Plasma levels of soluble E-cadherin and the keratinocytes growth factor in intrauterine growth restriction
Aim. To investigate plasma levels of soluble E-cadherin (sE-cad) and the keratinocytes growth factor (KGF) in women with fetal growth restriction (FGR).Krasnyi A.M., Khachaturyan A.A., Vtorushina V.V., Krechetova L.V., Kan N.E., Tyutyunnik V.L.
Materials and methods. The study included 25 pregnant women with fetal growth restriction and 19 women with a healthy pregnancy. Plasma levels of sE-cad and KGF were determined by ELISA.
Results. In women with FGR, the plasma level of sE-cad was decreased (p=0.006), while the KGF level was increased (p=0.037). ROC analysis assessing the diagnostic accuracy of sE-cad and KGF showed the AUC of 0.74 and 0.69, respectively. A low plasma level of sE-cad in women with FGR reflects impaired mobility and proliferative activity of trophoblast cells. In growth-restricted pregnancy, higher levels of KGF, as a proliferation factor, may be associated with the activation of compensatory mechanisms aimed to improve trophoblast cell proliferation.
Conclusion. Plasma levels of sE-cad and KGF in pregnant women reflect the underlying pathological process interfering with the healthy growth of the placenta and may, therefore, be used as markers of fetal growth restriction.
Financing. This study was supported by the state task of the Ministry of Health of the Russian Federation (state registration number AAAA-A18-118053190026-6).
Keywords
Fetal growth restriction (FGR) is one of the disorders known collectively as the Great Obstetric Syndromes. It is detected in 10% of pregnancies in the general population and is one of the leading causes of perinatal mortality and morbidity in newborns [1, 2]. It is essential to identify pregnant women with FGR to predict and timely diagnose this pregnancy complication [1, 3].
In the development of pregnancy and fetal growth, of particular importance is the placental barrier regulating the exchange of various substances from maternal to fetal blood and vice versa. The placental barrier is composed of trophoblast and chorionic villi covered by syncytium, the connective tissue of the villous stroma, and capillary endothelium, which ensure the normal functioning of the maternal-fetal–placental unit [4].
The soluble form of E-cadherin (sE-cad) and keratinocyte growth factor (KGF) are mitogens that regulate the migration and differentiation of epithelial cells [5, 6]. In the placenta, these factors target trophoblast cell receptors. Besides, sE-cad can interact with E-cadherins that form intercellular contacts, thus disrupting adhesive compounds. Therefore, the integrity and surface area of the placental barrier depends on sE-cad and KGF. It has been previously found that in FGR pregnancies, the placental levels of sE-cad are reduced, which is a possible cause of low cell proliferation and apoptosis of trophoblast cells, leading to FGR [7]. sE-cad and KGF are markedly expressed in the placenta and thus may be the primary source of these factors in blood plasma. Therefore, measuring these factors may have diagnostic value in FGR syndrome.
The present study aims to investigate plasma levels of soluble E-cadherin and KGF, and their diagnostic performance in FGR.
Material and methods
The study included 44 pregnant women who were referred for a delivery to V.I. Kulakov NMRC for OG&P of Minzdrav of Russia. The patients were divided into the study group and the control group. The study group consisted of 25 pregnant women diagnosed with FGR, confirmed after giving birth. The control group included 19 women with a healthy pregnancy. This study has been approved by the local ethics committee. All women were acquainted with the objectives of the study and signed an informed consent to participate. Criteria for inclusion in the study were: singleton pregnancy, delivery at 36–40 weeks, FGR at the gestational age from 34–40 weeks (for the study group). Exclusion criteria included preeclampsia, severe extragenital pathology, multiplegestation pregnancy, fetal malformations, and acute infectious diseases of the mother.
The study was carried out using commercial a human E-Cadherin ELISA Kit, Cusabio (USA), and human KGF-7 (KGF) ELISA Kit, Thermo Scientific (USA). Blood sampling was performed before delivery using Vacutainer® EDTA tubes. After that, the plasma was isolated by one-hour centrifugation in two stages at 4°C, the first for10 min at 200 g, and the second for 10 min at 4500 g. Plasma samples were stored at –80°C. Immediately before analysis, blood plasma samples were thawed at room temperature. An enzyme-linked immunosorbent assay was performed according to the manufacturer's instructions.
Statistical analysis
Variables not showing normal distribution were expressed as the median (Me) and the quartiles Q1 and Q3 and compared by non-parametric Mann-Whitney test. Categorical variables were analyzed using Fisher's exact test. Differences between the groups were considered statistically significant at p<0.05. To determine the diagnostic performance of the studied model, ROC curve analysis was performed by evaluating the area under the ROC curve with a 95% confidence interval. Statistical analysis was performed using Attestat (Russia) and OriginPro 8.5 (USA) software.
Results
The findings of clinical data, features, and outcomes of pregnancies of the study participant are presented in the table.
As can be seen from the data presented in the table, the groups did not differ in age, body mass index, and gestational age at delivery. It is noteworthy that only 60% of patients with FGR uteroplacental or fetoplacental blood flow disorders were diagnosed antenatally with transvaginal color Doppler ultrasonography; also, there was low placenta weight (p <0.001).
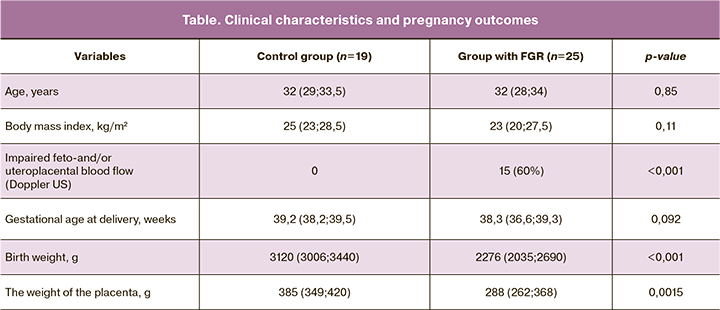
In the patients of the study group, plasma levels of sE-cad were lower than those in control subjects (p = 0.006) (Fig. 1).
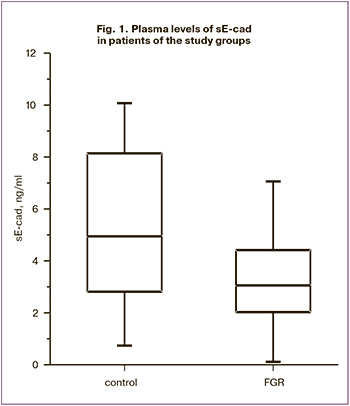
Plasma levels of sE-cad the control and study group were 4.9 (2.8; 6.3) and 3.1 (2.0; 3.9) ng/ml, respectively. ROC analysis showed good diagnostic accuracy: AUC was 0.74 (95% CI 0.63-0.98), sensitivity, specificity, the predictive value of positive and negative results of 63%, 80%, 74.1%, and 70.6%, respectively; the optimal threshold was 4.6 ng/ml (Fig. 2).
Patients in the study group had higher plasma levels of KGF than control subjects (p = 0.037) (Fig. 3).
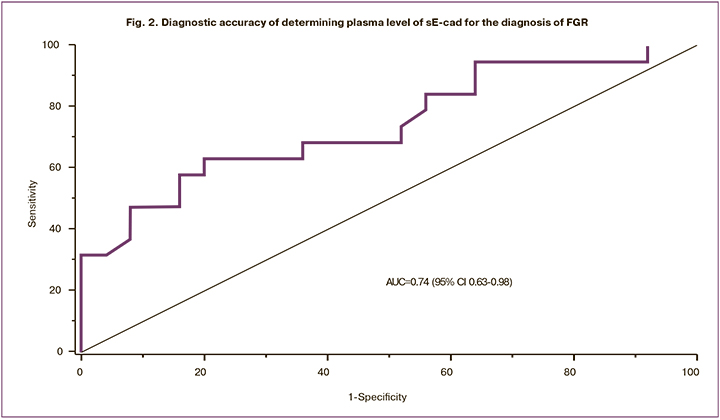
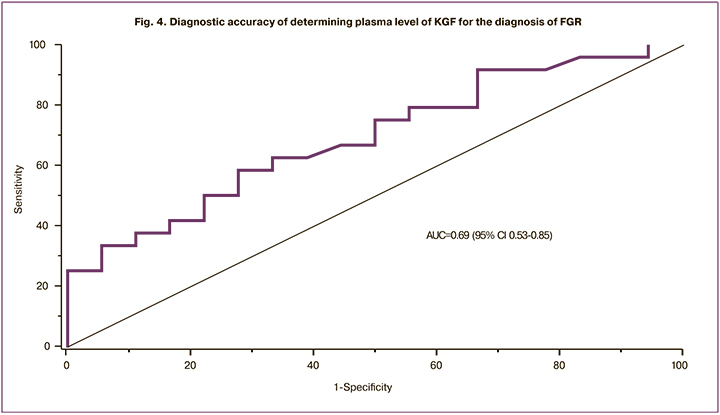
 The results for the control and groups were 0.13 (0.11; 0.15) and 0.15 (0.12; 5.78) ng/ml, respectively. ROC analysis assessing the diagnostic accuracy of KGF showed a moderate diagnostic accuracy, including AUC of 0.69 (95% CI 0.53-0.85), the sensitivity of 58%, the specificity of 72%, the predictive value of positive and negative results of 70% and 54.2%, respectively; the optimal threshold was 0.15 ng/ml (Fig. 4).
The results for the control and groups were 0.13 (0.11; 0.15) and 0.15 (0.12; 5.78) ng/ml, respectively. ROC analysis assessing the diagnostic accuracy of KGF showed a moderate diagnostic accuracy, including AUC of 0.69 (95% CI 0.53-0.85), the sensitivity of 58%, the specificity of 72%, the predictive value of positive and negative results of 70% and 54.2%, respectively; the optimal threshold was 0.15 ng/ml (Fig. 4).
Discussion
According to published data, FGR is characterized by impaired uteroplacental and fetoplacental blood flow [1, 3, 4]. However, these data are contradictory. Therefore, the search for the most accurate diagnostic markers for the detection of FGR is of great practical interest.
One of the causes of placental insufficiency is the slow development of the chorion of the placenta, which is reflected in the smaller size of placentas, as shown in our study. These processes are thought to occur under the influence of the vascular endothelial growth factor, vasoconstrictors, and vasodilators [8]. In our study, we investigated the factors determining the growth of placental trophoblast cells that form the placental barrier. Impaired development of the cells of the placental barrier makes it impossible for placental vessels to form. The studied factors (sE-cad and KGF) are associated with the proliferation, differentiation, and migration of trophoblast cells in the placenta. The decrease in plasma level of sE-cad during FGR is correlated with a lower level of sE-cad in the placentas of women with FGR-complicated pregnancies [7]. An insufficient level of sE-cad in epithelial cells is known to lead to a decrease in proliferation and motility, differentiation and apoptosis of epithelial cells [9]. The results suggest that the lack of sE-cad may impede the development of the placental chorion, resulting in the formation of FGR. ROC analysis showed excellent diagnostic performance of measuring plasma level of the soluble form of E-cadherin, which reflects the ongoing processes in the placenta and can be used to diagnose FGR. In this study, pregnant women with FGR-complicated pregnancies were found to have an increase in the plasma level of KGF.
In growth-restricted pregnancy, higher levels of KGF, as a proliferation factor, may be associated with the activation of compensatory mechanisms aimed to improve trophoblast cell proliferation. ROC analysis showed excellent diagnostic performance of measuring plasma level of the soluble form of E-cadherin and satisfactory performance for KGF. Therefore, determining sE-cad alone or in combination with KGF is the most appropriate test for the diagnosis of FGR.
Conclusion
Plasma levels of sE-cad and KGF in pregnant women may reflect the underlying pathological process interfering with the healthy growth of the placenta and may, therefore, be used for timely diagnosis of FGR.
References
- Стрижаков А.Н., Игнатко И.В., Тимохина Е.В., Белоцерковцева Л.Д. Синдром задержки роста плода. Патогенез. Диагностика. Лечение. Акушерская тактика. М.: ГЭОТАР-Медиа; 2012. 120 с. [Strizhakov A.N., Ignatko I.V., Timohina E.V., Belotserkovtseva L.D. Fetal growth retardation. Pathogenesis. Diagnostics. Treatment. Obstetric tactics. Moscow: GEOTAR-Media; 2012. 120 р. (in Russian)].
- Unterscheider J., Daly S., Geary M.P., Kennelly M.M., McAuliffe F.M., O’Donoghue K. et al. Optimizing the definition of intrauterine growth restriction: the multicenter prospective PORTO Study. Am. J. Obstet. Gynecol. 2013; 208(4): 290. e1-6. https://dx.doi.org/10.1016/j.ajog.2013.02.007.
- Gardosi J., Madurasinghe V., Williams M., Malik A., Francis A. Maternal and fetal risk factors for stillbirth: population based study. BMJ. 2013; 346: f108. https://dx.doi.org/10.1136/bmj.f108.
- Sharma D., Shastri S., Sharma P. Intrauterine growth restriction: antenatal and postnatal aspects. Clin. Med. Insights Pediatr. 2016; 10: 67-83. https://dx.doi.org/10.4137/CMPed.S40070. eCollection 2016.
- Andreadis S.T., Hamoen K.E., Yarmush M.L., Morgan J.R. Keratinocyte growth factor induces hyperproliferation and delays differentiation in a skin equivalent model system. FASEB J. 2001; 15(6): 898-906. https://dx.doi.org/10.1096/fj.00-0324com.
- Najy A.J., Day K.C., Day M.L. The ectodomain shedding of E-cadherin by ADAM15 supports ErbB receptor activation. J. Biol. Chem. 2008; 283(26): 18393-401. https://dx.doi.org/10.1074/jbc.M801329200.
- Красный А.М., Хачатурян А.А., Кан Н.Е., Хачатрян З.В., Тютюнник В.Л., Волгина Н.Е., Ганичкина М.Б., Мантрова Д.А., Садекова А.А. Роль Е-кадгерина в формировании задержки роста плода. Акушерство и гинекология. 2018; 6: 38-43. [Krasnyi A.M., Khachaturyan A.A., Kan N.E., Khachatryan Z.V., Tyutyunnik V.L., Volgina N.E., Ganichkina M.B., Mantrova D.A., Sadekova A.A. The role E-kadherin in the formation of intrauterine growth restriction. Akusherstvo i ginekologiya / Obstetrics and Gynecology. 2018; (6): 38-43. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.6.38-43.
- Szukiewicz D., Szewczyk G., Watroba M., Kurowska E., Maslinski S. Isolated placental vessel response to vascular endothelial growth factor and placenta growth factor in normal and growth-restricted pregnancy. Gynecol. Obstet. Invest. 2005; 59(2): 102-7. https://dx.doi.org/10.1159/000082622.
- Brouxhon S.M., Kyrkanides S., Raja V., Silberfeld A., Teng X., Trochesset D. et al. Ectodomain-specific E-cadherin antibody suppresses skin SCC growth and reduces tumor grade: a multitargeted therapy modulating RTKs and the PTEN-p53-MDM2 axis. Mol. Cancer Ther. 2014; 13(7): 1791-802. https://dx.doi.org/10.1158/1535-7163.MCT-13-0971.
Received 27.01.2020
Accepted 07.02.2020
About the Authors
Aleksey M. Krasnyi, Ph.D. (bio.sci.), Head of the Cytology Laboratory, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia; Tel.: +7(495)438-22-72. E-mail: alexred@list.ru.117997, Russia, Moscow, Ac. Oparina str., 4.
Anuta A. Khachaturyan, Ph.D. Student at the V.I. Kulakov NMRC for OG&P of Minzdrav of Russia. Tel.: +7(926)845-39-53. E-mail: x.anyt37@mail.ru.
117997, Russia, Moscow, Ac. Oparina str., 4.
Valentina V. Vtorushina, Ph.D., Laboratory Physician at the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Tel.: +7(495)438-11-83. E-mail: v_vtorushina@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Lubov V. Krechetova, Dr.Med.Sci., Head of the Immunology Laboratory, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia. Tel.: +7(495)438-11-83.
E-mail: l_krechetova@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Natalia E. Kan, Dr.Med.Sci., Head Physician of the Perinatal Center of European Medical Center; Professor at the Department of Obstetrics and Gynecology, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia. Tel.: +7(926)220-86-55. E-mail: kan-med@mail.ru. Researcher ID: B-2370-2015. ORCID ID: 0000-0001-5087-5946.
125040, Russia, Moscow, Pravda str., 15/1; 117997, Russia, Moscow, Ac. Oparina str., 4.
Victor L. Tyutyunnik, Dr.Med.Sci., Deputy Head Physician of the Perinatal Center of European Medical Cente. Tel.: +7(903)969-50-41. E-mail: tioutiounnik@mail.ru.
Researcher ID: B-2364-2015. ORCID ID: 0000-0002-5830-5099. 125040, Russia, Moscow, Pravda str., 15/1.
For reference: Krasnyi A.M., Khachaturyan A.A., Vtorushina V.V., Krechetova L.V., Kan N.E., Tyutyunnik V.L. Plasma levels of soluble E-cadherin
and the keratinocytes growth factor in intrauterine growth restriction.
Akusherstvo i Ginekologiya / Obstetrics and Gynecology. 2020; 6: 37-42 (in Russian)
http://dx.doi.org/10.18565/aig.2020.6.37-42



