fibroids (50–70% of cases) [3]. Despite the benign course, uterine fibroids reduce the quality of life of patients, and also have a significant economic impact on the healthcare system around the world [4].
The mechanisms underlying uterine fibroid development and growth remain unclear and are still debatable. The role of immune disorders in the pathogenesis of fibroids is under intense research nowadays [5, 6]. The growth of fibroids has been proven to be accompanied by the weakened immune protection, as well as a decrease in the level of anti-inflammatory and increase in the level of pro-inflammatory cytokines [7, 8]. Moreover, one of the important aspects of the uterine fibroid pathogenesis is impaired regulation of apoptosis [9–11]. Tumor growth is caused by an imbalance between cell proliferation and cell death. Cytokines, in particular interleukins, have a significant effect on cell proliferation, differentiation and death [12]. Some interleukins (IL1, IL2, IL3, IL4, IL-5, IL10) are able to launch a program for protecting cells from apoptosis by enhancing the functions of proteins Bcl-2, Bcl-xL, etc.; other interleukins (IL10, IL1, IL17), on the contrary, have the ability to induce apoptosis [10, 13]. Therefore, the polymorphic loci of interleukin genes selected for the study (A/C IL10 rs1800872, C/G IL6 rs1800795, T/C IL5 rs2069812, C/T IL4 rs2243250, T/C IL1ß rs16944, C/T IL1α rs1800587) can directly or indirectly influence cell apoptosis, and this way affect pathophysiology of uterine fibroids.
A lot of Russian and foreign studies have been devoted to the research of genetic factors involved in the development of uterine fibroids [3, 7, 14]. The scientists have estimated the role of polymorphic variants of different candidate genes, namely progesterone and estrogen receptors, growth factors, metalloproteinases, chemokines and folate cycle genes and others in uterine fibroid formation [14–19]. Despite the obvious importance of interleukin genes in pathophysiology of uterine fibroids, there are only a few studies devoted to the research of the role of interleukin gene polymorphism in the development of the disease and their results are often contradictory [3, 11]. The role of intergenic interactions of interleukin genes in the development of uterine fibroids has not been studied.
The objective of the study is to evaluate the role of polymorphic loci of interleukin genes in the development of uterine fibroids.
Materials and methods
The groups of the study participants were formed on the basis of the Gynecology Department in the Saint Joseph Belgorod Regional Clinical Hospital. The women included in the study came from the Central Chernozem region of Russia, they were not related to each other. The study included 492 patients. The main group consisted of 109 patients suffering from uterine fibroids. The diagnosis was verified by echographic, hysteroscopic methods followed by histological examination of the obtained material. The criteria for exclusion from the main group were pregnancy, the presence of confirmed malignant diseases of the endometrium, ovaries, and uterine body. The remaining 383 women without fibroids composed the control group. Women without diseases of the reproductive system were recruited for the control group.
The age of the patients of the main group (42.37 (6.02) years) and the control group (41.23 (5.72) years) was comparable according to the Mann–Whitney U test (p>0.05).
The study was approved by the Ethical Review Board of Belgorod National Research University (protocol No. 8 dated May 26, 2016). The informed consent was obtained from all the women who took part in the study before clinical, instrumental, and genetic studies were carried out.
Genomic DNA was extracted from peripheral blood leukocytes using phenol-chloroform method.
The following DNA markers were selected for the analysis: A/C IL10 rs1800872, C/G IL6 rs1800795, T/C IL5 rs2069812, C/T IL4 rs2243250, Т/С IL1β rs16944, С/Т IL1α rs1800587 [17, 19, 20, 21]. The analysis of molecular genetic loci C/G IL6 rs1800795 and C/T IL4 rs2243250 was carried out using the Real-Time PCR method and oligonucleotide primers and probes (LLC Syntol, Russia). The study of polymorphic loci A/C IL10 rs1800872, T/C IL1ß rs16944, T/C IL5 rs2069812, S/T IL-1α rs1800587 was carried out using the method of restriction fragment length polymorphism (RFLP) analysis of PCR amplification products of specific genome sites with the help of appropriate restriction enzymes (SibEnzym, Novosibirsk, Russia).
Statistical analysis
The frequency distribution of alleles and genotypes of interleukin genes in the main group and the control group was evaluated with the help of two-way contingency tables using the χ2 criterion with Yates correction for continuity. The odds ratio (OR) and 95% confidence interval (CI) were used to search for associations of DNA markers with the development of uterine fibroids.
Statistical data analysis was carried out using the program STATISTICA for Windows 10.0.
The association of combinations of the gene alleles and genotypes with the development of uterine fibroids was evaluated on the basis of the APSampler software (https://sourceforge.net/projects/apsampler/) using the Monte Carlo Markov chains method and Bayesian nonparametric statistics [22]. Permutation analysis (pperm) was used to verify the obtained associations in multiple comparisons. pperm<0.05 was considered a statistically significant level [23].
Results and discussion
The frequency of alleles and genotypes of DNA markers of interleukins in patients of the main and control groups is shown in Tables 1 and 2.
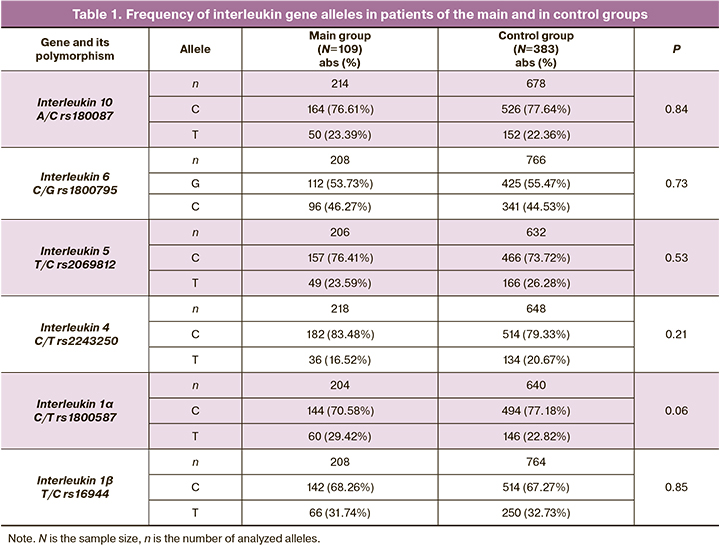
The comparative analysis revealed that genotype TT rs1800587 IL1α is more common in the main group (10.78%) than in the control group (4.39%, 2=5.70, p=0.017, OR=2.64, 95% CI: 1.15–6.02).
The combinations of alleles of the polymorphic loci of interleukins were revealed with the help of the APSampler software (https://sourceforge.net/projects/apsampler/). These combinations are presented in Table 3. Thus, the combination of alleles T rs2243250 IL4, T rs1800587 IL1α and T rs16944 IL1ß occurred in 32.67% of patients in the main group, and this indicator was 15.72% in the control group (OR=2.60, pperm=0.012).
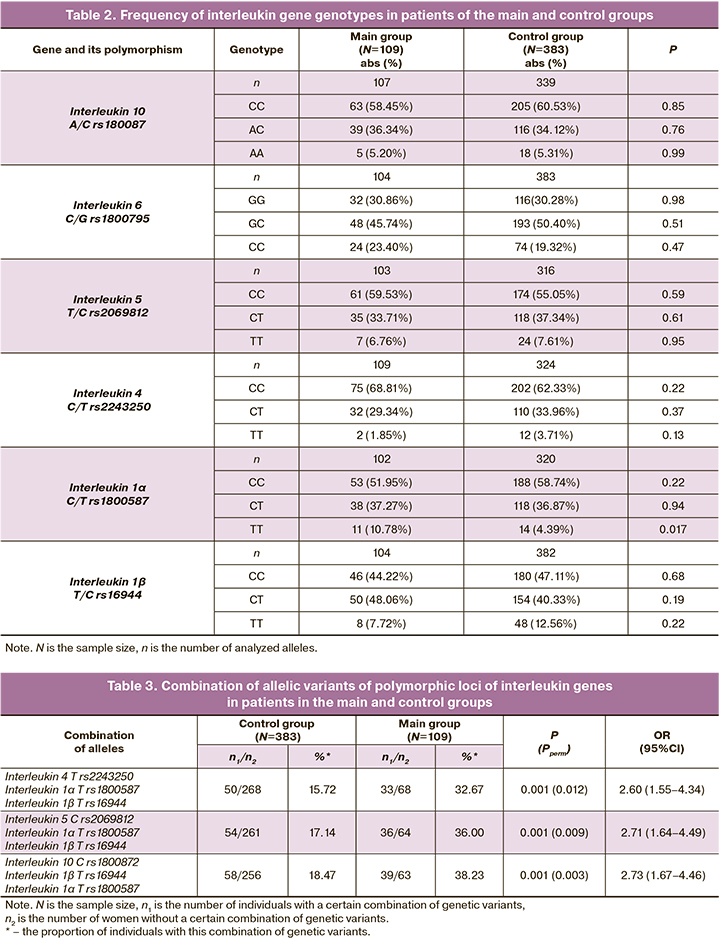
The combination of polymorphic loci C rs2069812 IL5, T rs1800587 IL1α and T rs16944 IL1ß was twice as common in the main group compared to the control group (OR=2.71, pperm=0.009). Along with this, the combination of alleles T rs16944 IL1ß, T rs1800587 IL1α and C rs1800872 IL10 occurred in 38.23% of patients in the main group, and in 18.47% of women in the control group (OR=2.73, pperm=0.003). The identified combinations of polymorphic interleukin loci are markers of the risk for uterine fibroids.
Allele T rs1800587 IL1α and allele T rs16944 IL1β have been shown to be risk factors for the development of uterine fibroids, so they are included in all the combinations identified by us. According to literature data, the IL-1 family is involved in the development of estrogen-dependent diseases. IL 1A and IL 1B are responsible for the production of neutrophils, natural killers, macrophages and monocytes [6]. Some tumor cells are capable of producing IL 1A and IL 1B, and it gives reason to believe that a high level of these interleukins may contribute to tumor proliferation [24].
Brodzikowska A. et al. (2019) showed that allele T rs1800587 IL1α and allele T rs16944 IL1ß are associated with high levels of IL1A and IL-1B in plasma in healthy individuals [25]. Thus, it can be assumed that alleles T rs16944 IL1ß and T rs1800587 IL1α cause the induction of apoptosis, impaired antitumor response and increased pro-inflammatory action through high levels of plasma IL1A and IL1B.
Moreover, the identified combinations had allele T rs2243250 IL4, which causes a decrease in the antitumor and anti-inflammatory effects of IL4. The production of the plasma level of interleukin 4 is due to the polymorphism of rs2243250 IL4. The polymorphic variant C IL 4 determines a high level of transcription of this gene. But allele T IL4 binds weakly to transcription factors therefore its productivity is decreased [26]. Interleukin 4 is involved in many biological processes. For example, it takes part in the regulation of inflammatory reactions, immune response, and mechanisms of cell protection from apoptosis [27], so it may play an important role in the pathogenesis of uterine fibroids.
According to the data from literature, IL5 provides anti-inflammatory reactions and antitumor action due to its ability to participate in apoptosis. Mestiri S. et al. (2020) reported that allele T rs2069812, which is located in the promoter region of gene IL5, is highly productive, and is responsible for a higher level of IL5; however, allele C rs2069812, on the contrary, is associated with a reduced level of IL5 in human blood [28]. Since allele C rs2069812 demonstrates a low productivity, it causes a decrease in the antitumor and anti-inflammatory effects of IL5, and thus may affect the development of uterine fibroids.
As the main member of the cytokine family, IL10 plays an important role in the regulation of proliferation and differentiation of various immune cells; this cytokine is included in the combinations which were obtained in our study. Polymorphism of the IL10 promoter region has been reported to affect its transcription and translation leading to abnormal cell proliferation and influencing fibroid development. Allele C rs1800872 is associated with a higher level of interleukin 10 [29]. When specific activation of T cells in the tumor microenvironment is absent, overexpression of IL10 impairs the production of Th1 cytokines and this way contributes to the development of the tumor process. Wang K. et al. (2021) found that variant AC/AA + AC rs1800872 has a protective effect in the development of cervical cancer [30]. Thus, the available literature data do not provide an unambiguous answer about the role of interleukin 10 level in the pathogenesis of gynecological pathology, and it is necessary to conduct further research in this area.
It should be noted that polymorphism С/Т IL1α rs1800587, which demonstrates an independent association with uterine fibroids, makes the main contribution to the development of the disease. However, polymorphic variants of the other four interleukin genes (A/C IL10 rs1800872, T/C IL5 rs2069812, C/T IL4 rs2243250, T/C IL1ß rs16944) are associated with uterine fibroids only during intergenic interactions and play an additional role in the development of the disease.
Conclusion
Molecular genetic markers A/C IL10 rs1800872, T/C IL5 rs2069812, C/T IL4 rs 2243250, T/C IL1ß rs16944, C/T IL1α rs1800587 play a significant role in the development of uterine fibroids.
Such genotype as TT rs1800587 IL1α (OR=5.70, p=0.017), as well as a combination of polymorphic variants of T rs2243250 IL4, T rs1800587 IL1α and T rs16944 IL1ß (OR=2.60, pperm=0.012), T rs1800587 IL1α, C rs2069812 IL5 and T rs16944 IL1ß (OR=2.60, pperm=0.012), T rs1800587 IL1α, C rs2069812 IL5 and T rs16944 IL1ß (OR=2.71, pperm=0.009), T rs1800587 IL1α, C rs1800872 IL10 and T rs16944 IL1ß (OR=2.73, pperm=0.003) should be considered risk factors for the development of uterine fibroids. The identified combinations of allelic variants of polymorphic loci of interleukin genes are associated with an increased risk of uterine fibroids. These combinations indicate the importance of inter-locus interactions of candidate genes in the development of this pathology. In practical medicine they can be used to identify risk groups and to carry out preventive measures.
References
- Alsudairi H.N., Alrasheed A.T., Dvornyk V. Estrogens and uterine fibroids: an integrated view. Research Results in Biomedicine. 2021; 7(2): 156-63. https://dx.doi.org/10.18413/2658-6533-2021-7-2-0-6.
- Aninye I.O., Laitner M.H. Uterine fibroids: assessing unmet needs from bench to bedside. J. Womens Health (Larchmt). 2021; 30(8): 1060-7.https://dx.doi.org/10.1089/jwh.2021.0280.
- Giuliani E., As-Sanie S., Marsh E.E. Epidemiology and management of uterine fibroids. Int. J. Gynaecol. Obstet. 2020; 149(1): 3-9. https://dx.doi.org/10.1002/ijgo.13102.
- Baranov V.S., Osinovskaya N.S., Yarmolinskaya M.I. Pathogenomics of uterine fibroids development. Int. J. Mol. Sci. 2019; 20(24): 6151.https://dx.doi.org/10.3390/ijms20246151.
- Manta L., Suciu N., Toader O., Purcărea R.M., Constantin A., Popa F. The etiopathogenesis of uterine fibromatosis. J. Med. Life. 2016; 9(1): 39-45.
- Aggarwal R., Jain A.K., Mittal P., Kohli M., Jawanjal P., Rath G. Association of pro‐ and anti‐inflammatory cytokines in preeclampsia. J. Clin. Lab. Anal. 2019; 33(4): e22834. https://dx.doi.org/10.1002/jcla.22834.
- McWilliams M.M., Chennathukuzhi V.M. Recent advances in uterine fibroid etiology. Semin. Reprod. Med. 2017; 35(2): 181-9.https://dx.doi.org/10.1055/s-0037-1599090.
- Олейник Н.С. Современные представления о морфо- и патогенезе миомы матки (обзор литературы). Таврический медико-биологический вестник. 2011; 14 (3-2): 251-4. [Oleynik N.S. Modern ideas about the morpho- and pathogenesis of uterine fibroids (literature review). Taurian Medical Biological Bulletin. 2011; 14 (3-2): 251-4.(in Russian)].
- Shen Z., Li S., Sheng B., Shen Q., Sun L.Z., Zhu H., Zhu X. The role of atorvastatin in suppressing tumor growth of uterine fibroids. J. Transl. Med. 2018; 16(1): 53. https://dx.doi.org/10.1186/s12967-018-1430-x.
- Place D.E., Kanneganti T.-D. Cell death-mediated cytokine release and its therapeutic implications. J. Exp. Med. 2019; 216(7): 1474-86.https://dx.doi.org/10.1084/jem.20181892.
- Ulin M., Ali M., Chaudhry Z.T., Al-Hendy A., Yang Q. Uterine fibroids in menopause and perimenopause. Menopause. 2020; 27(2): 238-42. https://dx.doi.org/10.1097/GME.0000000000001438.
- Радзинский В.Е., Алтухова О.Б. Молекулярно-генетические детерминанты бесплодия при генитальном эндометриозе. Научные результаты биомедицинских исследований. 2018; 4(3): 28-37. [Radzinsky V.E.,Altukhova O.B. Molecular-genetic determinants of infertility in genital endometryosis. Research Results in Biomedicine. 2018; 4(3): 28-37.(in Russian)]. https://dx.doi.org/10.18413/2313-8955-2018-4-3-0-3.
- Watza D., Lusk C.M., Dyson G., Purrington K.S., Chen K., Wenzlaff A.S. et al. Prognostic modeling of the immune-centric transcriptome reveals interleukin signaling candidates contributing to differential patient outcomes. Carcinogenesis. 2018; 39(12): 1447-54. https://dx.doi.org/10.1093/carcin/bgy119.
- Алтухова О.Б., Радзинский В.Е., Полякова И.С., Чурносов М.И. Вовлеченность полиморфизма генов рецепторов эстрогенов и прогестерона в развитие миомы матки. Акушерство и гинекология. 2020; 3: 127-32. [Altukhova O.B., Radzinsky V.E., Polyakova I.S., Churnosov M.I. Involvement of estrogen and progesterone receptor gene polymorphisms in the development of uterine fibroids. Obstetrics and gynecology. 2020; 3:127-132. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.3.127-132.
- Vang Q., Mas A., Diamond M.P., Al-Hendy A. The mechanism and function of epigenetics in uterine leiomyoma development. Reprod. Sci. 2016; 23(2):163-75. https://dx.doi.org/10.1177/1933719115584449.
- Пономаренко И.В., Полоников А.В., Чурносов М.И. Полиморфные локусы гена LHCGR, ассоциированные с развитием миомы матки. Акушерство и гинекология. 2018;(10):86-91. [Ponomarenko I.V., Polonikov A.V., Churnosov M.I. Polymorphic loci of the LHCGR gene associated with the development of uterine fibroids. Obstetrics and gynecology. 2018;(10):86–91. https://dx.doi.org/10.18565/aig.2018.10.86-91.]
- Machado-Lopez A., Simón C., Mas A. Molecular and cellular insights into the development of uterine fibroids. Int. J. Mol Sci. 2021;22(16):8483.https://dx.doi.org/10.3390/ijms22168483.
- Пономаренко И.В., Полоников А.В., Чурносов М.И. Ассоциация полиморфизма rs4986938 гена ESR2 с развитием гиперплазии эндометрия. Акушерство и гинекология. 2019; 4: 66-72. [Ponomarenko I.V., Polonikov A.V., Churnosov M.I. Association of polymorphism rs4986938 of the ESR2 gene with the development of endometrial hyperplasia. Obstetrics and gynecology. 2019; 4: 66-72. (In Russian)]. https://dx.doi.org/10.18565/aig.2019.4.66-72.
- Пономаренко И.В., Чурносов М.И. Современные представления об этиопатогенезе и факторах риска лейомиомы матки. Акушерство и гинекология. 2018;8: 27-32. [Ponomarenko I.V., Churnosov M.I. Modern ideas about the etiopathogenesis and increased risk of uterine leiomyoma. Obstetrics and gynecology.2018;8:27-32.(in Russian)]. https://dx.doi.org/10.18565/aig.2018.8.27-32.
- McWilliams M.M., Chennathukuzhi V.M. Recent advances in uterine fibroid etiology. Semin. Reprod. Med. 2017; 35(2): 181-9. https://dx.doi.org/10.1055/s-0037-1599090.
- Konenkov V.I., Koroleva E.G., Orlov N.B., Prokof'ev V.F., Shevchenko A.V., Novikov A.M., Dergacheva T.I. Blood serum levels of proinflammatory cytokines (IL-1β, IL-6, TNFα, IL-8, IL-12p70, and IFNγ) in patients with uterine myoma. Bull. Exp. Biol. Med. 2018; 165(5): 698-701.https://dx.doi.org/10.1007/s10517-018-4245-0.
- Favorov A.V., Andreewski T.V., Sudomoina M.A., Favorova O.O., Parmigiani M.F. A Markov chain Monte Carlo technique for identification of combinations of allelic variants underlying complex diseases in humans. Genetics. 2005; 171(4): 2113-21. https://dx.doi.org/10.1534/genetics.105.048090.
- Che R., Jack J.R., Motsinger-Reif A.A., Brown C.C. An adaptive permutation approach for genome-wide association study: evaluation and recommendations for use. BioData. Min. 2014; 7: 9. https://dx.doi.org/10.1186/1756-0381-7-9.
- Baker K.J., Houston A., Brint E. IL-1 family members in cancer: two sides to every story. Front. Immunol. 2019; 10: 1197. https://dx.doi.org/10.3389/fimmu.2019.01197.
- Brodzikowska А., Górska R., Kowalski J. Interleukin-1 genotype in periodontitis. Arch. Immunol. Ther. Exp. (Warsz). 2019; 67(6): 367-73.https://dx.doi.org/10.1007/s00005-019-00555-4.
- Chen G., Hu C., Song Y., Zhang H., Li S., Lai P. et al. Effects of IL-4-590C/T (rs2243250) polymorphism on the susceptibility of smoking-related cancer: a meta-analysis involving 11,407 subjects. Biomed. Res. Int. 2019; 2019: 3104176. https://dx.doi.org/10.1155/2019/3104176.
- Celik M.Ö., Labuz D., Keye J., Glauben R., Machelska H. IL-4 induces M2 macrophages to produce sustained analgesia via opioids. JCI Insight. 2020; 5(4): e133093. https://dx.doi.org/10.1172/jci.insight.133093.
- Mestiri S., Zaaber I., Inoubli O., Abid N., Omrani A., Nejehi H., Marmouch H. Association of cytokine Th2 gene polymorphisms with autoimmune thyroid diseases in Tunisian population. Int. J. Immunogenet. 2020; 47(3): 294-308. https://dx.doi.org/10.1111/iji.12472.
- Gallegos-Arreola M.P., Zúñiga González G.M., Figuera L.E., Puebla Pérez A.M., Delgado Saucedo J.I. Association of the IL-10 gene rs1800872 (-592 C>A) polymorphism with breast cancer in a Mexican population. J. BUON. 2019; 24(6): 2369-76.
- Wang K., Jiao Z., Chen H., Liu X., Lu J. et al. The association between rs1800872 polymorphism in interleukin-10 and risk of cervical cancer: a meta-analysis. Medicine (Baltimore). 2022; 100(3): e23892. https://dx.doi.org/10.1097/MD.0000000000023892.
Received 28.04.2022
Accepted 08.07.2022
About the Authors
Oksana B. Altukhova, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology, Medical Institute, Belgorod State National Research University,+7(4722)30-13-83, kristalinka@yandex.ru, 308015, Russia, Belgorod, Victory str., 85.
Viktor E. Radzinsky, Dr. Med. Sci., Professor, Honored Scientist of the Russian Federation, Academician of the International Academy of Sciences of the Higher School,
Head Department of Obstetrics and Gynecology, Faculty of Medicine, RUDN University, +7(495)360-46-69, radzinskiy-ve@rudn.ru,
117198, Russia, Moscow, Miklukho-Maklaya str., 6.
Svetlana S. Sirotina, PhD (Bio), Associate Professor at the Department of Biomedical Disciplines, Belgorod State National Research University, +7(4722)30-13-83,
sirotina@bsu.edu.ru, https://orcid.org/0000-0002-4163-7863, 308015, Russia, Belgorod, Victory str., 85.
Mikhail I. Churnosov, Dr. Med. Sci., Professor, Head of the Department of Biomedical Disciplines of the Medical Institute, Belgorod State National Research University, +7(4722)30-13-83, churnosov@bsu.edu.ru, https://orcid.org/0000-0003-1254-6134, 308015, Russia, Belgorod, Victory str., 85.
Valentina S. Orlova, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology of the Medical Institute, Belgorod State National Research University, +7(4722)30-13-83, orlova@bsu.edu.ru, https://orcid.org/0000-0003-3882-9191, 308015, Russia, Belgorod, Pobedy str., 85.
Irina V. Batlutskaya, Dr. Med. Sci., Associate Professor, Head of the Department of Biotechnology and Microbiology, Belgorod State National Research University, +7(4722)30-13-83, bat@bsu.edu.ru, https://orcid.org/0000-0003-0068-6586, 308015, Russia, Belgorod, Pobedy str., 85.
Olga A. Efremova, Dr. Med. Sci., Associate Professor, Head of the Department of Faculty Therapy of the Medical Institute, Belgorod State National Research University, +7(4722)30-13-83, efremova@bsu.edu.ru, https://orcid.org/0000-0003-4967-2556, 308015, Russia, Belgorod, Pobedy str., 85.
Authors’ contributions: Churnosov M.I., Altukhova O.B., Radzinsky V.E. – developing the concept and design of the study; Altukhova O.B., Churnosov M.I., Batlutskaya I.V. – collecting and reviewing the material; Sirotina S.S., Efemova O.A. – writing the text; Churnosov M.I., Altukhova O.B., Orlova V.S. – editing the article.
Conflicts of interest: The authors declare that they have no competing interests.
Funding: The study was performed without external funding.
Ethical Approval: The study was approved by the Ethical Review Board of Belgorod National Research University, Belgorod, Russia (protocol No. 8 dated May 26, 2016).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Altukhova O.B., Radzinsky V.E., Sirotina S.S., Churnosov M.I., Efremova O.A., Batlutskaya I.V., Orlova V.S. Interleukin gene polymorphism and risk of uterine fibroids.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 7: 81-87 (in Russian)
https://dx.doi.org/10.18565/aig.2022.7.81-87



