Balance of serum cytokines regulating inflammatory activity in patients with leiomyoma
Konenkov V.I., Shevchenko A.V., Koroleva E.G., Prokofiev V.F., Timofeeva Yu.S., Aidagulova S.V., Marinkin I.O.
Objective: To analyze the concentrations and balance of serum cytokines that activate (IL-1β, IL-6) and inhibit (IL-4, IL-10) inflammation in healthy individuals and in patients with leiomyoma in general, with and without endometrial lesions.
Materials and methods: The study included 109 women aged 23 to 61 years with uterine fibroids and 92 women of similar age (22–61 years) without uterine fibroids. All women had general clinical, standard instrumental and laboratory examinations. The concentrations of pro-inflammatory and anti-inflammatory cytokines were determined by enzyme-linked immunosorbent assay (ELISA) for ELx800 Plates (BioTek, Taiwan) using Vector-Best (Russia) kits. Descriptive statistical analysis and nonparametric statistical methods were used.
Results: The course of the tumor process in uterine fibroids is accompanied by a fivefold increase in pro-inflammatory cytokines IL-1β and IL-6 in the serum of female patients in comparison with healthy individuals (p<0.001); the concentrations of cytokines with anti-inflammatory activity, namely IL-4, are not so remarkable, but they are also higher (4.09(2.36-6.94) versus 2.42(0.97-5.86) in healthy individuals, p<0.001). Significant increase in IL-6 concentration in all patients with uterine fibroids (8.93(4.95-14.11), p<0.001) was found to be maximal among women with endometrial pathology (9.67(5.22-14.60), p<0.05).
Conclusion: Proliferation of tumor myocytes and development of microcirculatory channel components in patients with uterine fibroids are accompanied by the prevalence of pro-inflammatory activity of the cytokine network and a shift in the balance of its functional state towards dysregulation of control over the processes of myometrial remodeling.
Authors’ contributions: Konenkov V.I., Marinkin I.O. – developing the concept and design of the study; Koroleva E.G., Timofeeva Yu.S. – collecting and processing the primary material; Timofeeva Yu.S., Aidagulova S.V. – analysis of the literature data; Shevchenko A.V. – immunoenzyme analysis; Prokofiev V.F. – statistical processing and data analysis; Konenkov V.I., Aidagulova S.V., Marinkin I.O. – writing and editing the text.
Conflicts of interest: The authors declare no possible conflicts of interest.
Funding: The research was carried out within the framework of the State Assignment of Research Institute of Clinical and Experimental Lymphology, Institute of Cytology and Genetics, Siberian Branch of Russian Academy of Sciences, topic No. 1021060908897-3 and the State Assignment of the Novosibirsk State Medical University, for Research and Development No. 121021700349-8.
Ethical Approval: The study was approved by the Ethical Review Board of the Research Institute of Clinical and Experimental Lymphology, Institute of Cytology and Genetics, Siberian Branch of Russian Academy of Sciences (protocol No. 115 dated by 24.12.2015) and by the Ethical Review Board of the Novosibirsk State Medical University, Ministry of Health of Russia (protocol No. 107 dated by 31.05.2018).
Patient Consent for Publication: The patients provided an informed consent for the participation in the study and publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Konenkov V.I., Shevchenko A.V., Koroleva E.G., Prokofiev V.F., Timofeeva Yu.S., Aidagulova S.V., Marinkin I.O. Balance of serum cytokines regulating inflammatory activity in patients with leiomyoma.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (6): 84-90 (in Russian)
https://dx.doi.org/10.18565/aig.2024.74
Keywords
Leiomyoma, also known as uterine fibroids, is a benign monoclonal tumor arising from smooth muscle cells of the cervix or body of the uterus. It is one of the most common benign tumors of the female genital tract, which occurs in 20-40% of reproductive-aged women [1].
The most important aspect about uterine fibroid etiology is that the initiator of tumor growth remains unknown, although theories of tumorogenesis exist. One of them confirms that increased levels of estrogen and progesterone lead to increased mitotic activity of myocytes with myoma nodule formation and raise the likelihood of somatic mutations. Another hypothesis suggests the presence of congenital genetically determined pathology of myometrium in women with uterine fibroids, expressed in the increased number of estrogen receptors in myometrium [1].
There is also another concept, namely dysregulation of control over the processes of proliferation, differentiation, cell division and related processes of neoangiogenesis, vasculogenesis, inflammation activity, remodeling of extracellular matrix and fibrogenesis [2–4].
All of these processes are regulated by a superfamily of regulatory biomolecules, including cytokines, chemokines, and growth factors. These molecules form a complex regulatory network with autocrine and paracrine connections. Additionally, the intensity of cytokine production is under genetic control [5–7].
In a previous study, we analyzed the concentrations of biomolecules belonging to this group of 27 regulatory factors in the blood serum of a small group of patients with leiomyoma [8–11]. This analysis was conducted using the Bio-Rad kit (USA), namely Bio-Plex Pro by flow fluorimetry. The results indicated a number of statistically significant deviations in the serum concentrations of regulatory factors in the patients’ blood from those observed in women without uterine fibroids. This led to the question of how to interpret the obtained data in relation to the variants of the course of the disease.
The aim of the study was to carry out a comparative complex analysis of the concentrations and balance of serum cytokines that activate (IL-1β, IL-6) and inhibit (IL-4, IL-10) inflammation in healthy individuals and in patients with leiomyoma in general, with and without endometrial lesions.
Materials and methods
Patient Group Description
The patients were grouped on the basis of the diagnostic criteria formulated in the clinical recommendations «Uterine fibroids», which were approved by the Scientific and Practical Council of the Ministry of Health of the Russian Federation in 2020 [1]. According to these regulations, «the diagnosis of uterine fibroids is made due to the complaints, anamnestic data, physical examination, ultrasound and MRI of the pelvic organs (if necessary)».
The clinical study included 109 patients aged 23 to 61 years with the diagnosis of uterine fibroids (ICD-10 D25), all of them underwent surgical treatment. This is the main group of subjects, that will be referred to hereafter as «patients»; the characteristics of the group are described in Table 1.
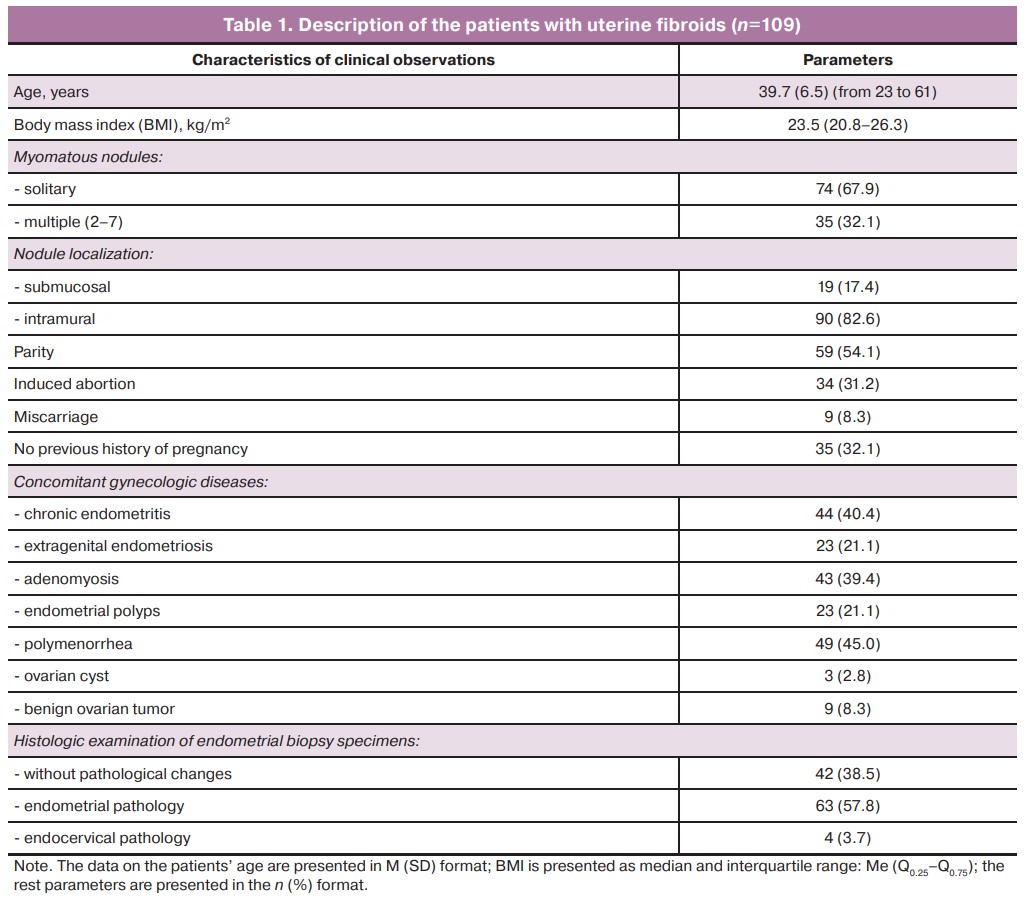
The minimum diameter of myomatous nodule was 12 mm (0.7%) and the maximum diameter reached 300 mm. The diameter of solitary myomatous nodules ranged from 12 to 300 mm, and the diameter of multiple myomatous nodules varied from 20 to 100 mm. The most common sizes of the nodules were 40–60 and 80 mm and accounted for up to 50.8% of all observed nodule size variants.
The most frequent pathologic processes detected by hysteroscopy were chronic endometritis, endometrial polyps, intrauterine synechiae, endocervical hyperplasia and endometrial hyperplasia without atypia.
During three months prior to myomectomy, 78/109 women (71.6%) did not use hormonal drugs, while the rest of the patients 31/109 (28.4%) took them to manage pain and menorrhagia. The patients were more often prescribed gonadotropin-releasing hormone agonists – 9.3%, the levonorgestrel-releasing intrauterine system (Mirena) – 3.6%, antigestagens – 2.1%, estradiol and dydrogesterone (Femoston) – 1.4%. The rest of the patients (12.1%) chose combined oral contraceptives by themselves, which had no effect on uterine fibroid progression.
The control group consisted of 92 women comparable in age (22–61 years) with the group of patients without leiomyoma and other gynecologic diseases.
Methods for determining cytokines
The concentrations of proinflammatory and anti-inflammatory cytokines were determined by enzyme-linked immunosorbent assay (ELISA) for ELx800 Plates (BioTek, Taiwan) using standardized kits, namely IL-1β, IL-4, IL-6, IL-10 produced by Vector-Best JSC (Russia) according to the manufacturer’s protocol. The concentration of the cytokines was expressed in pg/ml.
Statistical analysis
The results were processed and statistically analyzed using IBM SPSS Statistics 23. The normality of the data distribution was determined using the Shapiro–Wilk W-test. Mean (M) and standard deviation (SD) values were calculated for indicators measured on interval scales, with normal distribution, and presented in M (SD) format. The median (Me) and interquartile range, quartiles Q1 and Q3 (Q0.25–Q0.75), were calculated for indicators with improper law of Gaussian distribution, such as BMI and concentrations of cytokines IL-1β, IL-4, IL-6, IL-10. The Mann-Whitney U test was used to compare quantitative indices in independent groups. The correlation analysis was performed by calculating Spearman’s rank correlation coefficient (rs). The differences were considered statistically significant (α-error) at p<0.05.
Results
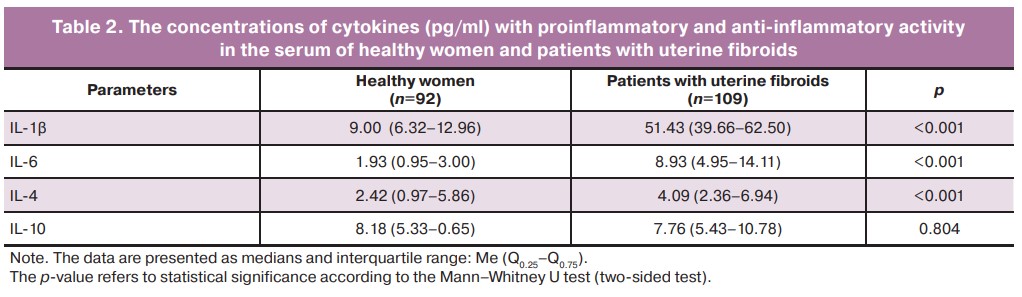
A significant excess of IL-1β concentrations in the serum of patients with uterine fibroids in comparison with healthy women was detected using ELISA. Thus, the content of this cytokine in the serum of the patients amounted to 51.43 (39.66–62.50) pg/ml, which significantly exceeds the control values (almost 6 times) obtained in the study of blood sera of healthy women, both in our studies (p<0.001) and in the studies of other laboratories using the same test systems of the Vector-Best company [12, 13]. When the blood sera of healthy women were examined, the normal values for this cytokine were 9.00 (6.32–12.96) pg/ml (Table 2).
The clinical and immunologic analysis of the whole group of patients with uterine fibroids revealed the dependence of the level of IL-1β concentration in serum on the condition of the endometrium which was determined by the results of preoperative hysteroscopy. The group of patients with pathologic changes included women with the confirmed signs of chronic endometritis, endometrial polyps and endometrial hyperplasia without atypia (Table 3).
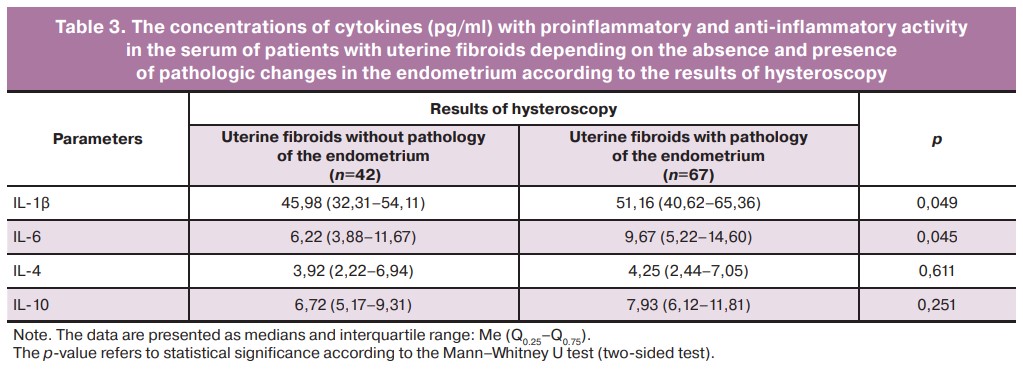
The results presented in Table 3 demonstrate that higher values of IL-1β concentration are detected in the group of women with different variants of endometrial pathology (p=0.049). This is clearly associated with the development of an inflammatory process in endometrial pathologic conditions. Although it should be noted that there is a significant increase in the concentration of this cytokine with proinflammatory activity in patients with uncomplicated uterine fibroids; this concentration probably characterizes the increased proliferative activity of the myometrium itself.
The presented data also show that changes in serum concentrations of another cytokine with proinflammatory activity, IL-6, follow the same patterns. The concentration of this regulatory factor in the whole group of patients with leiomyoma (p<0.001) is increased to the maximum among the patients with endometrial pathology. Serum IL-6 concentration in patients with uterine fibroids is more than four times greater than its content in the serum of healthy women; this suggests an increase in the proinflammatory activity of both cytokines in patients with uterine fibroids (Table 2).
Simultaneous changes in the concentrations of both proinflammatory cytokines in serum are also confirmed by the results of correlation analysis, which shows a significant positive relationship between the concentrations of IL-1β and IL-6, both in healthy women (rs=0.58, p<0.01) and, especially, in patients with uterine fibroids (rs=0.63, p<0.01).
Table 2 shows that the absolute content of cytokines with anti-inflammatory activity differs from the normative values to a lesser extent. Serum IL-4 concentration in patients with uterine fibroids is 4.09 (2.36–6.94) pg/ml, which is only slightly, although statistically significantly, higher than the values in the serum of women without tumor processes in the uterus. These data are also confirmed by the results of the studies conducted in other national laboratories that use chemicals produced by the Vector-Best company [13, 14]. The only exception is serum IL-10 concentration in healthy women, which amounted to 8.18 (5.33–10.65) pg/ml and slightly exceeded the normative values published in the work of Prof. A.S. Simbirtsev [13]. However, it should be noted that our laboratory findings were obtained on significantly larger samples of examined sera from both healthy women and patients with uterine fibroids.
It is worth noting that there were no differences in the concentrations of IL-4 and IL-10 as representatives of the anti-inflammatory part of the cytokine network regardless of the presence and absence of pathologic changes in the endometrium. This indicates that proinflammatory activity is more prevalent than anti-inflammatory activity in patients with uterine fibroids.
The correlation analysis also revealed a direct relationship between the concentrations of both cytokines with anti-inflammatory activity in healthy women (rs=0.70, p<0.01) and in patients with uterine fibroids (rs=0.42, p<0.01).
Discussion
The analysis of our own data in comparison with the data obtained in the national laboratories using the chemicals produced by the same Russian manufacturer allows us to state that the course of benign process in the uterus is accompanied by a significant excess (5.2-fold) of proinflammatory cytokines IL-1β and IL-6 in the blood serum of the patients with uterine fibroids and by a less marked increase in the concentrations (1.3-fold) of cytokines with obvious anti-inflammatory activity IL-4 and IL-10.
The increased levels of proinflammatory cytokines result in a variety of tissue effects. In particular, IL-1β increases the expression level of a number of cytokines, chemokines and growth factors, activates matrix metalloproteinases and adhesion molecules, increases leukocyte infiltration of tissues, platelet activation, changes in blood flow, initiates neoangiogenesis, etc. [15].
IL-6 also has a dramatic pleiotropic effect on muscle tissue remodeling by initiating the acute-phase response and hematopoietic processes, predominantly in an autocrine or paracrine manner. When IL-6 activates target genes, it does not only serve as a differentiation and growth factor for hematopoietic cells, B cells, T cells, osteoclasts, and endothelial cells, but it also plays an important role in the growth, differentiation, regeneration, and degradation of peripheral and endothelial cells. IL-6 activates and recruits neutrophils and monocytes, stimulates vascular endothelial cells to secrete adhesion molecules and other inflammatory factors, and enhances the local inflammatory response [16]. Systemic and local increase in IL-6 concentration induced by tissue hypoxia leads to a pre-thrombotic state, which can induce the production of platelet growth factor, fibroblast growth factor, tumor necrosis factor (TNF)-α, macrophage colony-stimulating factor, and promote smooth muscle cell proliferation [17].
Increased IL-6 levels, in turn, stimulate the production of anti-inflammatory cytokines such as IL-1ra (interleukin-1 receptor antagonist) and IL-10, and inhibit the production of the proinflammatory cytokine TNF-α. Decreased TNF-α expression may promote muscle cell proliferation by inhibiting the extrinsic pathway of apoptosis [18].
Our results show that the level of cytokines IL-4 and IL-10 in the blood of patients with uterine fibroids is increased, though less significant. IL-10 is generally considered as an anti-inflammatory cytokine whose level increases in the late phase of inflammation and contributes to the resolution of inflammation [19].
Anti-inflammatory immune responses promote muscle repair and conversely, type 1 inflammatory response causes muscle damage and is counter-regulated by anti-inflammatory cytokines such as IL-4 and IL-10. IL-4 is an anti-inflammatory cytokine that can induce type 2 immune response, which can subsequently promote angiogenesis, repair and remodeling of muscle tissue [20]. The obtained data indicate the necessity of a systematic approach to the analysis of interdependent changes in the cytokine network that is under polygenic control during the development of benign tumors of the reproductive organs.
Conclusion
Proliferation of tumor myocytes and development of microcirculatory channel components of a new extensive blood and lymphatic vascular network in patients with uterine fibroids are accompanied by the predominance of proinflammatory activity of the cytokine network and a shift in the balance of its functional state towards dysregulation of control over the processes of myometrial remodeling. The current level of development of immunology, molecular biology and genetics makes it possible to use the available data for a deeper analysis of the pathogenetic role of the revealed disorders of the normal functioning of the cytokine network for the development of prognostic algorithms and therapeutic schemes for the correction of these disorders even before the development of pathology.
References
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Миома матки. 2020. [Ministry of Health of the Russian Federation. Clinical guidelines. Uterine fibroids. 2020. (in Russian)].
- Dudley A.C., Griffioen A.W. Pathological angiogenesis: mechanisms and therapeutic strategies. Angiogenesis. 2023; 26(3): 313-47. https://dx.doi.org/10.1007/s10456-023-09876-7.
- Mlodawska O.W., Saini P., Parker J.B., Wei J.J., Bulun S.E., Simon M.A. et al. Epigenomic and enhancer dysregulation in uterine leiomyomas. Hum. Reprod. Update. 2022; 28(4): 518-47. https://dx.doi.org/10.1093/humupd/dmac008
- Koltsova A.S., Efimova O.A., Pendina A.A. A view on uterine leiomyoma genesis through the prism of genetic, epigenetic and cellular heterogeneity. Int. J. Mol. Sci. 2023; 24(6): 5752. https://dx.doi.org/10.3390/ijms24065752.
- Liu C., Chu D., Kalantar-Zadeh K., George J., Young H.A., Liu G. Cytokines: from clinical significance to quantification. Adv. Sci. (Weinh). 2021; 8(15): e2004433. https://dx.doi.org/10.1002/advs.202004433.
- Fajgenbaum D.C., June C.H. Cytokine storm. N. Engl. J. Med. 2020; 383(23): 2255-73. https://dx.doi.org/10.1056/NEJMra2026131.
- Weyand C.M., Watanabe R., Zhang H., Akiyama M., Berry G.J., Goronzy J.J. Cytokines, growth factors and proteases in medium and large vessel vasculitis. Clin. Immunol. 2019; 206: 33-41. https://dx.doi.org/10.1016/j.clim.2019.02.007.
- Коненков В.И., Королева Е.Г., Орлов Н.Б., Прокофьев В.Ф., Шевченко А.В., Новиков А.М., Дергачева Т.И. Уровни провоспалительных цитокинов (IL-1β, IL-6, TNFα, IL-8, IL-12p70, IFNγ) в сыворотке крови с миомой матки. Бюллетень экспериментальной биологии и медицины. 2018; 165(5): 648-52. [Konenkov V.I., Koroleva E.G., Orlov N.B., Prokof'ev V.F., Shevchenko A.V., Novikov A.M., Dergacheva T.I. Blood serum levels of proinflammatory cytokines (IL-1β, IL-6, TNFα, IL-8, IL-12p70, and IFNγ) in patients with uterine myoma. Bulletin of Experimental Biology and Medicine. 2018; 165(5): 648-52. (in Russian)].
- Коненков В.И., Королева Е.Г., Орлов Н.Б., Прокофьев В.Ф., Шевченко А.В., Новиков А.М., Дергачева Т.И., Останин А.А. Противовоспалительная активность цитокинов сыворотки крови (IL-4, IL-10, IL-13) и природного антагониста рецептора IL-1β (IL-1ra) у женщин с миомой матки. Акушерство и гинекология. 2018; 10: 80-5. [Konenkov V.I., Koroleva E.G., Orlov N.B., Prokof'ev V.F., Shevchenko A.V., Novikov A.M., Dergacheva T.I., Ostanin A.A. Anti-inflammatory activity of serum cytokines (IL-4, IL-10, IL-13) and the natural IL-1β receptor antagonist (IL-1RA) in women with uterine myoma. Obstetrics and Gynecology. 2018; (10): 80-5. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.10.80-85.
- Коненков В.И., Королева Е.Г., Орлов Н.Б., Прокофьев В.Ф. Шевченко А.В. Новиков А.М. Сывороточные уровни факторов роста гемопоэза и ангиогенеза (IL-5, IL-7, IL-9, FGF-β, G-CSF, VEGF и PDGF) у женщин с миомой матки. Медицинская иммунология. 2018; 20(5): 691-8. [Konenkov V.I., Koroleva E.G., Orlov N.B., Prokofiev V.F., Shevchenko A.V., Novikov A.M. Serum levels of hemopoietic and angiogenesis growth factors (IL-5, IL-7, IL-9, FGF-β, G-CSF, VEGF and PDGF) in women with uterine myoma. Medical Immunology. 2018; 20(5): 691-8. (in Russian)]. https://dx.doi.org/10.15789/1563-0625-2018-5-691-698.
- Коненков В.И., Королева Е.Г., Орлов Н.Б., Прокофьев В.Ф., Шевченко А.В., Новиков А.М., Дергачева Т.И. Изменения концентраций CCL-хемокинов (MCP-1, MIP-1α, MIP-1β, RANTES и EOTAXIN) в сыворотке крови женщин с миомой матки. Акушерство и гинекология. 2019; 8: 107-11. [Konenkov V.I., Koroleva E.G., Orlov N.B., Prokofev V.F., Shevchenko A.V., Novikov A.M., Dergacheva T.I. Changes in the serum concentrations of CCL chemokines (MCP-1, MIP-1A, MIP-1Β, RANTES and EOTAXIN) in women with uterine myoma. Obstetrics and Gynecology. 2019; (8): 107-11. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.8.107-111.
- Хворостухина Н.Ф., Островская А.Е., Новичков Д.А., Степанова Н.Н. Цитокиновый профиль при осложнениях гормонотерапии миомы матки. Медицинская иммунология. 2017; 19(6): 739-48. [Khvorostukhina N.F., Ostrovskaya A.E., Novichkov D.A., Stepanova N.N. Cytokine profile in complications of hormone therapy administered for uterine fibroids. Medical Immunology. 2017; 19(6): 739-48. (in Russian)]. https://dx.doi.org/10.15789/1563-0625-2017-6-739-748.
- Стагниева И.В., Симбирцев А.С. Эффективность иммуномодулирующей терапии у больных риносинуситом. Медицинская иммунология. 2015; 17(5): 423‑30. [Stagnieva I.V., Simbirtsev A.S. Immunotherapy efficiency in rhinosinusitis patients. Medical Immunology. 2015; 17(5): 423-30. (in Russian)]. https://dx.doi.org/10.15789/1563‑0625‑2015‑5‑423‑430.
- Зорина В.Н., Исакова О.В., Зорина Р.М., Баженова Л.Г., Зорин Н.А. Концентрации иммунорегуляторных белков и некоторых цитокинов в крови женщин при приеме менопаузальной терапии. Медицинская иммунология. 2016; 18(2): 177‑82. [Zorina V.N., Isakova O.V., Zorina R.M., Bazhenova L.G., Zorin N.A.Concentrations of immunoregulatory proteins and some cytokines in blood of women during menopausaltherapy. Medical Immunology. 2016; 18(2): 177-82. (in Russian)]. https://dx.doi.org/10.15789/1563‑0625‑2016‑2‑177‑182.
- Zhu H., Hu S., Li Y., Sun Y., Xiong X., Hu X. et al. Interleukins and ischemic stroke. Front. Immunol. 2022; 13: 828447. https://dx.doi.org/10.3389/fimmu.2022.828447.
- Uyama N., Tsutsui H., Wu S., Yasuda K., Hatano E., Qin X.Y. et al. Anti-interleukin-6 receptor antibody treatment ameliorates postoperative adhesion formation. Sci. Rep. 2019; 9(1): 17558. https://dx.doi.org/10.1038/s41598-019-54175-1.
- Zimmermann M., Aguilera F.B., Castellucci M., Rossato M., Costa S., Lunardi C. et al. Chromatin remodelling and autocrine TNFα are required for optimal interleukin-6 expression in activated human neutrophils. Nat. Commun. 2015; 6: 6061. https://dx.doi.org/10.1038/ncomms7061.
- Ayari S., Abellard A., Carayol M., Guedj É., Gavarry O. A systematic review of exercise modalities that reduce pro-inflammatory cytokines in humans and animals' models with mild cognitive impairment or dementia. Exp. Gerontol. 2023; 175: 112141. https://dx.doi.org/10.1016/j.exger.2023.112141.
- Liberale L., Ministrini S., Carbone F., Camici G.G., Montecucco F. Cytokines as therapeutic targets for cardio- and cerebrovascular diseases. Basic Res. Cardiol. 2021; 116(1): 23. https://dx.doi.org/10.1007/s00395-021-00863-x.
- Raimondo T.M., Mooney D.J. Anti-inflammatory nanoparticles significantly improve muscle function in a murine model of advanced muscular dystrophy. Sci. Adv. 2021; 7(26): eabh3693. https://dx.doi.org/10.1126/sciadv.abh3693.
Received 29.03.2024
Accepted 13.06.2024
About the Authors
Vladimir I. Konenkov, Dr. Med. Sci., Professor, Academician of the RAS, Head of the Clinical Immunogenetics Laboratory, Research Institute of Clinical and Experimental Lymрhology – Branch of the Institute of Cytology and Genetics, Siberian Branch of Russian Academy of Sciences, 2 Timakova str., Novosibirsk, 630060, Russia,+7(383)333-64-09, vikonenkov@gmail.com, https://orcid.org/0000-0001-7385-6270
Alla V. Shevchenko, Dr. Biol. Sci., Leading Researcher at the Clinical Immunogenetics Laboratory, Research Institute of Clinical and Experimental Lymрhology – Branch of the Institute of Cytology and Genetics, Siberian Branch of Russian Academy of Sciences, 13 Frunze str./15 Michurina str., Novosibirsk, 630091, Russia, +7(383) 373-96-01,
https://orcid.org/0000-0001-5898-950X
Elena G. Koroleva, gynecologist, Junior Researcher, Laboratory of Cellular Biology and Fundamental Basis of Reproduction of Central Scientific Laboratory, Novosibirsk State Medical University, 52 Krasny prospect str., Novosibirsk, 630091, Russia, +7(383)226-35-60, korlex71@mail.ru, https://orcid.org/0000-0002-8522-4382
Viktor F. Prokofiev, PhD, Leading Researcher at the Clinical Immunogenetics Laboratory, Research Institute of Clinical and Experimental Lymрhology – Branch of the Institute of Cytology and Genetics, Siberian Branch of Russian Academy of Sciences, 13 Frunze str./15 Michurina str., Novosibirsk, 630091, Russia, +7(383) 373-96-01,
vf_prok@mail.ru, https://orcid.org/0000-0001-7290-1631
Yulia S. Timofeeva, PhD, Assistant at the Obstetrics and Gynecology Department, Novosibirsk State Medical University, 52 Krasny prospect str., Novosibirsk,
630091, Russia, +7(961)220-31-13, dr.j.timofeeva@yandex.ru, https://orcid.org/0000-0002-5379-9296
Svetlana V. Aidagulova, Dr. Biol. Sci., Professor, Head of the Laboratory of Cellular Biology and Fundamental Basis of Reproduction of Central Scientific Laboratory, Novosibirsk State Medical University, +7(913)909-22-51, asvetvlad@yandex.ru, https://orcid.org/0000-0001-7124-1969
Igor O. Marinkin, Dr. Med. Sci., Professor, Head of the Obstetrics and Gynecology Department, Rector, Novosibirsk State Medical University, 52 Krasny prospect str., Novosibirsk, 630091, Russia, +7(383)222-32-04, rector@ngmu.ru, https://orcid.org/0000-0002-9409-4823
Corresponding author: Vladimir I. Konenkov, vikonenkov@gmail.com



