The role of the folate cycle genes in development of uterine fibroids
Aim: To study association between polymorphic variants of the folate cycle genes and uterine fibroids.Altukhova O.B., Radzinsky V.E., Polyakova I.S., Sirotina S.S., Churnosov M.I.
Materials and methods: 977 women were examined. Among them, 188 women had uterine fibroids alone, and 789 were included in the control group. Five polymorphic loci of the folate cycle genes were selected for the study: МТRR c.66А>G rs1801394, SHМТ1 c.1420С>Т rs1979277, ТYМS c.*19С>Т rs699517, ТYМS c.*89А>G rs2790, МТR c.2756А>G rs180508. The study was carried out using Thermal Cycler CFX96 Real-Time System for polymerase chain reaction.
Results: Associations between molecular and genetic markers in genes SHМТ1 c.1420С>Т rs1979277, ТYМS c.*19С>Т rs699517, ТYМS c.*89А>G rs2790, МТR c.2756А>G rs180508 and formation of uterine fibroids alone were detected. The frequency of SHМТ1 rs1979277 C allele in patients with uterine fibroids was higher (70.92%) versus the control group (OR=1.30, р=0.04). Risk factor for development of uterine fibroids alone was combination of SHMT1 rs1979277 TT genotype and MTR rs180508 G allele (OR=0.29, р=0.01), as well as the combination of SHМТ1 rs1979277 СС genotype and of ТYМS rs699517 C allele (OR=1.41, р=0.02). The combination of SHMT1 rs1979277 TT genotype and TYMS rs2790 G allele was a protective factor for development of uterine fibroids alone (OR=0.26, р=0.03).
Conclusion: Polymorphic loci of the folate cycle genes SHМТ1 c.1420С>Т rs1979277, ТYМS c.*19С>Т rs699517, ТYМS c.*89А>G rs2790, МТR c.2756А>G rs180508 are associated with development of uterine fibroids alone.
Keywords
Uterine fibroids are neoplasms of uterine smooth muscle. The incidence rate of uterine fibroids in women of reproductive age is 25–30% [1, 2]. Management strategies for uterine fibroids mainly involve surgical interventions [3, 4], and this is associated with significant economic costs of health care for women of reproductive age.
Many studies are devoted to the mechanisms of development of proliferative diseases of the uterus [5, 6]. There are some published data on the genetic factors for development of this disease, but they have not yet been fully studied [7–9]. The folate cycle genes can be associated with the incidence of uterine fibroids. This may be due to reduced enzymatic activity of the folate cycle leading to impairment of folic acid metabolism and high level of homocysteine, which has an independent mutagenic and prooxidative effect [10, 11]. Moreover, in hyperhomocysteinemia, prostacyclin synthesis decreases and proliferation in vascular smooth muscle cells increases [12–14], that may increase the risk of uterine fibroids.
The aim of research was to study association between polymorphic variants of the folate cycle genes and uterine fibroids.
Materials and methods
The study included women with uterine fibroids alone (n=188) and women in the control group (n=789), who are Russian residents of the Central Black Earth Region of Russia and have no blood kinship.
The studied groups were formed in the Department of Gynecology of St. Joasaph Clinical Hospital in Belgorod Region of Russia. The inclusion criteria were the patients with uterine fibroids alone verified by ultrasound scan and hysteroscopy followed by histological examination of the obtained material. The control group included women, who had no proliferative diseases of the uterus and underwent prophylactic medical examination in the Diagnostic Department of the Perinatal Center. All participants of the study have signed informed consent for publication of their data. The study was carried out under control of the Ethics Committee of the Medical Institute of Belgorod State National Research University.
Genomic DNA isolation from the peripheral blood leukocytes was performed by standard procedure based on the phenol-chloroform extraction. Genotyping of 5 polymorphic loci of genes of the folate cycle was performed: МТRR c.66А>G rs1801394, SHМТ1 c.1420С>Т rs1979277, ТYМS c.*19С>Т rs699517, ТYМS c.*89А>G rs2790, МТR c.2756А>G rs180508. According to published data [15] these polymorphisms have a significant regulatory potential.
Analysis of genomic polymorphic loci of the folate cycle genes was performed by real-time polymerase chain reaction using synthesized oligonucleotide primers and probes (LLC Syntol, Russia) [16].
Statistical analysis
Distribution of alleles and genotypes in the 2 groups was evaluated using chi-square test (χ2 test) with 2×2 contingency tables and the Yates continuity correction. Odds ratio (OR) and 95% confidence interval (95% CI) were used for detection of associations between allelic variants and formation of uterine fibroids.
Software for Windows STATISTICA 10.0 was used for statistical data processing. Analysis of associations between the combination of alleles and genotypes of the studied polymorphic variants of genes and the incidence of uterine fibroids was carried out by АРSampler (https://sourceforge.net/projects/apsampler/) using Markov chain Monte Carlo and Bayesian nonparametric statistics [17, 18]. Permutation test (p-value=pperm) was used to validate the detected associations. Statistically significant p-value was pperm<0.05.
Evaluation of the effect of polymorphic variants on gene expression was performed in silico [19] using the Genotype-Tissue Expression (GTEx) portal data base (https://www.gtexportal.org/). The results with p<8×10-5, FDR ≤0.05 were included in the study. Canalization and relationship between the variants of polymorphic alleles and gene transcription was analyzed using coefficient β of the linear regression, which is indicative of normalized gene expression changes per one polymorphic (alternative) genetic variant. [20].
Regulatory effects of polymorphic loci were detected using web-based SNP selection tools SNPinfo (https:// npinfo.niehs.nih.gov/).
Results and discussion
Comparative analysis of allelic and genotypic frequencies in the studied groups is shown in Tables 1 and 2. Distribution of the frequencies of alleles and genotypes for all studied polymorphic loci was in conformity with Hardy-Weinberg equilibrium (Tables 1, 2).
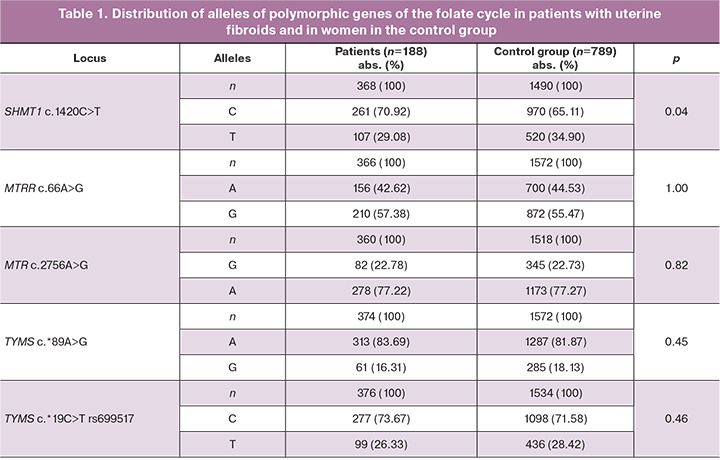
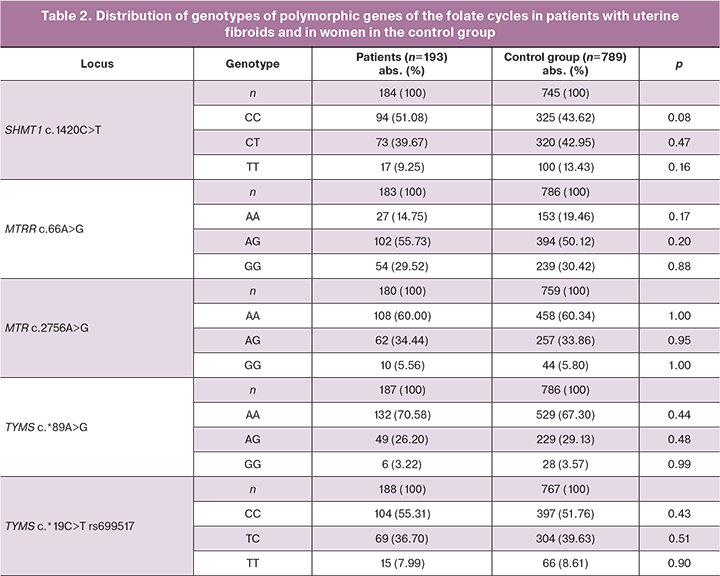
Analysis of distribution of alleles and genotypes of SHМТ1 c.1420С>Т rs1979277, МТRR c.66А>G rs1801394, МТR c.2756А>G rs180508, ТYМS c.*19С>Т rs699517, ТYМS c.*89А>G rs2790 showed differences in allelic frequency in locus SHМТ1 rs1979277 C among the patients with uterine fibroids (261/368, 70,92%) and women in the control group (970/1490, 65.11%, χ2=4.21, р=0.04, OR=1.30, 95% CI 1.01;1.69).
Statistically significant differences in other studied genes of the folate cycle were not detected in the two groups (p>0.05).
Analysis of allelic and genotypic combinations at the studied loci revealed low occurrence of the combination of the SHMT1 rs1979277 TT genotype and МТR rs180508 G allele among the patients with uterine fibroids (3/176, 1.71%) versus the control group (40/721, 5.54%, р=0.01 OR=0.29, 95% CI 0.09; 0.96).
It was found that among the patients with uterine fibroids, the frequency of combination of SHМТ1 rs1979277 СС genotype and ТYМS rs699517 С allele (90/184, 48.91%) was significantly high versus the control group (294/730, 40.27%, р=0.02, OR=1.41 95% CI 1.02; 1.96).
At the same time, the combination of SHMT1 rs1979277 TT genotype and ТYМS rs699517 С allele occurs 3.7 times less often in women with uterine fibroids (2/183, 1.09%) versus the control group (30/742, 4.04%) and is a protective factor for development of uterine fibroids alone (р=0.03, OR=0.26, 95% CI 0.06;1.10).
Three polymorphisms (p<8×10-5, FDR≤0.05) related to mRNA expression level (cis-eQTL) of four genes in different organs were detected using in silico the GTEx portal data base (http://www.gtexportal.org/).
It was found that MTRR gene polymorphic variant rs1801394 A was associated with low levels of MTRR expression in ovaries (β=-0.42, FDR≤0.05), thyroid gland (β =-0.13, FDR≤0.05), fibroblasts (β=0.26, FDR≤0.05), basal ganglia β=-0.47, FDR≤0.05) and subcutaneous adipose tissue (β=-0.16, FDR≤0.05).
TYMS gene polymorphic variant rs699517 Т was associated with a high level of genes expression: RP11-806L2.6 in fibroblasts (β=0.23, FDR≤0.05), thyroid gland (β=0.38, FDR≤0.05), adrenal glands (β=0.38, FDR≤0.05), ENOSF1 in fibroblasts (β=0.24, FDR≤0.05) and low level of gene transcription: ENOSF1 in skeletal muscles (β=-0.40, FDR≤0.05), and TYMS in ovaries (β=-0.28, FDR≤0.05).
ТYМS gene polymorphic variant rs2790 G was associated with a high level of genes expression: ENOSF1 in fibroblasts (β=0.29, FDR≤0.05) and adrenal glands (β=0.43, FDR≤0.05), RP11-806L2.6 in thyroid gland (β=0.40, FDR≤0.05) and low level of genes expression: ENOSF1 in skeletal muscles (β=-0.49, FDR≤0.05), thyroid gland (β=-0.90, FDR≤0.05), uterus (β=-0,51, FDR≤0,05), and ТYМS in ovaries (β=-0.29, FDR≤0.05).
Thus, GTEx portal data base indicate a significant effect of the studied polymorphisms on expression levels of MTRR, RP11-806L2.6, ENOSF1, TYMS genes in female reproductive organs (ovaries, etc.) and organs that regulate their activity (thyroid gland, adrenal glands, subcutaneous adipose tissue, etc.) and respectively are involved in the pathogenesis of uterine fibroids [21–24].
Polymorphic locus МТRR c.66А>G rs1801394 is located in the region of exon splicing enhancer (ESE) of the soluble cytokine receptor TNFα (spr55) (score=2.82). Regulatory potential (RegPotential) of locus MTRR rs1801394 equals to 0.25 in GTEx portal data base (SNPinfo https://manticore.niehs.nih.gov/). It should be noted that the value of the regulatory potential is indicative of the level at which the locus is involved in regulation of gene expression. We detected in silico that the value of the regulatory potential for locus rs1801394 was 0.25. This indicates its significant role in regulation of transcription activity of МТRR gene.
Polymorphic locus ТYМS c.*19С>Т rs699517 participates in synthesis of microRNA (miRNA) hsa-miR-151-3p (Score=144 points, Energy=-18.84), hsa-miR-28-5p (Score=165 points, Energy=-18.04), hsa-miR-338-5p (Score=156 points, Energy=-17.16), hsa-miR-498 (Score=141 points, Energy=-18.51), hsa-miR-548a-3p (Score=141 points, Energy=-15.15), hsa-miR-708 (Score=156 points, Energy=-17.16), hsa-miR-548e (Score=140 points, Energy=-12.36). The regulatory potential of the studied polymorphism ТYМS c.*19С>Т rs699517 was 0.12.
Polymorphic locus ТYМS c.*89А>G rs2790 participates in synthesis of miRNA hsa-miR-1248 (Score=151 points, Energy=-13.54), hsа-miR-192 (Scоre=140 points, Energy=-9.42), hsа-miR-215 (Scоre=140 points, Energy=-9.70), hsа-miR-515-3p (Scоre=143 points, Energy=-21.97). The regulatory potential of the studied polymorphism ТYМS c.*89А>G rs2790 was 0.20.
In the course of this study we found that the combination of polymorphic variants of the folate cycle genes are associated with formation of uterine fibroids. There are published data about association of МТR rs180508 G allele with a high homocysteine level [25]. In addition, polymorphic variants МТR c.2756А>G rs180508 and МТRR c.66А>G rs1801394 were found in women with rapidly growing uterine fibroids.
The data in literature confirm association of SHМТ1 c.1420С>Т (rs1979277) with development of uterine fibroids alone [26]. This polymorphic locus can affect the catalytic activity of the enzyme serine hydroxymethyltransferase, which is a cofactor for thymilate synthase and glycine [27]. This can lead to impaired biosynthesis of thymidylate and, as a consequence, cause pathological cell proliferation [28]. A number of studies have shown association between the SHMT1 c.1420C> T (rs1979277) polymorphism and development of intrauterine growth retardation [29], non-Hodgkin's malignant lymphomas [30], and rectal cancer [31].
Conclusion
Our study showed that the folate cycle genes: ТYМS c.*19С>Т rs699517, ТYМS c.*89А>G rs2790, SHМТ1 c.1420С>Т rs1979277, МТR c.2756А>G rs180508, МТRR c.66А>G rs1801394 play important role in formation of uterine fibroids alone. SHМТ1 rs1979277 C allele is a risk factor for development of uterine fibroids (OR=1.30). Also, a risk factor for development of uterine fibroids is the combination of SHМТ1 rs1979277 СС genotype and ТYМS rs699517 C allele (OR=1.41). However, the combination of the SHMT1 rs1979277 TT genotype and TYMS rs2790 G allele is a protective factor for development of uterine fobroids alone (OR=0.26).
References
- Адамян Л.В., ред. Cочетанные доброкачественные опухоли и гиперпластические процессы матки (миома, аденомиоз, гиперплазия эндометрия). Проект клинических рекомендаций по ведению больных. М.: Изд-во Научного центра акушерства, гинекологии и перинатологии им. В.И. Кулакова; 2015. 92с. [Adamyan L.V., ed. Combined benign tumors and hyperplastic processes of the uterus (fibroids, adenomyosis, endometrial hyperplasia). Draft clinical guidelines for the management of patients. Adamyan L.V., Andreeva E.N., Apolikhina I.A., Balan V.E., Bezhenar V.F., Gevorkyan M.A. and others. M.: Publishing house of the Scientific Center for Obstetrics, Gynecology and Perinatology them. V.I. Kulakov; 2015. 92p. (in Russian)].
- De La Cruz M.S., Buchanan E.M. Uterine fibroids: diagnosis and treatment. Am. Fam. Physician. 2017; 95(2): 100-17.
- Giuliani E., As-Sanie S., Marsh E.E. Epidemiology and management of uterine fibroids. Int. J. Gynaecol. Obstet. 2020; 149(1): 3-9. https://dx.doi.org/10.1002/ijgo.13102.
- Stewar E.A., Cookson C.L., Gandolfo R.A., Schulze-Rath R. Epidemiology of uterine fibroids: a systematic review. BJOG. 2017; 124(10): 1501-12. https://dx.doi.org/10.1111/1471-0528.14640.
- McWilliams M.M., Chennathukuzhi V.M. Recent advances in uterine fibroid Eeiology. Semin. Reprod. Med. 2017; 35(2): 181-9. https://dx.doi.org/10.1055/s-0037-1599090.
- Pavone D., Clemenza S., Sorbi F., Fambrini M., Petraglia F. Epidemiology and risk factors of uterine fibroids. Best Pract. Res. Clin. Obstet. Gynaecol. 2018; 46: 3-11. https://dx.doi.org/10.1016/j.bpobgyn.2017.09.004.
- Churnosov M.I., Altuchova O.B., Demakova N.A., Batlutskaya I.V., Polonikov A.V. Associations of cytokines genetic variants with myomatous knots sizes. Res. J. Pharm. Biol. Chem. Sci. 2014; 5(6):1344-7.
- Пономаренко И.В., Чурносов М.И. Современные представления об этиопатогенезе и факторах риска лейомиомы матки. Акушерство и гинекология. 2018; 8: 27-32. [Ponomarenko I.V., Churnosov M.I. Current views on the etiopathogenesis and risk factors of uterine leiomyoma. Akusherstvo I Ginekologiya/Obstetrics and Gynecology. 2018;8: 27-32. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.8.27-32.
- Пономаренко И.В., Полоников А.В., Чурносов М.И. Полиморфные локусы гена LHCGR ассоциированы с развитием миомы матки. Акушерство и гинекология. 2018; 10: 86-91. [Ponomarenko I.V., Polonikov A.V., Churnosov M.I. Polymorphic loci of the LHCGR gene are associated with the development of uterine leiomyma. Obstetrics and Gynecology/Akusherstvo i ginekologiya. 2018; (10): 86-91. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.10.86-91.
- Иванов А.М., Гильманов А.Ж., Малютина Н.Н., Ховаева Я.Б., Ненашева О.Ю., Элькин Г.И., Соснин Д.Ю. Полиморфизм генов фолатного цикла как фактор риска формирования гипергомоцистеинемии. Анализ риска здоровью. 2020; 4: 137-46. [Ivanov A.V., Gilmanov A.J., Malyutina N.N., Hovaeva Ya.B., Nenasheva O.Y., Elkin G.I., Sosnin D.Y. Polymorphism of folate cycle genes as a risk factor for the formation of hyperhomocysteinemia. Health risk analysis: 2020; 4: 137-46. (in Russian)].
- Мальцев Д.В. Клинический полиморфизм генетического дефицита энзимов цикла фолиевой кислоты. Український неврологічний журнал. 2016; 2: 7-16. [Maltsev D.V. Clinical polymorphism of genetic deficiency of enzymes of the folic acid cycle. Ukrainian Neurological Journal: 2016; 2:7-16.]
- Суховольская М.А., Субботина Т.Н. Концентрация гомоцистеина в сыворотке крови спортсменов-разрядников с мутациями в генах МТHFR и МТR. Гематология и трансфузиология. 2012; 57(Приложение 3): 81. [Sukhovolskaya M.A., Subbotina T.N. The concentration of homocysteine in the blood serum of athletes-dischargers with mutations in the MTHFR and MTR genes. Hematology and Transfusiology: 2012; 57(Suppl 3): 81. (in Russian)].
- Фетисова И.Н. Полиморфизм генов фолатного цикла и болезни человека. Вестник Ивановской медицинской академии. 2006; 11(1-2): 77-82. [Fetisova I.N. Polymorphism of folate cycle genes and human disease. Bulletin of the Ivanovo Medical Academy: 2006; 11 (1-2): 77-82. (in Russian)].
- Шмелева В.М. Гипергомоцистеинемия в патогенезе тромботических заболеваний. Трансфузиология. 2006; 1: 33-47. [Shmeleva V.M. Hyperhomocysteinemia in the pathogenesis of thrombotic diseases. Transfusiology. 2006; 1: 33-47. (in Russian)].
- Krivoshei I.V., Altuchova O.B., Golovchenko O.V., Polonikov A.V., Churnosov M.I. Genetic factors of hysteromyoma. Res. J. Med. Sci. 2015; 9(4): 182-5. https://dx.doi.org/10.3923/rjmsci.2015.182.185.
- Skibola C.F., Forrest C., Agana L., Hubbard A., Smith M.T., Bracci P.M., Holly E.A. Polymorphisms and haplotypes in folate-metabolizing genes and risk of non-Hodgkin lymphoma. Blood. 2004; 104(7): 2155-62. https://dx.doi.org/10.1182/blood-2004-02-0557.
- Favorov A.V., Andreewski T.V., Sudomoina M.A., Favorova O.O., Parmigiani M.F. A Markov chain Monte Carlo technique for identification of combinations of allelic variants underlying complex diseases in humans. Genetics. 2005; 171(4): 2113-21. https://dx.doi.org/10.1534/genetics.105.048090.
- Lvovs D., Favorova O.O., Favorov A.V. A polygenic approach to the study of polygenic diseases. Acta Nat. 2012; 4(3): 59-71.
- Пономаренко И.В., Решетников Е.А., Полоников А.В., Чурносов М.И. Полиморфный локус rs314276 гена LIN28B ассоциирован с возрастом менархе у женщин Центрального Черноземья России. Акушерство и гинекология. 2019; 2: 98-104. [Ponomarenko I.V., Reshetnikov E.A., Polonikov A.V., Churnosov M.I. The rs314276 polymorphic locus of the LIN28B gene is associated with the age of menarche in women in the Central Black Earth Region of Russia. Obstetrics and Gynecology/Akusherstvo i ginekologiya. 2019; 2: 98-104. (in Russian)]. https://dx.doi. org/10.18565/aig.2019.2.98-104.
- The GTEx Consortium. Genetic effects on gene expression across human tissues. Nature. 2017; 550: 204-13.
- Kim M.H., Park Y.R., Lim D.J., Yoon K.H., Kang M.I., Cha B.Y., Lee K.W., Son H.Y. The relationship between thyroid nodules and uterine fibroids. Endocr. J. 2010; 57(7): 615-21. https://dx.doi. org/10.1507/endocrj.k10e-024.
- Manta L., Suciu N., Toader O., Purcărea R.M., Constantin A., Popa F. The etiopathogenesis of uterine fibromatosis. J. Med. Life: 2016; 9(1): 39-45.
- Alsudairi H.N., Alrasheed A.T., Dvornyk V. Estrogens and uterine fibroids: an integrated view. Res. Results Biomed. 2021; 7(2): 156-63. https://dx.doi. org/10.18413/2658-6533-2021-7-2-0-6.
- Spinos N., Terzis G., Crysanthopoulou A., Adonakis G., Markou K.B., Vervita V. et al. Increased frequency of thyroid nodules and breast fibroadenomas in women with uterine fibroids. Thyroid. 2007; 17(12): 1257-9. https://dx.doi. org/10.1089/thy.2006.0330.
- Дюжев Ж.А. Полиморфизм генов фолатного обмена у женщин с лейомиомой матки. Мать и дитя в Кузбассе 2011; 1: 215-9. [Dyuzhev Zh.A. Polymorphism of folate metabolism genes in women with uterine leiomyoma. Mother and child in Kuzbass: 2011; 1: 215-9. (in Russian)].
- Niclot S. Implication of the folate-methionine metabolism pathways in susceptibility to follicular lymphomas. Blood: 2006;108(1): 278-85.
- Кох Н.В., Слепухина А.А., Лифшиц Г.И. Фолатный цикл: обзор и практические рекомендации по интерпретации генетических тестов. Медицинская генетика. 2015; 14(11): 3-8. [Kokh N.V., Slepukhina A.A., Lifshits G.I. The Folate Cycle: An Overview and Practical Guidelines for the Interpretation of Genetic Tests. Medical Genetics: 2015; 14(11): 3-8. (in Russian)].
- Ефремова О.А. Изучение ассоциации полиморфных локусов генов фолатного цикла с развитием синдрома задержки роста плода 2-3 степени. Научные результаты биомедицинских исследований. 2020; 6(1): 37-50. [Efremova O.A. Study of the association of polymorphic loci of folate cycle genes with the development of fetal growth retardation syndrome of 2-3 degrees. Research Results in Biomedicine: 2020; 6(1): 37-50 (in Russian)]. https://dx.doi. org/10.18413/2658-6533-2020-6-1-0-4.
- Березина О.В. Ассоциация полиморфных вариантов генов фолатного цикла с риском развития агрессивных и индолентных лимфом. Сибирский научный медицинский журнал. 2011; 31(2): 20-5. [Berezina O.V. Association of polymorphic variants of folate cycle genes with the risk of developing aggressive and indolent lymphomas. Siberian Scientific Medical Journal: 2011; 31(2): 20-5. (in Russian)].
- Березина О.В. Гены фолатного цикла и системы биотрансформации ксенобиотиков как маркеры предрасположенности к развитию неходжкинских злокачественных лимфом у жителей г. Новосибирска. Сибирский научный медицинский журнал. 2014; 34(2): 10-7. [Berezina O.V. Folate cycle genes and biotransformation systems of xenobiotics as markers of predisposition to the development of non-Hodgkin malignant lymphomas in residents of Novosibirsk. Siberian Scientific Medical Journal: 2014; 34(2): 10-7. (in Russian)].
- Komlósi V., Hitre E., Pap E., Adleff V., Réti A., Székely E. et al. SHМТ1 1420 and МТHFR 677 variants are associated with rectal but not colon cancer. BMC Cancer. 2010; 10: 525. https://dx.doi.org/10.1186/1471-2407-10-525.
Received 26.07.2021
Accepted 08.11.2021
About the Authors
Oksana B. Altukhova, Dr. Med. Sci., Associate Professor of the Department of Obstetrics and Gynecology, Medical Institute, Belgorod State National Research University,+7(4722)30-13-83, 308015, Russia, Belgorod, Victory str., 85.
Viktor E. Radzinsky, Dr. Med. Sci., Professor, Honored Scientist of the Russian Federation, Academician of the International Academy of Sciences of the Higher School,
Head of the Department of Obstetrics and Gynecology, Faculty of Medicine, RUDN University, +7(495)360-46-69, 117198, Russia, Moscow, Miklukho-Maklaya str., 6.
Irina S. Polyakova, PhD (Bio), Associate Professor of the Department of Biomedical Disciplines, Belgorod State National Research University, +7(4722)30-13-83, polyakovairina@bsu.edu.ru, https://orcid.org/0000-0002-0228-3513, 308015, Russia, Belgorod, Victory str., 85.
Svetlana S. Sirotina, PhD (Bio), Associate Professor of the Department of Biomedical Disciplines, Medical Institute, Belgorod State National Research University,
+7(4722)30-13-83, http://orcid.org/0000-0002-4163-7863, 308015, Russia, Belgorod, Victory str., 85.
Mikhail I. Churnosov, Dr. Med. Sci., Professor, Head of the Department of Biomedical Disciplines, Medical Institute, Belgorod State National Research University,
+7(4722)30-13-83, churnosov@bsu.edu.ru, http://orcid.org/0000-0003-1254-6134, 308015, Russia, Belgorod, Victory str., 85.
Corresponding author: Irina S. Polyakova, polyakovairina@bsu.edu.ru
Author’s contributions: Radzinsky V.E., Churnosov M.I., Altukhova O.B. – the concept and design of the study, editing the article; Altukhova O.B., Churnosov M.I., Sirotina S.S. – material collection and processing; Polyakova I.S. – writing the article.
Conflicts of interest: The authors declare that they have no conflicts of interest.
Funding: The study was carried out without any sponsorship.
Patients’ Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Altukhova O.B., Radzinsky V.E., Polyakova I.S., Sirotina S.S., Churnosov M.I. The role of the folate cycle genes in development of uterine fibroids.
Akusherstvo i Gynecologia/Obstetrics and Gynecology. 2021; 12: 96-101
https://dx.doi.org/10.18565/aig.2021.12.96-101



