The role of chemokine genes in the development of adenomyosis with concurrent endometrial hyperplastic processes
Relevance: Adenomyosis and endometrial hyperplastic processes (EHPs) are among the leading problems in gynecology. The causes and underlying mechanisms of the concurrent development of adenomyosis and EHPs are studied by various research teams. Hormonal and genetic disorders are known to be involved in the development of this pathology. Chemokines are involved in angiogenesis, hematopoiesis, embryological development, B- and T-cell development, dendritic cell maturation, inflammation, infection, tumor growth and metastasis, so they may be associated with the development of adenomyosis and its combination with EHPs. The role of intergenic chemokine gene interactions in the formation of this disease remains poorly understood.Altukhova O.B., Radzinsky V.E., Polyakova I.S., Sirotina S.S., Batlutskaya I.V., Orlova V.S., Efremova O.A., Churnosov M.I.
Objective: To investigate the relationship between chemokine genes and adenomyosis with concurrent EHPs.
Materials and methods: The study included 91 women with adenomyosis and concurrent EHPs and 789 control women. Genotyping of six chemokine genes SDF1 c.*519 G>А rs1801157, RАNТES c.-471 G>А rs2107538, I-ТАС c.*1539 Т>С rs4512021, МСР1 c.77-109 G>С rs2857657, МIР1β c.*524 А>Т rs1719153, IL-8 c.-352 А>Т rs4073 was performed by real-time PCR. The odds ratio (OR) and the 95% confidence interval (CI) were used to evaluate associations. ARSampler software was used to assess the association of combinations of alleles and genotypes of the analyzed genes with the development of adenomyosis with concurrent EHPs.
Results: Associations of the studied genes with the development of the disease were revealed. Patients with adenomyosis and concurrent EHPs were more likely to have the T allele of the rs4073 IL-8 gene (86/142, 60.56%) than controls (379/744, 50.94%; p=0.04, OR=1.48 95% CI 1.02–2.40). The prevalence of the rs1801157 SDF A allele (38/152, 25.0%) was higher compared with controls (126/730, 17.26%; p=0.03, OR=1.59 95% CI 1.06–2.40). The combination of TT genotype rs4073 IL-8 with A allele rs1801157 SDF1, A allele rs4512021 I-TAC, and G allele rs2857657 MSR1 (OR=4.55, 95% CI 2.10–9.86, p=0.0002) was a risk factor for disease. The combination of the A allele rs4073 IL-8 with the G allele rs2107538 RANTES and the T allele rs1719153 MIR1β is protective against the development of adenomyosis with concurrent EHPs (OR=0.36 95% CI 0.18–0.73, p=0.002).
Conclusion: Polymorphic chemokine gene loci SDF1 c.*519 G>A rs1801157, RANTES c.-471 G>A rs2107538, I-TAS c.*1539 T>C rs4512021, MSR1 c.77-109 G>C rs2857657, MIR1β c.*524 A>T rs1719153, IL-8 c.-352 A>T rs4073 are associated with the development of adenomyosis with concurrent EHPs.
Keywords
Adenomyosis and endometrial hyperplastic processes (EHPs) are one of the leading problems in gynecology. They affect women's reproductive health, reduce their quality of life, and affect their ability to work at all ages [1–4]. Adenomyosis occurs in 7-12% and EHPs in 10– 15% of reproductive-aged women [5]. The number of patients with a combination of these diseases is increasing. This problem is a significant medical and social issue as adenomyosis and its combination with EHPs account for 12 to 50% of operations in gynecological hospitals [6–8].
The causes and mechanisms of the concurrent development of adenomyosis and EHPs are studied by various research teams [9– 12]. Hormonal and genetic disorders are known to be involved in the development of this pathology [11, 13, 14]. There is a growing number of studies aimed at determining the influence of genetic factors in the formation of this pathology [15, 16]. Among the candidate genes for uterine hyperplastic diseases, chemokine genes are of great importance. Chemokines are a family of small proteins involved in many biological processes, including the ability to act as chemotactic mediators by transmitting signals through G-protein coupled receptors. They are involved in angiogenesis, hematopoiesis, embryological development, B- and T-cell development, dendritic cell maturation, inflammation, infection, tumor growth, and metastasis [17, 18], so they can be associated with the development of adenomyosis and its combination with EHPs. The role of intergenic chemokine gene interactions in the formation of this disease remains poorly understood.
This study aimed to investigate the relationship between chemokine genes and adenomyosis with concurrent EHPs.
Materials and methods
The study was conducted at the Department of Gynecology of Belgorod Regional Clinical Hospital of St. Joasaph and included 91 women diagnosed with adenomyosis in combination with EHPs and 789 women without reproductive tract diseases. All subjects were native Russian residents of the Central Federal District of Russia [19]. The mean age of the patients in the study group [40.89 (9.81) years] was comparable with that in the control group [37.89 (7.76) years (Mann–Whitney U-test, p>0.05).
Criteria for inclusion in the group of patients were the diagnosis of adenomyosis in combination with EHPs, confirmed echographically, hysteroscopically and morphologically. The study was approved by the Research Ethics Committee of the BelSU Medical Institute. Before starting the study, all women signed an informed consent for diagnostic and therapeutic and manipulative procedures. Peripheral blood samples were drawn into EDTA-containing tubes and subjected to DNA using phenol chloroform.
Six single nucleotide polymorphisms of chemokine genes were selected for analysis including chemokine ligand-5 (RANTES rs2107538), interferon-γ-inducible T-cell chemoattractant alpha (I-TAC rs4512021), macrophage inflammatory protein-1 (MP1β rs1719153), interleukin-8 (IL-8 rs4073), monocyte chemoattractant protein-1 (MCP1 rs2857657), stromal cell factor-1 (SDF1 rs1801157). Candidate genes selected for the study have important medical and biological implications in humans and may be involved in the pathogenesis of adenomyosis combined with endometrial hyperplasia. Molecular genetic loci were analyzed by real-time polymerase chain reaction using oligonucleotide primers and probes (Syntol LLC, Russia).
Statistical analysis
To compare allele frequencies and genotypes between the study and control groups, we used 2×2 contingency tables and the χ2 test with Yates correction for continuity. The empirical distribution of genotype frequencies in the patient and control groups compared to the theoretically expected was determined according to the Hardy-Weinberg equilibrium with Bonferroni correction for multiple comparisons according to the number of studied polymorphisms (n=6); pbonf<0.05:6=0.009 was taken as statistically significant.
Odds ratio (OR) and its 95% confidence interval (95% CI) were used to identify associations of the studied genes with the formation of adenomyosis and EHPs. Statistical analysis was performed using STATISTICA for Windows 10.0 software.
Combinations of chemokine alleles and genotypes associated with the development of the disease were searched using APSampler software (https://sourceforge.net/projects/apsampler/) using the Monte Carlo– Markov chain method (MCMC) and Bayesian statistical methods [20, 21]. Permutation analysis (pperm) was used to validate the associations found in multiple comparisons. Differences were considered statistically significant at pperm<0.05.
Using the GTExportal database (https://www.gtexportal.org/), we evaluated the effect of the studied polymorphic variants on the level of gene transcriptional activity [22]. For this purpose, we used the results of regression analysis presented in the GTExportal online database [23]. The association of alleles with the level of gene expression was evaluated using the linear regression coefficient (β), which determines the change in the normalized gene expression index per polymorphic (alternative) genetic variant [24]. To minimize the errors associated with false positives, we used data with p<8×10-5 and False Discovery Rate (FDR) ≤0.05. The regulatory effects of the genes studied were evaluated using data from the SNPinfo program (https://npinfo.niehs.nih.gov/).
Results and discussion
The clinical and demographic characteristics and comorbidities of the patients in the study groups are shown in Table 1.
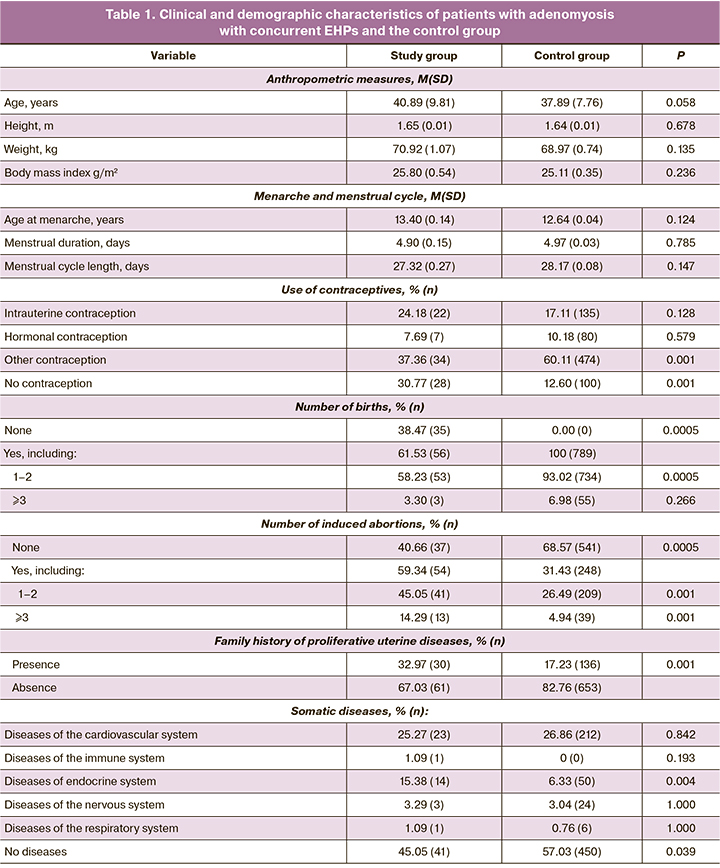
The study and control groups did not differ in the main biomedical characteristics, such as age (p=0.058), height (p=0.678), weight (p=0.135), body mass index (p=0.236), age at menarche (p=0.124), menstrual duration (p=0.785), and menstrual cycle length (p=0.147). At the same time, patients in the study group were more likely to have a family history of hereditary disorders (p=0.001), history of abortion (p=0.0005), endocrine diseases (p=0.004), fewer births (p=0.0005) and fewer women without comorbidities (p=0.039).
The allele and genotype frequencies of the chemokines in the study group and controls are presented in Tables 2 and 3. It should be noted that for the polymorphic locus rs4073 of the IL-8 gene, a higher level of observed heterozygosity was recorded in the control group than expected, according to the Hardy–Weinberg equilibrium (H0=0.546, HE=0.500, PHWE=0.012). At the rs2857657 locus of the MSR1 gene, a lower level of observed heterozygosity was recorded in the control group than expected (H0=0.275, HE=0.300, PHWE=0.015), but when the Bonferroni correction, which accounts for multiple comparisons based on the number of polymorphisms studied (n=6, pbonf<0.009), these differences were not significant and the polymorphic loci tested passed the HWE test. The distributions of the genotype frequencies at the remaining loci were consistent with the Hardy–Weinberg equilibrium (Tables 2, 3).
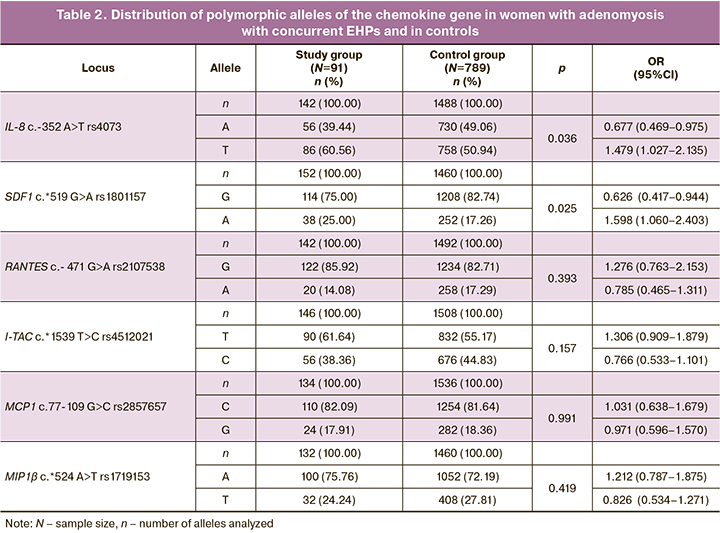
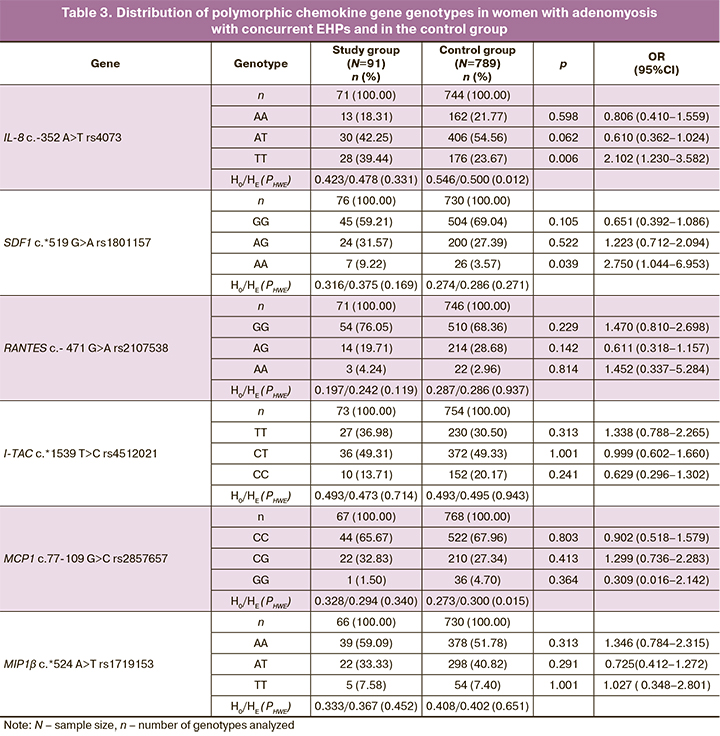
The comparative analysis revealed that the T rs4073 IL-8 allele was more common in the study group (86/142, 60.56%) than in the control group (379/744, 50.94%, χ2=3.32, p=0.04, OR=1.479 95% CI 1.027–2.135). The TT rs4073 IL-8 genotype was 1.7 times more common (28/71, 39.44%) in the study group compared to the control group (88/372, 23.67%, χ2=5.74, p=0.01, OR=2.102 95% CI 1.230–3.582). A higher frequency of the rs1801157 SDF A allele (38/152, 25.00%) was detected in women with adenomyosis with concurrent EHPs compared with controls (126/730, 17.26%, χ2=4.47, p=0.03, OR=1.598, 95%CI 1.060–2.403).
Combinations of alleles and genotypes important in the development of the disease were identified using the ARSampler program (https://sourceforge.net/projects/apsampler/). The combination of alleles of A rs1801157 SDF1, A rs4512021 I-TAC and G rs2857657 MCP1 alleles with the TT rs4073 IL-8 genotype occurred significantly more frequently (22.95%) than in the control group (6.13%, p=0.0002, pperm=0.001, OR=4.55 95%CI 2.10–9.86). This combination is a risk factor for the concurrent development of adenomyosis and EHPs. The combination of the A rs4073 IL-8, G rs2107538 RANTES, and T rs1719153 MIR1β alleles is a protective factor for the development of the disease. The prevalence of this combination in the group of patients with endometriosis with concurrent EHPs is 18.33%, in the control group – 37.85% (p=0.002, OR=0.36 95% CI 0.18–0.73).
We identified four chemokine genes affecting mRNA expression of eight genes in different organs (http://www.gtexportal.org/). The T allele rs2107538 of the RANTES gene was found to be associated with increased expression of the C17ORF50 gene in the basal ganglia of the brain (β=0.82, FDR≤0.05), pituitary gland (β=0.39, FDR≤0.05), hippocampus (β=0.63, FDR≤0.05), thyroid (β=0.28, FDR≤0.05), adrenal gland (β=0.46, FDR≤0.05), and with low levels of RDM1 gene transcription in the basal ganglia of the brain (β=-0.41, FDR≤0.05), hypothalamus (β=-0.51, FDR≤0.05), AC015849 gene 16 in the basal ganglia of the brain (β=-0.41, FDR≤0.05). The T allele of rs4073 of the IL-8 gene reduces the expression level of the CXCL6 gene in subcutaneous adipose tissue (β=-0.13, FDR≤0.05).
The G allele of the rs4512021 I-TAC gene is associated with low levels of expression of the NAAA gene in the ovaries (β=-0.72, FDR≤0.05), uterus (β=-0.62, FDR≤0.05), vagina (β=-0.61, FDR≤0.05), thyroid (β=-0.34, FDR≤0.05), fibroblasts (β=-0.85, FDR≤0.05), skeletal muscle (β=-0.67, FDR≤0, 05), mammary gland (β=-0.50, FDR≤0.05), adrenal gland (β=-0.59, FDR≤0.05), and with high expression of the ART3 gene (β=0.78, FDR≤0.05) in the pituitary gland, NUP54 gene in thyroid (β=0.24, FDR≤0.05), uterus (β=0.46, FDR≤0.05), skeletal muscle (β=0.18, FDR≤0.05), and pituitary (β=0.44, FDR≤0.05). The polymorphic variant of the rs1719153 MIP1β gene was associated with high levels of CCL4 gene expression in the mammary gland (β=0.30, FDR≤0.05), skeletal muscle (β=0.26, FDR≤0.05), thyroid gland (β=0.24, FDR≤0.05).
The data of GTExportal database indicate a significant influence of the studied polymorphisms on the expression level of CCL4, ART3, CXCL6, C17ORF50, RDM1, AC015849.16, NUP54, NAAA in organs of the female reproductive tract (uterus, vagina, ovaries) and in organs directly involved in the regulation of their activity (thyroid gland, adrenal glands, subcutaneous fatty tissue, skeletal musculature) and involved in the pathogenesis of adenomyosis with concurrent EHPs, respectively.
The polymorphic SDF1 locus c. *519 G>A rs1801157 is involved in the synthesis of hsa-miR-149 (Score=153, Energy=-21.42), hsa-miR-1827 (Score=140, Energy=-14.97), miR-298 (Score=149, Energy=-28.51), miR-631 (Score=152, Energy=-21.69), miR-650 (Score=146, Energy=-25.03), miR-892b (Score=158, Energy=-24.45), miR-940 (Score=153, Energy=-29.24). In the SNPinfo database, the regulatory potential (RegPotential) of the rs1801157 locus of the SDF1 gene is 0.43 (SNPinfo https://manticore.niehs.nih.gov/). It should be noted that the indicator of regulatory potential characterizes the degree of participation of the locus in the regulation of gene expression. The in silico value of the regulatory potential for the rs1801157 locus, which is equal to 0.43, indicates its significant role in the regulation of the transcriptional activity of the SDF1 gene.
The C allele of the RANTES c.-471 G>A gene rs2107538 is a binding site for transcription factors ETS_Q6 (Core Match Score=0.86), GATA4_Q3 (Core Match Score=0.68), GRE_C (Core Match Score=0.71), HAND1E47_01 (Core Match Score=0.86), PAX4_04 (Core Match Score=0.86), etc. The G allele of the MSR1 c.77-109 G>C gene rs2857657 serves as a binding site for transcription factors AR_Q2 (Core Match Score=0.75), GATA_C (Core Match Score=0.60), GRE_C (Core Match Score=0.76), PAX4_04 (Core Match Score=0.86), HNF3B_01 (Core Match Score=0.93). Polymorphic T variant of IL-8 gene rs4073 - transcription factor binding site CDPCR3_01 (Core Match Score=0.74), CDP_02 (Core Match Score=0.68), EBOX_Q6_01 (Core Match Score=0.86), FXR_Q3 (Core Match Score=0.90).
Our findings demonstrate the important role of chemokines in the body [25]. IL-8 chemokine initiates directed migration of neutrophils and is able to trigger angiogenesis processes by increasing the proliferation of endothelial and muscle cells. IL-8 is a growth factor for endometrial cells [26].
According to the available literature, high concentrations of RANTES are observed in the peritoneal fluid of patients with adenomyosis. The RANTES gene exhibits important regulatory activity toward angiogenesis during several pathophysiological processes. RANTES is released by many cell types, such as platelets or smooth muscle cells. This chemokine interacts with GPCR (G-protein coupled receptor) and GAG (glycosaminoglycan) chains associated with HSPG, and participates in angiogenesis by increasing endometrial cell proliferation and differentiation and apoptosis, supporting the development of the vascular blood supply at the inflammation site [27].
The I-TAC gene, according to the literature, is a risk factor for endometriosis, having a stimulating effect on endometrial stromal cells [28]. MSR-1 plays an important role in the processes of tissue infiltration by monocytes, and also serves as a marker of the activity of inflammatory reactions. Analysis of the MSR-1 chemokine concentration in peritoneal fluid in patients with endometriosis shows a significant increase in comparison with the control group [29]. The SDF1 gene enhances endometrial angiogenesis and increases cell survival, inhibits apoptosis, and increases pathological endometrial proliferation [30].
Conclusion
This study findings suggest a significant role of molecular genetic markers SDF1, c.*519 G>A rs1801157, RANTES c.-471 G>A rs2107538, I-TAC c.*1539 T>C rs4512021, MCP1 c.77-109 G>C rs2857657, MIP1β c.*524 A>T rs1719153, IL-8 c.-352 A>T rs4073 in the formation of adenomyosis with concurrent EHPs. The T allele of the rs4073 IL-8 gene (p=0.04, OR=1.49 95% CI 1.027–2.135) and the A allele of rs1801157 SDF (p=0.03, OR=1.598, 95%CI 1.060–2.403) are risk factors for disease. Moreover, the combination of the TT rs4073 IL-8 genotype with the A rs1801157 SDF1, A rs4512021 I-TAC, and G rs2857657 MSP1 alleles was a risk factor for disease (OR=4.55 95% CI 2.10–9.86, p=0.0002). The combination of the A allele rs4073 IL-8 with the G allele rs2107538 RANTES and the T allele rs1719153 MIP1β is protective for the development of this pathology (OR=0.361 95% CI 0.181–0.730, p=0.002).
Our findings can be used to identify risk groups for the development of adenomyosis with concurrent EHPs and to take preventive measures.
References
- Адамян Л.В., Гарданова Ж.Р., Яроцкая Е.Л., Овакимян А.С., Козаченко И.Ф. Особенности болевого синдрома, психоэмоционального состояния и качества жизни женщин с наружным генитальным эндометриозом. Проблемы репродукции. 2016; 22(3): 77-83. [Adamyan L.V., Gardanova Zh.R., Iarotskaia E.L., Ovakimian A.S., Kozachenko I.Ph. The characteristics of pain syndrome and the women’s psycho-emotional status as well as life quality in women with external genital endometriosis. Russian Journal of Human Reproduction. 2016; 22(3): 77 83. (in Russian)]. https://doi.org/10.17116/repro201622377-83.
- Churnosov M.I., Altuchova O.B., Demakova N.A., Krivoshei I.V., Evdokimov V.I., Batlutskaya I.V., Polonikov A.V. Associations of cytokines genetic variants with myomatous knots sizes. Res. J. Pharm. Biolog. Chem. Sci. 2014; 5(6): 1344-7.
- Толибова Г.Х., Траль Т.Г., Кахиани М.И., Коган И.Ю. Гиперплазия эндометрия у женщин с наружным генитальным эндометриозом и миомой матки. Российский вестник акушера-гинеколога. 2020; 20(6): 90-5. [Tolibova G.Kh., Tral T.G., Kakhiani M.I., Kogan I.Yu. Endometrial hyperplasia in women with external genital endometriosis and uterine myoma. Russian Bulletin of Obstetrician-Gynecologist. 2020; 20(6): 90 5. (in Russian)].https://doi.org/10.17116/rosakush20202006190.
- Saunders P.T.K., Horne A.W. Endometriosis: etiology, pathobiology, and therapeutic prospects. Cell. 2021; 184(11): 2807-24. https://dx.doi.org/10.1016/j.cell.2021.04.041.
- Zondervan K.T., Becker C.M., Koga K., Missmer S.A., Taylor R.N., Viganò P. Endometriosis. Nat. Rev. Dis. Primers. 2018; 4(1): 9. https://dx.doi.org/10.1038/s41572-018-0008-5.
- Gante I., Мedeiros-Borges С., Águas F. Hysterectomies in Рortugal (2000–2014): what has changed? Eur. J. Obstet. Gynecol. Reprod. Biol. 2017; 208: 97-102. https://dx.doi.org/10.1016/j.ejogrb.2016.11.021.
- Abrao M.S., Andres M.P., Miller C.E., Gingold J.A., Rius M., Neto J.F. et al. AAGL 2021 endometriosis classification: an anatomy-based surgical complexity score. J. Minim. Invasive Gynecol. 2021; 28(11): 1941-1950.e1. https://dx.doi.org/10.1016/j.jmig.2021.09.709.
- Пучков К.В., Попов А.А., Федоров А.А., Федотова И.С. Эндометриоз-ассоциированные злокачественные опухоли, связанные с глубоким инфильтративным эндометриозом: обзор литературы и клинические наблюдения. Российский вестник акушера-гинеколога. 2019; 19(4): 42-6. [Puchkov K.V., Popov A.A., Fedorov A.A., Fedotova I.S. Endometriosis-associated malignant tumors associated with deep infiltrative endometriosis: review of the literature and clinical observations. Russian Bulletin of Obstetrician-Gynecologist. 2019; 19(4): 42-6. (in Russian)]. https://dx.doi.org/10.17116/rosakush20191904142.
- Габидуллина Р.И., Смирнова Г.А., Нухбала Ф.Р., Валеева Е.В., Орлова Ю.И., Шакиров А.А. Гиперпластические процессы эндометрия: современная тактика ведения пациенток. Consilium Medicum. 2019; 21(6): 53-8. [Gabidullina R.I., Smirnova G.A., Nuhbala F.R., Valeeva E.V., Orlova Yu.I., Shakirov A.A. Hyperplastic processes of the endometrium: modern tactics of patient management. Consilium Medicum. 2019; 21(6): 53-8. (in Russian)]. https://doi.org/10.26442/20795696.2019.6.190472.
- Krivoshei I.V., Altuchova O.B., Polonikov A.V., Churnosov M.I. Bioinformatic analysis of the liability to the hyperplastic processes of the uterus. Res. J. Pharm. Biolog. Chem. Sci. 2015; 6(5): 1563-6.
- Asghari S., Valizadeh A., Aghebati-Maleki L., Nouri M., Yousefi M. Endometriosis: perspective, lights, and shadows of etiology. Biomed. Pharmacother. 2018; 106: 163-74. https://doi.org/10.1016/j.biopha.2018.06.109.
- Пономаренко И.В., Полоников А.В., Чурносов М.И. Молекулярные механизмы и факторы риска развития эндометриоза. Акушерство и гинекология. 2019; 3: 26-31. [Ponomarenko I.V., Polonikov A.V., Churnosov M.I. Molecular mechanisms of and risk factors for endometriosis. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; 3: 26-31. (in Russian)].https://dx.doi.org/10.18565/aig.2019.3.26-31.
- Szymanowski K., Мikołajczyk М., Wirstlein Р. Мatrix metalloproteinase-2 (ММР-2), ММР-9, tissue inhibitor of matrix metalloproteinases (ТIМР-1) and transforming growth factor-β2 (ТGF-β2) expression in eutopic endometrium of women with peritoneal endometriosis. Аnn. Аgric. Environ. Мed. 2016; 23(4): 649-53. https://dx.doi.org/10.5604/12321966.1226861.
- Пономаренко И.В., Полоников А.В., Чурносов М.И. Ассоциация полиморфизма rs4986938 гена ESR2 с развитием гиперплазии эндометрия. Акушерство и гинекология. 2019; 4: 66-72. [Ponomarenko I.V., Polonikov A.V., Churnosov M.I. Association of ESR2 RS4986938 polymorphism with the development of endometrial hyperplasia. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; 4: 66-72. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.4.66-72.
- Krivoshei I.V., Altuchova O.B., Golovchenko O.V., Orlova V.S., Polonikov A.V., Churnosov M.I. Genetic factors of hysteromyoma. Res. J. Med. Sci. 2015; 9(4): 182-5. https://dx.doi.org/10.3923/rjmsci.2015.182.185.
- Пономаренко И.В., Полоников А.В., Чурносов М.И. Гиперпластические процессы эндометрия: этиопатогенез, факторы риска, полиморфизм генов-кандидатов. Акушерство и гинекология. 2019; 1: 13-8. [Ponomarenko I.V., Polonikov A.V., Churnosov M.I. Endometrial hyperplastic processes: etiopathogenesis, risk factors, polymorphism of candidate genes. Akusherstvo I Ginekologiya/Obstetrics and Gynecology. 2019; 1: 13-8. (in Russian)].https://dx.doi.org/10.18565/aig.2019.1.13-18.
- Радзинский В.Е., Алтухова О.Б. Молекулярно-генетические детерминанты бесплодия при генитальном эндометриозе. Научные результаты биомедицинских исследований. 2018; 4(3): 28-37. [Radzinsky V.E., Altuchova O.B. Molecular-genetic determinants of infertility in genital endometryosis. Research Results in Biomedicine. 2018; 4(3): 28-37 (in Russian)]. https://dx.doi.org/10.18413/2313-8955-2018-4-3-0-3.
- Miller M.C., Mayo K.H. Chemokines from a structural perspective. Int. J. Mol. Sci. 2017; 18(10): 2088. https://dx.doi.org/10.3390/ijms18102088.
- Полоников А.В., Клёсова Е.Ю., Азарова Ю.Э. Биоинформатические инструменты и интернет-ресурсы для оценки регуляторного потенциала полиморфных локусов, установленных полногеномными ассоциативными исследованиями мультифакториальных заболеваний (обзор). Научные результаты биомедицинских исследований. 2021; 7(1): 15-31. [Polonikov A.V., Klyosova E.Yu., Azarova I.E. Bioinformatic tools and internet resources for functional annotation of polymorphic loci detected by genome wide association studies of multifactorial diseases (review). Research Results in Biomedicine. 2021;7(1):15-31. (in Russian)]. https://dx.doi.org/10.18413/2658-6533-2020-7-1-0-2.
- Favorov A.V., Andreewski T.V., Sudomoina M.A., Favorova O.O., Parmigiani M.F., Ochs M.F. A Markov chain Monte Carlo technique for identification of combinations of allelic variants underlying complex diseases in humans. Genetics. 2005; 171(4): 2113-21. https://dx.doi.org/10.1534/genetics.105.048090.
- Lvovs D., Favorova O.O., Favorov A.V. A polygenic approach to the study of polygenic diseases. Acta Naturae. 2012; 4(3): 59-71.
- Battle A., Brown C.D., Engelhardt B.E., Montgomery S.B.; GTEx Consortium. Genetic effects on gene expression across human tissues. Nature. 2017; 550(7675): 204-13. https://dx.doi.org/10.1038/nature24277.
- Пономаренко И.В., Решетников Е.А., Полоников А.В., Чурносов М.И. Полиморфный локус rs314276 гена LIN28B ассоциирован с возрастом менархе у женщин Центрального Черноземья России. Акушерство и гинекология. 2019; 2: 98-104. [Ponomarenko I.V., Reshetnikov Е.А., Polonikov A.V., Churnosov M.I. The polymorphic locus RS314276 of the LIN28B gene is associated with the age of menarche in women of the central black earth region of Russia. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; 2: 98-104 (in Russian)]. https://dx.doi.org/10.18565/aig.2019.2.98-104.
- Пономаренко И.В., Полоников А.В., Верзилина И.Н., Чурносов М.И. Молекулярно-генетические детерминанты развития эндометриоза. Вопросы гинекологии, акушерства и перинатологии. 2019; 18(1): 82-6. [Ponomarenko I.V., Polonikov A.V., Verzilina I.N., Churnosov M.I. Molecular-genetic determinants of the development of endometriosis. Voprosy Ginekologii, Akusherstva i Perinatologii/Issues of Gynecology, Obstetrics and Perinatology. 2019; 18(1): 82-6. (in Russian)]. https://dx.doi.org/10.20953/1726-1678-2019-1-82-86.
- Nasibullin T.R., Yagafarova L.F., Yagafarov I.R., Timasheva Y.R., Erdman V.V., Tuktarova I.A., Mustafina O.E. Combinations of polymorphic markers of chemokine genes, their receptors and acute phase protein genes as potential predictors of coronary heart diseases. Acta Naturae. 2016; 8(1): 111-6.
- Rabbanizadeh F., Kohan L., Najib F. Аssociation between Interleukin-8-251Т/А polymorphism and endometriosis in Iranian women. Res. Мol. Мed. 2015; 3(2): 45-9. https://dx.doi.org/10.7508/rmm.2015.02.007.
- Корытина Г.Ф., Ахмадишина Л.З., Кочетова О.В., Азнабаева Ю.Г., Загидуллин Ш.З., Викторова Т.В. Роль генов сывороточного амилоидного белка А1, молекул адгезии, хемокинов и их рецепторов в развитии хронической обструктивной болезни легких. Генетика. 2019; 55(1): 100-9. [Korytina G.F., Akhmadishina L.Z., Kochetova O.V., Viktorova T.V., Aznabaeva Y.G., Zagidullin Sh.Z., Viktorova T.V. The role of serum amyloid A1, adhesion molecules, chemokines, and chemokine receptors genes in chronic obstructive pulmonary disease. Russian Journal of Genetics. 2019; 55(1):100-9. (in Russian)]. https://dx.doi.org/10.1134/S0016675818120056.
- Hirota Y., Osuga Y., Koga K., Yoshino O., Hirata T., Morimoto C. et al. The expression and possible roles of chemokine CXCL11 and its receptor CXCR3 in the human endometrium. J. Immunol. 2006; 177(12): 8813-21.https://dx.doi.org/10.4049/jimmunol.177.12.8813.
- Heidari S., Kolahdouz-Mohammadi R., Khodaverdi S., Tajik N., Delbandi A.A. Expression levels of MCP-1, HGF, and IGF-1 in endometriotic patients compared with non-endometriotic controls. BMC Womens Health. 2021; 21(1): 422. https://dx.doi.org/10.1186/s12905-021-01560-6.
- Liu Y., Ren C.C., Yang L., Xu Y.M., Chen Y.N. Role of CXCL12-CXCR4 axis in ovarian cancer metastasis and CXCL12-CXCR4 blockade with AMD3100 suppresses tumor cell migration and invasion in vitro. J. Cell. Physiol. 2019; 234(4): 3897-909. https://dx.doi.org/10.1002/jcp.27163.
Received 29.06.2022
Accepted 12.07.2022
About the Authors
Oksana B. Altukhova, Dr. Med. Sci., Associate Professor at the Department of Obstetrics and Gynecology, Medical Institute, Belgorod State National Research University, +7(4722)30-13-83, 308015, Russia, Belgorod, Pobedy str., 85.Viktor E. Radzinsky, Dr. Med. Sci., Professor, Merited Scholar of the Russian Federation, Academician of the International Academy of Sciences of the Higher School,
Head of the Department of Obstetrics and Gynecology, Faculty of Medicine, RUDN University, +7(495)360-46-69, 117198, Russia, Moscow, Miklukho-Maklaya str., 6.
Irina S. Polyakova, PhD (Bio), Associate Professor at the Department of Biomedical Disciplines, Belgorod State National Research University, +7(4722)30-13-83, polyakovairina@bsu.edu.ru, https://orcid.org/0000-0002-0228-3513, 308015, Russia, Belgorod, Pobedy str., 85.
Mikhail I. Churnosov, Dr. Med. Sci., Professor, Head of the Department of Biomedical Disciplines of the Medical Institute, Belgorod State National Research University, +7(4722)30-13-83, churnosov@bsu.edu.ru, https://orcid.org/0000-0003-1254-6134, 308015, Russia, Belgorod, Pobedy str., 85.
Valentina S. Orlova, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology of the Medical Institute, Belgorod State National Research University, +7(4722)30-13-83, orlova@bsu.edu.ru, https://orcid.org/0000-0003-3882-9191, 308015, Russia, Belgorod, Pobedy str., 85.
Irina V. Batlutskaya, Dr. Bio. Sci., Associate Professor, Head of the Department of Biotechnology and Microbiology, Belgorod State National Research University,
+7(4722)30-13-83, bat@bsu.edu.ru, https://orcid.org/0000-0003-0068-6586, 308015, Russia, Belgorod, Pobedy str., 85.
Olga A. Efremova, Dr. Med. Sci., Associate Professor, Head of the Department of Faculty Therapy of the Medical Institute, Belgorod State National Research University, +7(4722)30-13-83, efremova@bsu.edu.ru, https://orcid.org/0000-0003-4967-2556, 308015, Russia, Belgorod, Pobedy str., 85.
Svetlana S. Sirotina, PhD (Bio), Associate Professor at the Department of Biomedical Disciplines, Belgorod State National Research University, +7(4722)30-13-83,
sirotina@bsu.edu.ru, https://orcid.org/0000-0002-4163-7863, 308015, Russia, Belgorod, Pobedy str., 85.
Corresponding author: Irina S. Polyakova, polyakovairina@bsu.edu.ru
Authors' contributions: Radzinsky V.E., Churnosov M.I., Altukhova O.B. – conception and design of the study; Altukhova O.B., Churnosov M.I., Batlutskaya I.V., Orlova V.S., Sirotina S.S. – data collection and processing; Polyakova I.S. – manuscript drafting; Radzinsky V.E., Churnosov M.I., Efremova O.A. – manuscript editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was approved by the Research Ethics Committee of the Medical Institute of BelSU.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Altukhova O.B., Radzinsky V.E., Polyakova I.S., Sirotina S.S., Batlutskaya I.V., Orlova V.S., Efremova O.A., Churnosov M.I. The role of chemokine genes in the development
of adenomyosis with concurrent endometrial hyperplastic processes.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 8: 76-84 (in Russian)
https://dx.doi.org/10.18565/aig.2022.8.76-84



