Difference between maternal risk factors for fetal growth restriction and small for gestational age
Ziyadinov A.A., Novikova V.A., Radzinsky V.E.
Objective: This study aimed to compare the maternal gestational risks of insufficient fetal growth (IFG), including small for gestational age (SGA) and fetal growth restriction (FGR).
Materials and methods: This retrospective cohort study was conducted at Perinatal center of N.A. Semashko Republican Clinical Hospital between 2018 and 2023. The study included 611 women with IFG, including 435 with FGR and 176 with SGA. The discriminators of FGR and SGA were studied. Statistical analysis was performed using Statistica 12.0 and Microsoft Excel 2007, and CHAID analysis was conducted using the Classification Trees module.
Results: Potential causes of IFG were hypertensive disorders during pregnancy (41.74%), including preeclampsia (PE) (25.21%), severe (22.59%) or moderate (2.62%), gestational hypertension (GAH) (8.67%), chronic arterial hypertension (CAH) (7.86%), and gestational diabetes mellitus (GDM) (12.77%). The cause of IFG was unknown in 45.49% of women. FGR was more likely to be associated with PE of unknown cause (OR=1.94); SGA was associated with GDM (OR=8.76), GAH (OR=4.38), and CAH (OR=3.93). Prematurity is not obligatory for IFG (24.22%) but is typical for FGR (34.02%). Preterm delivery was associated with severe PE (OR=14.89) and CAH (OR=2.43). The rate of cesarean section for IFG was 55.16% and was associated with FGR (OR=2.95), PE or CAH in FGR, GAH (OR=12.00), and an unknown cause (OR=2.05) in SGA infants. The incidence of iatrogenic prematurity in IFG due to FGR was 86.48 %. Low birth weight (LBW) was more common in the FGR group (OR=6.38).
Conclusion: The FGR and SGA differ in terms of risk factors. The causes of IFG are associated with the risk of iatrogenic prematurity and LBW. Prevention of gestational complications of cardiometabolic origin (hypertensive disorders and GDM) is a measure for preventing IFG. The association of PE with FGR, but not with SGA, confirms the similarity of their pathogenesis and the impossibility of uniform prevention of both IFG variants.
Authors' contributions: Ziyadinov A.A. – development of the concept and design of the study, data analysis, writing the text, editing, approval of the manuscript for publication; Novikova V.A. – development of the concept and design of the study, statistical data processing and interpretation of the results; Radzinsky V.E. – development of the concept and design of the study, editing, approval of the manuscript for publication.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of S.I. Georgievsky Medical Institute, V.I. Vernadsky Crimean Federal University.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Ziyadinov A.A., Novikova V.A., Radzinsky V.E.
Difference between maternal risk factors for fetal growth restriction and small for gestational age.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (5): 53-63 (in Russian)
https://dx.doi.org/10.18565/aig.2024.36
Keywords
The realization of fertility without compromising the health of the mother and newborn is not always feasible. The rapid increase in the incidence of infants born with insufficient fetal growth (IFG), low birth weight (LBW), or preterm birth has necessitated the identification of a special type of newborn, the small vulnerable newborn [1]. A small vulnerable newborn is one whose size does not correspond to the gestational age (small or large for gestational age), whose weight is outside the normal range (low or high), or whose gestational age at birth is considered vulnerable (preterm or small for gestational age). IFG, especially when associated with LBW and/or prematurity, carries the greatest risk of infant and child morbidity and mortality before the age of 5 years as well as long-term consequences [2].
Understanding the fundamental differences between small for gestational age (SGA) and fetal growth restriction (FGR) is important. SGA is defined as a heterogeneous group of fetuses with measurements below a predefined cut-off for gestational age, but a low risk of perinatal complications. This includes fetuses with estimated fetal weight/abdominal circumference values in the 3rd to 9th percentiles, normal blood flow measurements according to Doppler ultrasound data, and normal dynamics of increase in estimated fetal weight and/or abdominal circumference. It also includes constitutionally small fetuses [3]. The unshakable postulate is that SGA is constitutionally special [4, 5], meaning it is potentially a "normal" fetus that corresponds to its gestational age, which fundamentally distinguishes it from FGR [6]. On the other hand, FGR implies the failure of the fetus to achieve genetically determined growth potential, including the probability of falling below the 10th percentile of reference weight values [5, 7]. This failure in growth potential is associated with long-term consequences, similar to those observed in LBW and SGA infants [8].
The key prerequisites for SGA include maternal comorbidity (such as hypertensive disorders, subclinical hypothyroidism (especially in areas with iodine deficiency), chronic infections, and malaria) [9–11], placental dysfunction, and fetal factors (such as congenital anomalies, malformations, and genetic defects) [10]. Specific risk factors for FGR are numerous and varied, and can be categorized as maternal, placental, fetal, or genetic [8]. Global maternal risk factors for FGR include low levels of maternal healthcare, fasting during pregnancy, and low weight gain during pregnancy. Extragenital diseases also play a significant role, including hypertensive disorders (gestational and non-gestational), complications of pregnancy with vasculopathy (such as preeclampsia and diabetes mellitus), chronic kidney disease, systemic lupus erythematosus, bronchial asthma, antiphospholipid syndrome, sickle cell anemia, and infections (e.g., TORCH, malaria, tuberculosis, urinary tract infections, and bacterial vaginosis). The concepts of LBW and IFG (SGA or FGR) at birth are not interchangeable; however, they share a common pathogenesis. In international sources they are often designated by a single abbreviation, LBW/SGA/FGR (LBW, low birth weight; SGA, small-for-gestational age; FGR, fetal growth restriction) [13]. Many questions regarding the maternal gestational determinants of IFG remain unanswered, both in general and for specific types (SGA or FGR), which hinders the timely prevention and diagnosis of fetal disorders, as well as the prediction of the risk of delivering a small, vulnerable fetus.
This study aimed to compare the maternal gestational risks of insufficient fetal growth, including small for gestational age and fetal growth restriction.
Materials and methods
This retrospective cohort study was conducted between 2018 and 2023 in N.A. Semashko Republican Clinical Hospital and clinics of the Department of Obstetrics and Gynecology with the course of Perinatology, Medical Institute of Peoples' Friendship University of Russia. This study included 611 women with singleton pregnancies and IFG: FGR (n=435) and SGA (n=176). The main inclusion criterion for women in this study was the presence of no more than one pregnancy complication potentially related to IFG. Patients with two or more pregnancy complications competing with the potential risk of IFG were excluded from the study. The definitions of FGR and SGA were in accordance with national clinical guidelines [3].
Statistical analysis
Statistical analysis was performed using Statistica 12.0 and Microsoft Excel 2007. Pearson's chi-square test (χ2) was used to compare categorical variables. Yates correction was used when the expected frequencies were <10. The relationship between the studied factor and the outcome/group was assessed based on the odds ratio (OR) with a 95% confidence interval (CI). To classify the groups, CHAID (Chi Squared Automatic Interaction Detection) analysis was used in the Classification Trees module to effectively determine the relationship between predictor variables and categorical response. In the data sample, the forecasting variables (predictors) of the outcome being studied (categorical responses) were selected. The importance of the features was assessed, which reflects the importance of each feature from the point of view of obtaining solutions. The score ranged from 0 to 1 for each feature, where 0 meant “not used at all” and 1 meant “excellently predicts the target variable” [14]. The area under the ROC curve (AUC ROC) was used to evaluate the quality of the algorithm's classification of objects into two classes. Quality was considered high if the AUC ROC was greater than 0.8. The metric for the quality of predictive models in binary classification problems is the Gini coefficient [15]. Approximation of its values to "1" was considered as high quality of binary classification.
Results and discussion
IFG could be associated with gestational complications assessed as potential causes of IFG in only 54.51% (333/611) of the women. In almost half of the women (45.49%, 278/611), the cause of IFG was unknown because there were no gestational complications (Fig. 1a).
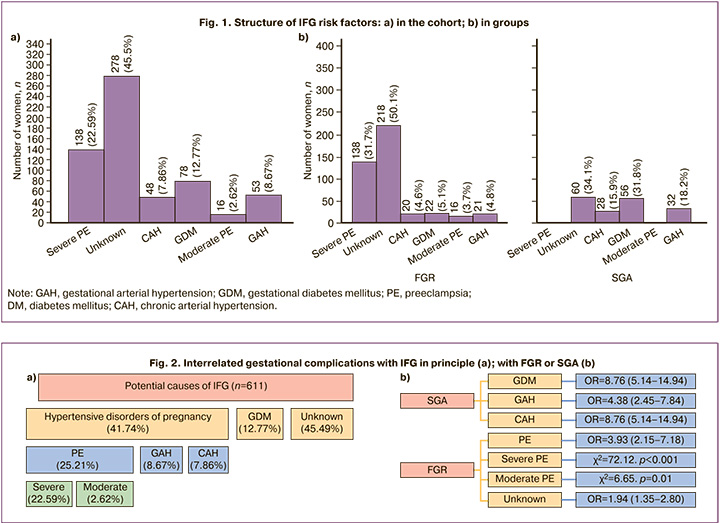
All established causes of IFG had cardiometabolic origins characteristic of gestation [16], and represented two groups of complications, including hypertensive disorders of pregnancy and gestational diabetes mellitus (GDM) (Fig. 2).
Hypertensive disorders in ¼ of women were represented by preeclampsia (PE) (25.21%, 154/611, including severe in 22.59% (138/154) and moderate in 2.62% (16/154), with a predominance of severe (89.61 %, 138/154), and gestational arterial hypertension (GAH) (8.67%, 53/611), and least of all chronic arterial hypertension (CAH) (7.86%, 48/611). DM was only represented by GDM (12.77%). The potential causes of IFG were predominantly of gestational origin, including PE, GDM, and GAH (92.14%, 563/611), with the exception of CAH.
FGR was distinguished by its exclusive involvement in PE, both severe and moderate, and it was also highly associated with an unknown cause of IFG (Fig. 2b). For SGA infants, other causes of IFG (DM, GAH, and CAH) were more common.
The gestational age at delivery in the cohort ranged from 25 to 41 weeks (Fig. 3a), but the median (Me) and interquartile range (Q1–Q3) corresponded to term delivery (Me=39 weeks, Q1–Q3=37–39 weeks). An earlier gestational age significantly distinguished FGR from SGA (Me=39 weeks (Q1–Q3=34–39 weeks) and Me=39 weeks (Q1–Q3=39–39 weeks), p<0.001) (Fig. 3b).
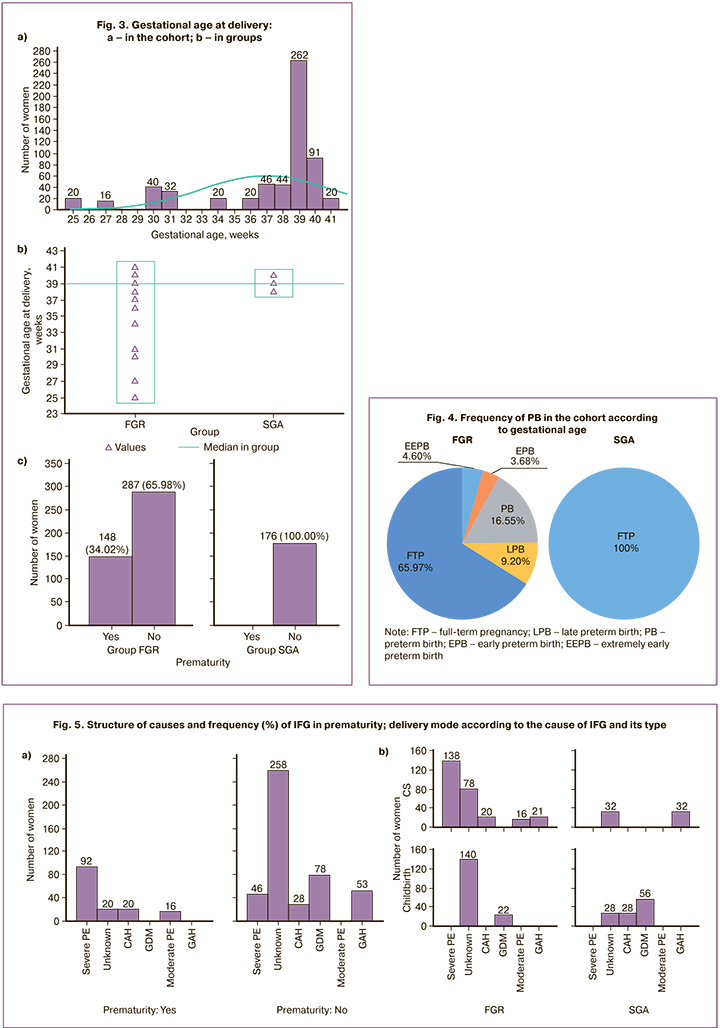
Prematurity was not a characteristic feature of the study cohort (24.22%, n=148). It was exclusively related to FGR (34.02%), which fundamentally distinguished it from SGA (c2=79.02, p<0.001). Of the 24.83% of women who gave birth before 34 weeks of gestation, 16.55% (n=72) were classified as preterm birth (PB), 3.68% (n=16) as early preterm birth (EPB), and 4.6% (n=20) as extremely early preterm birth (EEPB); only 9.2% (n=40) gave birth between 34 and 37 weeks of gestation (Fig. 4).
Prematurity showed not only an absolute association with FGR but also an association with certain maternal gestational complications (Fig. 5).
In the cohort, prematurity was most associated with severe PE (c2=174.96, p<0.001; OR=14.89, 95% CI 9.49–23.37) and significantly less with CAH (c2=8.64, p=0.004; OR=2.43, 95% CI 1.32–4.45). Prematurity was associated with moderate PE (c2=47.25, p<0.001) because it was absent in full-term deliveries. Full-term delivery was characteristic of unknown cause (c2=80.58, p<0.001; OR=8.06, 95% CI 4.86–13.36); it was an exclusive marker for GDM (c2=28.58, p<0.001) and GAH (c2=18.55, p<0.001).
The cohort had a dominantly high (55.16%, 337/611) need for cesarean section (CS), which was more common in FGR than in SGA (c2=35.03, p<0.001; OR=2.95, 95%CI 2.05–4.24). CS was the only method of delivery for women with FGR compared to SGA for PE (regardless of severity) and CAH. CS was a more common mode of delivery in women with SGA compared to FGR for GAH (OR=12.00, 95% CI 6.19–23.27) and IFG of unknown cause (OR=2.05, 95% CI 1.15–3.66). Vaginal delivery was the only method of delivery in SGA women with severe PE and CAH and all women with diabetes, but was significantly more often used in SGA (odds ratio [OR] =8.76, 95% CI 5.14–14.94).
Analysis of indications for CS demonstrated their association with FGR in severe PE (70/273 and 0/64, c2=19.18, p<0.001) and PE with critical fetal condition (68/273 and 0/64, c2= 18.46, p<0.001), progressive chronic placental insufficiency (CPI) (95/273 and 0/64, c2=29.319, p<0.001), immature cervix during pregnancy more than 40 weeks and 6 days (20/ 273 and 0/64, c2=3.76, p=0.053); to SGA – with a scar on the uterus after CS and an immature cervix in full-term pregnancy (32/64 and 0/273, c2=145.06, p<0.001); with 2 or more scars on the uterus after CS (32/64 and 20/273, c2=69.12, p<0.001) (Fig. 6a).
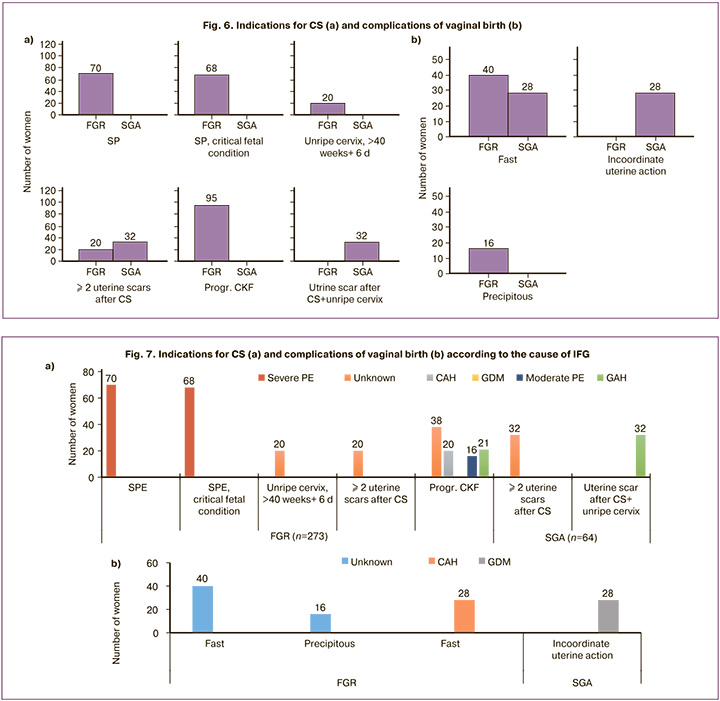
An assessment of complications of vaginal birth showed that rapid ones were more typical for FGR (40/56 and 28/56, c2=5.39, p=0.02; OR=2.5, 95% CI 1.14–5.46) childbirth. Absolute markers of FGR were rapid labor (40/56 and 0/56, c2=59.15, p<0.001), SGA – discoordination of labor (16/56 and 0/56, c2=16.41, p<0.001).
Taking into account the causes of IFG, it turned out that PE is expectedly associated with indications for CS as a severe course and the addition of a critical condition of the fetus; unknown cause – with progressive CPI, immature cervix at more than 40 weeks and 6 days of gestation, and 2 or more uterine scars after CS; GAH – with a uterine scar after 2 or more CS in history in combination with an immature cervix; CAH, with progressive CPI; moderate PE, with progressive CPI (Fig. 7a).
Vaginal delivery was complicated by precipitate labor exclusively due to an unknown cause of IFG, discoordination of labor with GDM, rapid labor with an unknown cause, and CAH (Fig. 7b).
Induction of labor (amniotomy) was required in 5.06% (22/435) of the women with FGR and exclusively in full-term pregnancies. Consequently, in 86.49% (128/148) of the women, prematurity turned out to be forced, “iatrogenic” (in those delivered by CS) (Fig. 8); only 13.51% were spontaneous.
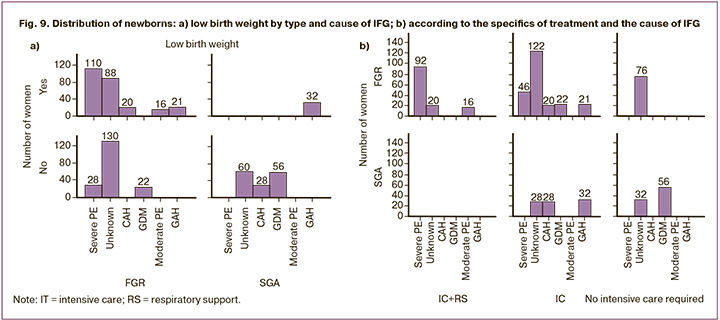
LBW was detected in the cohort with a frequency of 46.92% (n=287), more typical for FGR compared to SGA (255/435 and 32/176, respectively, c2=80.65, p<0.001; OR=6.38, 95% CI 4.16–9.78). LBW (low weight) was an exclusive marker of FGR in PE (severe and moderate), unknown cause and CAH (Fig. 9a), but was more associated with SGA compared to FGR in GAH (32/144 and 21/414, respectively, c2=26.55, p<0.001; OR=4.38, 95% CI 2.45–7.84); with GDM it was completely absent.
Analysis of the specific treatment of a newborn in accordance with the severity of his condition showed a relationship with the type of IFG, its potential cause, and prematurity (Table).
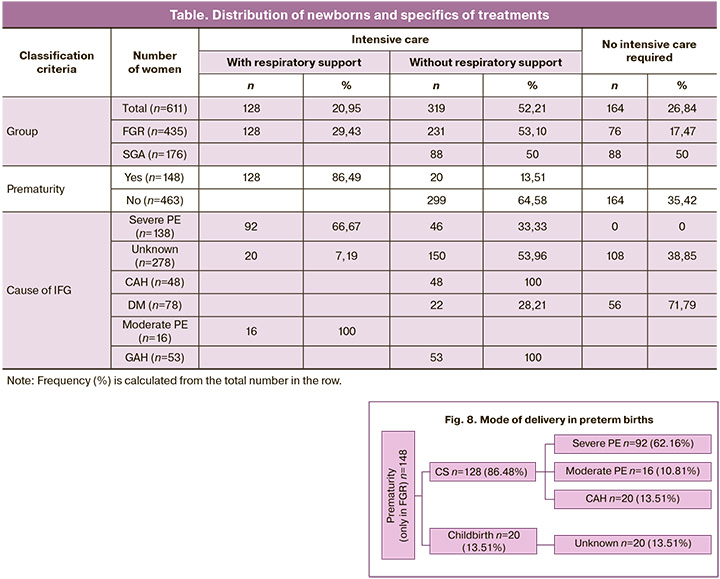
Intensive care was required for almost ¾ (73.16%) of the newborns in the cohort: more than ½ (52.21%) without respiratory support, 1/5 (20.95%) with respiratory support. Only neonates with FGR required transfer to the intensive care unit. FGR compared to SGA demonstrated a “monopoly” need for respiratory and intensive care of the newborn (29.43%, c2=63.75, p<0.001). No need for intensive care was more common in the SGA group than in the FGR group (50% vs. 17.47%, respectively; OR=4.72, 95% CI 3.21–6.95). Intensive care without respiratory support in FGR and SGA was required at a comparable rate (53.10% and 50%, respectively, c2=0.48, p=0.49).
The highest proportion of infants with IFG from mothers with severe PE required intensive care with respiratory support (66.67%, 92/138). Moderate PE required respiratory support, and GAH required intensive care. GDM had the highest proportion (71.79%, 56/78) of newborns who did not require intensive care.
Based on the data obtained, we found that the difference between FGR and SGA with high accuracy (AUC=0.93; Gini coefficient=0.86) determined, first, the cause of IFG, then prematurity, and LBW, which are much inferior in predictive value significance, and least of all, the method of delivery (Fig. 10a). When delivered by CS, the difference in predicting FGR or SGA was primarily determined by the indication for it (AUC=0.98; Gini coefficient=0.96), the second place followed by the cause of IFG; prematurity and LBW were the least important predictors (Fig. 10b). For vaginal delivery, the opposite trend was observed: complications during labor were the least important predictors of FGR or SGA, and the cause of IFG demonstrated paramount importance (AUC=1.0; Gini coefficient=1.0) (Fig. 10c).
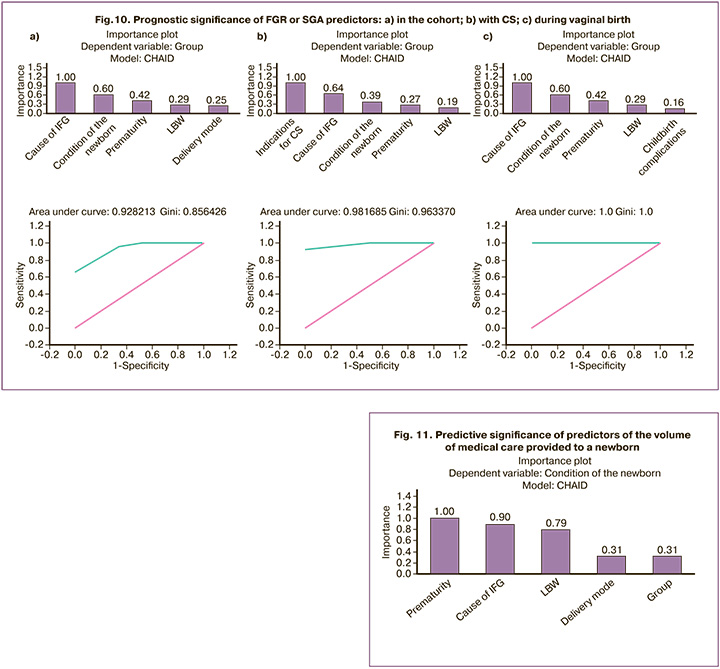
It is obvious that the cause of IFG has the greatest significance in predicting FGR or SGA, often exceeding prematurity, LBW, and method of delivery. The need for CS, justified by decompensation of a pregnancy complication (cause of IFG) or CPI, determined the distinction of FGR from SGA as an indication for CS.
A similar approach to distinguishing predictors of the volume of medical care provided to newborns demonstrated that prematurity was expected to be of greatest importance (Fig. 11). The cause IFG ranked second but competed in importance with prematurity and was dominant over LBW. Belonging to one of the IFG variants (FGR or SGA) was inferior for all reasons (significance=0.310), including method of delivery (significance=0.313).
Discussion
The cause of IFG is unclear in 45.49% of women, which is understandable. Modern research suggests a variety of disorders of a genetic nature, including placental disorders, such as reduced activity of placental 11β-hydroxysteroid dehydrogenase type 2 mRNA, insufficient expression of placental growth factor (PlGF), activation of SERPINA3, insufficient expression of homeobox (DLX3, DLX4, MSX2 and GAX, ESX1 L, HLX1), overexpression of Cullin (CUL4B and CUL7), STOX1, NEAT1 (nuclear assembly transcript 1), and overexpression of trophoblast miRNAs (miRNAs) (miRNA-424 and miRNA-141). Maternal disorders, such as overexpression of endothelin-1 (ET-1), leptin underexpression, visfatin overexpression, thrombophilia gene mutation (factor V G1691 A or factor II A (20210)), high levels of soluble vascular cell adhesion molecule-1 (sVCaM-1), and higher levels of soluble E-selectin (sE-selectin also play a role. Additionally, genetic factors in the fetus, such as high levels of urinary protein S100B, genetic deletion of IGF1 (insulin-like growth factor 1) and SHOX, and mutation of insulin-like growth factor receptor 1 (IGF-1R), contribute to the condition [17].
The structure of the IFG mono-risk factors we have identified is less diverse than those known globally, but their exclusively cardiometabolic genesis, which we have identified, deserves close attention. This result supports the stratification of high-risk groups for prevention and timely diagnosis of IFG. Moreover, most maternal IFG risk factors, except for CAH, occur during gestation, making them preventable if GAH and GDM are managed. When choosing a preventive strategy, it is essential to consider the specificity of the maternal risk factors for SGA and FGR. Predicting IFG in general is not sufficient, and specific risk factors for each variant need to be identified.
Prematurity, a known potential companion of IFG, was not an obligate marker according to our data (24.22%). It is exclusively characteristic of FGR (frequency 34.02%, with 24.83% for delivery before 34 weeks of pregnancy). The need for early delivery was most strongly associated with severe PE (OR=14.89, 95% CI 9.49–23.37), and to a lesser extent with CAH (OR=2.43, 95% CI 1.32–4.45). Conditions for carrying a pregnancy to term in cases of IFG of unknown cause (OR=8.06, 95% CI 4.86–13.36), GDM (p<0.001), and GAH (p<0.001) were available.
The present study showed that IFG did not significantly reduce the frequency of delivery by CS, with its rate (55.16%) far exceeding the WHO target level of 10–15%. CS was more necessary for FGR than SGA (OR=2.95, 95% CI 2.05–4.24). Considering the causes of IFG, CS appeared inevitable in cases of FGR combined with PE (regardless of severity) or CAH, and was more likely with SGA combined with GAH (OR=12.00, 95% CI 6.19–23.27) and unknown cause IFG (OR=2.05, 95% CI 1.15–3.66).
We identified six indications for CS in IFG cases: severe PE, a combination of severe PE with a critical condition of the fetus, progressive chronic renal failure, an immature cervix at a gestational age of more than 40 weeks and 6 days, a uterine scar after CS, and the presence of two or more scars on the uterus after CS. They can be divided into four groups: 1) severe PE, 2) progressive (decompensated) PN, 3) immature cervix, and 4) uterine scar after CS.
FGR was the leading cause of CS in urgent maternal and/or fetal conditions: severe PE (p<0.001), PE with a critical fetal condition (p<0.001), and progressive chronic renal failure (p<0.001). SGA was associated with a uterine scar after CS, with or without an immature cervix at full-term pregnancy (p<0.001).
Vaginal delivery remains an option for SGA infants with severe PE and CAH. GDM is a “guarantor” of vaginal delivery, especially in SGA (OR=8.76, 95% CI 5.14–14.94). The difference in labor complications in different IFG variants is notable: FGR with accelerated labor is associated with rapid (OR=2.5, 95% CI 1.14–5.46) and precipitous (p<0.001) labor, whereas SGA is associated with incoordinate uterine activity (p<0.001). This requires further investigation into the regulation of labor, IFG, and its variants.
The global trend of “iatrogenic” prematurity correlates highly with IFG, specifically FGR, at a frequency of 86.48%. This prematurity was primarily caused by hypertensive disorders during pregnancy, especially severe (62.16%) and moderate (10.81%) PE and CAH (13.51%). This result supports the well-established view of the inextricable connection between FGR and PE, demonstrating the role of pregestational hypertension in gestational risk for the mother and fetus/newborn.
LBW, like prematurity, is not an obligate marker of IFG, confirming the internationally accepted definitions of FGR and SGA that do not exclude normal fetal/newborn weight. The incidence of LBW in the cohort was less than half (46.92%), and was more characteristic of FGR (OR=6.38, 95% CI 4.16–9.78).
The connection with LBW was shown not only by the combination of FGR with PE (regardless of severity) and CAH, but also by unknown causes, confirming the complex and not always clear genesis of IFG. SGA was more strongly associated with LBW than FGR in cases of GAH (OR=4.38, 95% CI 2.45–7.84). The absence of LBW in GDM in women with IFG is explained by the “excess” fetal weight due to glycemic disturbances and possible macrosomia.
The negative risks of IFG necessitated intensive care in 52.21% of newborns and respiratory support in 20.95% of newborns. FGR and SGA are characterized by different scenarios for pregnancy and childbirth outcomes for the fetus, as evidenced by the specific treatment of newborns. FGR was closely associated with urgent maternal and fetal conditions requiring immediate delivery and respiratory and intensive care of the newborn (29.43%, p<0.001). SGA was characterized by the absence of disturbances in the newborn's vital functions and a lower need for intensive care compared to FGR (OR=4.72, 95% CI 3.21–6.95). Intensive care without respiratory support was required at a comparable rate for FGR and SGA (χ2=0.48, p=0.49).
The cause of IFG has demonstrated involvement in specific newborn conditions. Only full-term neonates with an unknown cause of IFG do not require intensive care. Severe PE most often required intensive care with respiratory support (66.67%) or intensive care only (33.33%), whereas moderate PE exclusively required intensive care. GAH also required intensive care. GDM had the lowest risk to the newborn compared with other causes of IFG, with the highest frequency (71.79%), allowing for the exclusion of intensive therapy.
Modern methods of statistical data analysis enable the prediction and timely diagnosis of FGR. We ranked the factors distinguishing FGR from SGA according to their importance. The leading cause of IFG was known maternal or unknown maternal/placental/fetal complications. Prematurity and LBW did not compete in predictive value with IFG causes, and mode of delivery was the least important among the selected predictors. The need for urgent delivery by CS overlapped with IFG causes, having the greatest predictive value for FGR and SGA, whereas the cause of IFG was the second most important. Prematurity and LBW were marginally significant discriminators of FGR and SGA infants. In vaginal delivery, the cause of IFG was the most important discriminator between FGR and SGA, while prematurity and LBW were the least important.
Supporting the debate about small vulnerable fetuses, we confirmed the importance of prematurity in newborn outcomes with IFG, justifying the necessary amount of medical care. However, the cause of IFG showed significant importance, slightly inferior to prematurity, but surpassed that of LBW. The IFG variant (FGR or SGA) was the least informative predictor of the amount of medical care required. This indicates that the newborn's outcome is determined not only by FGR or SGA but also by a cumulative set of interdependent gestational factors.
Conclusion
IFG manifests in two forms, FGR and SGA, which differ fundamentally in maternal gestational risk factors, risks of prematurity, low birth weight, delivery mode, indications for CS and complications of vaginal birth, and the need for intensive care and/or respiratory support for newborns. Maternal gestational risk factors of IFG are the key factors that determine the scenario of IFG formation (FGR or SGA) and the outcome of pregnancy not only for the mother, but also for the fetus or newborn. Prevention of maternal complications of gestation with a cardiometabolic profile (in particular, hypertensive conditions during pregnancy and GDM) appears to be a measure for the prevention of IFG. The close association of PE with FGR but not SGA (!) confirms the commonality of their pathogenesis and the impossibility of creating unified preventive measures for both IFG variants.
References
- Ashorn P., Black R.E., Lawn J.E., Ashorn U., Klein N., Hofmeyr J. et al. The Lancet Small Vulnerable Newborn Series: science for a healthy start. Lancet. 2020; 396(10253): 743-5. https://dx.doi.org/10.1016/S0140-6736(20)31906-1.
- Haksari E.L., Hakimi M., Ismail D. Neonatal mortality in small for gestational age infants based on reference local newborn curve at secondary and tertiary hospitals in Indonesia. BMC Pediatr. 2023; 23(1): 214. https://dx.doi.org/10.1186/s12887-023-04023-z.
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Недостаточный рост плода, требующий предоставления медицинской помощи матери (задержка роста плода). 2022. [Ministry of Health of the Russian Federation. Clinical guidelines. Insufficient fetal growth requiring maternal medical care (fetal growth restriction). 2022. (in Russian)].
- Colella M., Frérot A., Novais A.R.B., Baud O. Neonatal and long-term consequences of fetal growth restriction. Curr. Pediatr. Rev. 2018; 14(4): 212-8. https://dx.doi.org/10.2174/1573396314666180712114531.
- Gordijn S.J., Beune I.M., Ganzevoort W. Building consensus and standards in fetal growth restriction studies. Best Pract. Res. Clin. Obstet. Gynaecol. 2018; 49:117-26. https://dx.doi.org/10.1016/j.bpobgyn.2018.02.002.
- Osuchukwu O.O., Reed D.J. Small for gestational age. 2022. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2024.
- Melamed N., Baschat A., Yinon Y., Athanasiadis A., Mecacci F., Figueras F. et al. FIGO (international Federation of Gynecology and obstetrics) initiative on fetal growth: best practice advice for screening, diagnosis, and management of fetal growth restriction. Int. J. Gynaecol. Obstet. 2021; 152 Suppl 1(Suppl 1): 3-57. https://dx.doi.org/10.1002/ijgo.13522.
- McCowan L.M., Figueras F., Anderson N.H. Evidence-based national guidelines for the management of suspected fetal growth restriction: comparison, consensus, and controversy. Am. J. Obstet. Gynecol. 2018; 218(2S): S855-S868. https://dx.doi.org/10.1016/j.ajog.2017.12.004.
- Galvão R.B., Souza R.T., Vieira M.C., Pasupathy D., Mayrink J., Feitosa F.E. et al.; Preterm SAMBA study group. Performances of birthweight charts to predict adverse perinatal outcomes related to SGA in a cohort of nulliparas. BMC Pregnancy Childbirth. 2022; 22(1): 615. https://dx.doi.org/10.1186/s12884-022-04943-1.
- Konstantyner T., Areco K.C.N., Bandiera-Paiva P., Marinonio A.S.S., Kawakami M.D., Balda R.C.X. et al. The burden of inappropriate birth weight on neonatal survival in term newborns: a population-based study in a middle-income setting. Front. Pediatr. 2023; 11: 1147496. https://dx.doi.org/10.3389/fped.2023.1147496.
- Hokken-Koelega A.C.S., van der Steen M., Boguszewski M.C.S., Cianfarani S., Dahlgren J., Horikawa R. et al. International Consensus Guideline on small for gestational age: etiology and management from infancy to early adulthood. Endocr. Rev. 2023; 44(3): 539-65. https://dx.doi.org/10.1210/endrev/bnad002.
- Yang L., Feng L., Huang L., Li X., Qiu W., Yang K. et al. Maternal factors for intrauterine growth retardation: systematic review and meta-analysis of observational studies. Reprod. Sci. 2023; 30(6): 1737-45. https://dx.doi.org/10.1007/s43032-021-00756-3.
- Mansfield R., Cecula P., Pedraz C.T., Zimianiti I., Elsaddig M., Zhao R. et al. Impact of perinatal factors on biomarkers of cardiovascular disease risk in preadolescent children. J. Hypertens. 2023; 41(7): 1059-67. https://dx.doi.org/10.1097/HJH.0000000000003452.
- Шитиков В.К., Мастицкий С.Э. Классификация, регрессия и другие алгоритмы Data Mining с использованием R. 2017; 351 с. Доступно по: https://github.com/ranalytics/data-mining [Shitikov V.K., Mastitsky S.E. Classification, regression and other Data Mining algorithms using R. 2017; 351 p. (in Russian). Available at: https://github.com/ranalytics/data-mining].
- Дружилов М.А., Кузнецова Т.Ю., Гаврилов Д.В., Гусев А.В. Верификация субклинического каротидного атеросклероза в рамках риск-стратификации при избыточном весе и ожирении: роль методов машинного обучения в формировании диагностического алгоритма. Кардиоваскулярная терапия и профилактика. 2022; 21(7): 3222. [Druzhilov M.A., Kuznetsova T.Yu., Gavrilov D.V., Gusev A.V. Verification of subclinical carotid atherosclerosis as part of risk stratification in overweight and obesity: the role of machine learning in the development of a diagnostic algorithm. Cardiovascular Therapy and Prevention. 2022; 21(7): 3222. (in Russian)]. https://dx.doi.org/10.15829/1728-8800-2022-3222.
- Thong E.P., Ghelani D.P., Manoleehakul P., Yesmin A., Slater K., Taylor R. et al. Optimising cardiometabolic risk factors in pregnancy: a review of risk prediction models targeting gestational diabetes and hypertensive disorders. J. Cardiovasc. Dev. Dis. 2022; 9(2): 55. https://dx.doi.org/10.3390/jcdd9020055.
- Sharma D., Shastri S., Sharma P. Intrauterine growth restriction: antenatal and postnatal aspects. Clin. Med. Insights Pediatr. 2016; 10: 67-83. https://dx.doi.org/10.4137/CMPed.S40070.
- WHO. Statement on caesarean section rates. Geneva: World Health Organization, 2015. URL: http://www.who.int/reproductivehealth/ publications/maternal_perinatal_health/cs-statement/en/.
- Valencia C.M., Mol B.W., Jacobsson B.; FIGO Working Group for Preterm Birth. FIGO good practice recommendations on modifiable causes of iatrogenic preterm birth. Int. J. Gynaecol. Obstet. 2021; 155(1): 8-12. https://dx.doi.org/10.1002/ijgo.13857.
- Sławek-Szmyt S., Kawka-Paciorkowska K., Ciepłucha A., Lesiak M., Ropacka-Lesiak M. Preeclampsia and fetal growth restriction as risk factors of future maternal cardiovascular disease-A review. J. Clin. Med. 2022; 11(20): 6048. https://dx.doi.org/10.3390/jcm11206048.
- Абрамова М.Ю., Пономаренко И.В., Орлова В.С., Батлуцкая И.В., Ефремова О.А., Сорокина И.Н., Чурносов М.И. Генетические маркеры риска развития задержки роста плода у беременных с преэклампсией. Медицинский совет. 2023; 17(6): 150-6. [Abramova M.Yu., Ponomarenko I.V., Orlova V.S., Batlutskaya I.V., Efremova O.A., Sorokina I.N., Churnosov M.I. Genetic markers of the risk of fetal growth retardation in pregnant women with preeclampsia. Medical Council. 2023; 17(6): 150-6. (in Russian)]. https://dx.doi.org/10.21518/ms2022-006.
- Гуменюк Е.Г., Ившин А.А., Болдина Ю.С. Поиск предикторов задержки роста плода: от сантиметровой ленты до искусcтвенного интеллекта. Акушерство и гинекология. 2022; 12: 18-24. [Gumenyuk E.G., Ivshin A.A., Boldina Yu.S. Search for predictors of fetal growth retardation: from centimeter tape to artificial intelligence. Obstetrics and Gynecology. 2022; (12): 18-24. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.185.
Received 15.02.2024
Accepted 08.05.2024
About the Authors
Arsen A. Ziyadinov, PhD, Associate Professor at the Department of Obstetrics, Gynecology and Perinatology No. 1 of S.I. Georgievsky Medical Institute, V.I. Vernadsky Crimean Federal University; Obstetrician-Gynecologist at the Perinatal Center of N.A. Semashko Republican Clinical Hospital, 295017, Russia, Republic of Crimea, Simferopol, Semashko str., 8, ars-en@yandex.ruVladislava A. Novikova, Dr. Med. Sci., Professor of the Department of Obstetrics and Gynecology with the course of Perinatology, Medical Institute of Peoples’ Friendship University of Russia, 117198, Russia, Moscow, Miklukho-Maklaya str., 6, kafedra-aig@mail.ru
Victor E. Radzinsky, Dr. Med. Sci., Professor, Corresponding Member of the RAS, Head of the Department of Obstetrics and Gynecology with the course of Perinatology, Medical Institute of Peoples’ Friendship University of Russia, 117198, Russia, Moscow, Miklukho-Maklaya str., 6, kafedra-aig@mail.ru



