Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia, Moscow
Objective. To study the diagnostic significance of transforming growth factor (TGF)-β1, TGF-β2, and TGF-β3 isoforms in fetal growth restriction (FGR).
Subjects and methods. Plasma samples from 48 women were examined during this investigation. The pregnant women were divided into 2 groups: 1) 28 pregnant women with FGR; 2) 20 women with physiological pregnancy. All the patients underwent multiplex measurement of the concentrations of TGF-β1, TGF-β2, and TGF-β3 isoforms by using the standard 3-plex Bio-Plex Pro assay system on a Bio-Plex 200 System flow-through laser immunoassay device (Bio-Rad, USA), followed by the processing of the results obtained in the Bio-Plex Manager Software version 6.0 Properties (Bio-Rad, USA).
Results. The level of TGF-β3 was ascertained to ne significantly lower in pregnant women with FGR (41.42 pg/ml)
than in apparently healthy pregnant women (48.42pg/ml) (p = 0.01). The levels of TGF-β1 and TGF-β2 did not statistically differ in the study groups.
Conclusion. The findings indicate that studying the plasma levels of TGF-β3 is promising in predicting fetal growth restriction.
fetal growth restriction
transforming growth factor-β
placental insufficiency
В настоящее время задержка роста плода (ЗРП) остается одной из самых актуальных проблем современного акушерства и не имеет тенденции к снижению. Согласно данным Исследовательской группы по эпидемиологии детского здоровья, в структуре перинатальной заболеваемости доля детей, рожденных маловесными для гестационного возраста, в 2012 г. составила 22% [1]. В связи с отсутствием эффективных методов лечения перспективным является изучение молекулярных механизмов формирования ЗРП с целью разработки новых прогностических и диагностических методов. ЗРП характеризуется ограничением генетически запрограммированного роста плода [2]. Как известно, в основе ее развития может лежать сочетание материнских, плацентарных и плодовых факторов, которое в большинстве случаев приводит к нарушению процессов инвазии вневорсинчатого цитотрофобласта в спиральные артерии матки и их последующего преобразования [3–5]. В процессе формирования плацентарной недостаточности наблюдается не только нарушение процессов пролиферации и апоптоза клеток трофобласта, но и усиление окислительного стресса, который, в свою очередь, приводит к экспрессии провоспалительных цитокинов [6–10]. Среди них представляет интерес трансформирующий фактор роста бета (TGF-β). Члены суперсемейства TGF-β – TGF-β1, -2 и -3 выступают в качестве важных посредников для нормального формирования плаценты и роста плода путем регуляции процессов инвазии, пролиферации и апоптоза клеток трофобласта [11, 12]. Описаны различные механизмы, с помощью которых осуществляются вышеупомянутые процессы, но отсутствуют данные о роли отдельных изоформ TGF-β в патогенезе ЗРП [13–15].
Целью исследования было изучение изменения уровней изоформ TGF-β – TGF-β1, TGF-β2 и TGF-β3 для определения их диагностической значимости при ЗРП.
Материалы и методы
В исследование были включены 48 беременных, которые поступили и были родоразрешены в ФБГУ «НМИЦ АГП им. В.И. Кулакова» Минздрава России. Пациентки были разделены на 2 группы: 1-ю группу составили 28 беременных с ЗРП. Диагноз ЗРП в 1-й группе был подтвержден постнатально согласно таблицам Фентона для недоношенных и центильным таблицам ВОЗ для доношенных детей [16]. Во 2-ю группу вошли 20 соматически здоровых женщин без отягощенного акушерско-гинекологического анамнеза с физиологическим течением данной беременности. Всем пациенткам проведено клинико-лабораторное обследование согласно приказу Минздрава России от 01.11.2012 №572н. Данное исследование было одобрено локальным этическим комитетом ФБГУ «НМИЦ АГП им. В.И. Кулакова» Минздрава России, всеми пациентками подписано информированное согласие на участие. Критериями исключения из исследования послужили: многоплодная беременность, наличие миомы матки больших размеров, тяжелой экстрагенитальной патологии и острых инфекционных заболеваний у матери, наличие резус-конфликта, хромосомных аномалий и пороков развития у плода.
Для определения концентрации изоформ TGF-β (TGF-β1, TGF-β2, TGF β3) использовали образцы периферической венозной крови с этилендиаминтетрауксусной кислотой (ЭДТА). Забор венозной крови производился до начала родовой деятельности. Для получения образцов плазмы производилось двукратное центрифугирование образцов периферической крови при 1000 g в течение 15 минут при 40° и 10 000 g в течение 10 мин при 40° для полного удаления тромбоцитов и осадков согласно инструкции производителя тест-системы (Bio-Rad, США). Полученные образцы хранили при температуре -80°. Определение концентрации изоформ TGF-β (TGF-β1, TGF-β2, TGF-β3) в плазме проводилось с помощью мультиплексного метода с использованием стандартной 3-плексной тест-системы Bio-Plex Pro TGF-β Panel 3-Plex (Bio-Rad, США) на проточном лазерном иммуноанализаторе Bio-Plex 200 System (Bio-Rad, США). Далее проводилась последующая обработка полученных результатов с использованием приложения Bio-Plex Manager 6.0 Properties (Bio-Rad, США).
Для статистического анализа и построения графиков использовали пакеты статистических программ Statistica 10 и OriginPro 8.5 (США). Для проверки гипотезы о нормальном распределении использовали критерий Шапиро–Уилка, для проверки равенства дисперсий – критерий Левина. Статистический анализ проводили с помощью параметрического t-критерия Стьюдента при соблюдении нормального распределения и непараметрического критерия Манна–Уитни при несоблюдении условий нормального распределения. Сравнение групп по качественным признакам проводилось с помощью точного критерия Фишера. Для оценки диагностической эффективности исследуемого метода использовался ROC-анализ. Данные ROC-анализа представлены в виде площади под кривой с 95% доверительным интервалом. Различия между статистическими величинами считали статистически значимыми при уровне достоверности p<0,05.
Результаты и обсуждение
Анализ клинико-анамнестических данных как возможных конфаундеров показал, что средний возраст и средний индекс массы тела не различались в группах (p>0,05). Между группами не были получены статистически значимые различия по гинекологической и соматической заболеваемости. Было отмечено, что в основной группе чаще встречалось кровотечение в I триместре беременности (p=0,03). Гестационный срок в основной группе составил 36,2 (34,1; 38) недель, что было обусловлено досрочным родоразрешением путем операции кесарева сечения (КС) в связи с ухудшением состояния плода по данным инструментальных методов исследования. Клинико-анамнестические данные представлены в табл. 1.
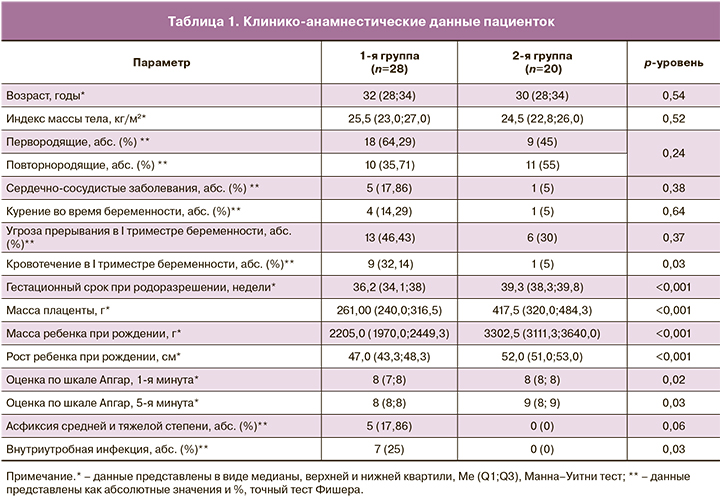
При оценке перинатальных исходов было выявлено, что масса детей при рождении в основной группе составила в среднем 2205,0 (1970,0; 2449,3) г, что было в 1,6 раза меньше, чем в группе сравнения (3302,5 (3111,3; 3640,0) г; p<0,001). Оценка состояния по шкале Апгар на 1-й минуте в основной группе составила 8 (7; 8), в группе сравнения – 8 (8; 8).
Было показано, что у детей с малыми размерами к гестационному возрасту постнатальный период чаще осложнялся внутриутробной инфекцией, по сравнению со 2-й группой (p=0,03).
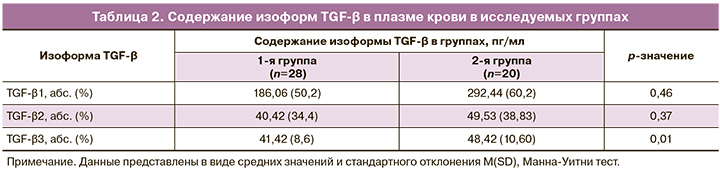
Результаты содержания изоформ TGF-β в плазме периферической крови в исследуемых группах беременных представлены в табл. 2. Согласно полученным данным, содержание TGF-β1 и TGF-β2 статистически не различалось в группах. Содержание изоформы TGF-β3 в основной группе составило в среднем 41,42 (8,6) пг/мл, что было статистически ниже, чем в группе сравнения (48,42 (10,60) пг/мл; p=0,01).
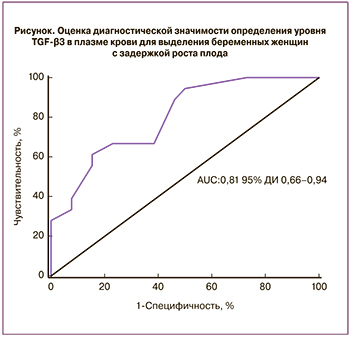 С целью определения значимости TGF-β3 в качестве диагностического теста при ЗРП нами был проведен ROC-анализ (рисунок). Согласно полученным данным, TGF-β3 является перспективным маркером для выявления групп беременных с риском развития ЗРП AUC=0,81 (95% ДИ 0,66–0,94) с чувствительностью 61%, специфичностью 85%. Это дает возможность предполагать, что именно изоформа TGF-β3 играет значимую роль в патогенезе ЗРП и может являться предиктором развития данного осложнения.
С целью определения значимости TGF-β3 в качестве диагностического теста при ЗРП нами был проведен ROC-анализ (рисунок). Согласно полученным данным, TGF-β3 является перспективным маркером для выявления групп беременных с риском развития ЗРП AUC=0,81 (95% ДИ 0,66–0,94) с чувствительностью 61%, специфичностью 85%. Это дает возможность предполагать, что именно изоформа TGF-β3 играет значимую роль в патогенезе ЗРП и может являться предиктором развития данного осложнения.
До настоящего времени исследований, направленных на изучение концентрации изоформ TGF-β1, -2 и -3 в плазме периферической крови при ЗРП не проводилось. Однако существуют работы, направленные на изучение экспрессии отдельных изоформ TGF-β в плаценте и плазме периферической крови при преэклампсии и ЗРП. Согласно нашим данным, содержание изоформ TGF-β1, -2 статистически не различалось в исследуемых группах. Эти данные согласуются с данными мировой литературы, в которых также не была выявлена связь между экспрессией TGF-β1, -2 и плацента- ассоциированными осложнениями беременности [17, 18].
Вместе с тем полученные нами данные о взаимосвязи снижения уровня TGF-β3 при ЗРП, по-видимому, свидетельствуют о его роли в патогенезе данного осложнения беременности. Вышеуказанное согласуется с исследованием Genbacev O. и соавт., которые продемонстрировали изменение экспрессии TGF-β3 в ответ на снижение оксигенации на ранних этапах физиологической беременности. Было показано, что TGF-β3 регулирует дифференцировку трофобласта, предотвращая его развитие по инвазивному фенотипу, и с началом формирования кровотока в межворсинчатом пространстве в 10–12 недель гестации, путем снижения его экспрессии, обеспечивает возможность развития инвазивного вневорсинчатого трофобласта [19, 20]. Гипоксия, которая наблюдается при плацента-ассоциированных осложнениях беременности, запускает ряд механизмов, которые приходят в действие путем активации индуцируемых гипоксией факторов 1 и 2 (HIF-1 и HIF-2) [21]. Существуют данные, согласно которым HIF-1 повышает транскрипцию TGF-β3 и приводит к нарушению процессов инвазии цитотрофобласта [22–24]. Гипоксия, наблюдаемая при плацента-ассоциированных осложнениях, приводит к активации HIF-1 и повышению экспрессии TGF-β3 в плаценте. Однако выявленное нами снижение уровня циркулирующего TGF-β3 может быть обусловлено его связыванием с растворимой формой эндоглина (Seng), являющегося изоформой корецептора TGF-β3 и обладающего антиангиогенным действием [25, 26].
Заключение
В нашей работе мы не получили значимых различий в содержании изоформ TGF-β1 и TGF-β2 в плазме крови исследуемых групп пациенток. Полученные результаты, указывающие на снижение уровня TGF-β3 в плазме женщин с ЗРП, могут являться следствием связывания его с растворимой формой эндоглина на фоне повышения экспрессии в плаценте. Эти данные подтверждают патогенетическую значимость изоформы TGF-β3 в формировании ЗРП и предопределяют возможность его использования в качестве диагностического маркера ЗРП уже на ранних сроках беременности.
- Lee A.C., Kozuki N., Cousens S., Stevens G.A., Blencowe H., Silveira M.F. et al. Estimates of burden and consequences of infants born small for gestational age in low and middle income countries with INTERGROWTH-21st standard: analysis of CHERG datasets. BMJ. 2017; 358: j3677. https://doi.org/10.1136/bmj.j3677.
- Alfirevic Z., Stampalija T., Dowswell T. Fetal and umbilical Doppler ultrasound in high-risk pregnancies. Cochrane Database Syst. Rev. 2017; 6(6): CD007529. https://doi.org/10.1002/14651858.
- Devaskar S.U., Chu A. Intrauterine growth restriction: hungry for an answer. Physiology (Bethesda). 2016; 31(2): 131-46. https://.doi.org/10.1152/physiol.00033.2015.
- Burton G.J., Jauniaux E. Pathophysiology of placental-derived fetal growth restriction. Am. J. Obstet. Gynecol. 2018; 218(2, Suppl.): S745-61. https://doi.org/10.1016/j.ajog.2017.11.577.
- Стрижаков А.Н., Мирющенко М.М., Игнатко И.В., Попова Н.Г., Флорова В.С., Кузнецов А.С. Прогнозирование синдрома задержки роста плода у беременных высокого риска. Акушерство и гинекология. 2017; 7: 34-44. [Strizhakov A.N., Miryushchenko M.M., Ignatko I.V., Popova N.G., Florova V.S., Kuznetsov A.S. Prediction of fetal growth restriction in high-risk pregnant women. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2017; (7): 34-44. (in Russian)]. https://dx.doi.org/10.18565/aig.2017.7.34-44
- Вишнякова П.А., Суханова Ю.А., Микаелян А.Г., Булатова Ю.С., Пятаева С.В., Балашов И.С., Боровиков П.И., Тетруашвили Н.К., Высоких М.Ю. Синдром задержки роста плода и маркеры митохондриальной дисфункции. Акушерство и гинекология. 2018; 6: 31-6. [Vishnyakova P.A., Sukhanova Yu.A., Mikaelyan A.G., Bulatova Yu.S., Pyataeva S.V., Balashov I.S., Borovikov P.I., Tetruashvili N.K., Vyssokikh M.Yu. Fetal growth restriction and markers for mitochondrial dysfunction. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2018; (6): 31-6. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.6.31-36
- Amarilyo G., Oren A., Mimouni F.B., Ochshorn Y., Deutsch V., Mandel D. Increased cord serum inflammatory markers in small-for-gestational-age neonates. J. Perinatol. 2011; 31(1): 30-2. https://doi.org/10.1038/jp.2010.53.
- Robb K.P., Cotechini T., Allaire C., Sperou A., Graham C.H. Inflammation-induced fetal growth restriction in rats is associated with increased placental HIF-1α accumulation. PLoS One. 2017; 12(4): e0175805. https://doi.org/10.1371/journal.pone.0175805.
- Lausten-Thomsen U., Olsen M., Greisen G., Schmiegelow K. Inflammatory markers in umbilical cord blood from small-for-gestational-age newborns. Fetal Pediatr. Pathol. 2014; 33(2): 114-8. https://doi.org/10.3109/15513815.2013.879239.
- Al-Azemi M., Raghupathy R., Azizieh F. Pro-inflammatory and anti-inflammatory cytokine profiles in fetal growth restriction. Clin. Exp. Obstet. Gynecol. 2017; 44(1): 98-103. https://doi.org/10.12891/ceog3295.2017.
- Caniggia I., Mostachfi H., Winter J., Gassmann M., Lye S.J., Kuliszewski M. et al. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3). J. Clin. Invest. 2000; 105(5): 577-87. https://doi.org/10.1172/JCI8316.
- Lash G.E., Otun H.A., Innes B.A., Bulmer J.N., Searle R.F., Robson S.C. Inhibition of trophoblast cell invasion by TGFB1, 2, and 3 is associated with a decrease in active proteases. Biol. Reprod. 2005; 73(2): 374-81. https://doi.org/10.1095/biolreprod.105.040337.
- Moser G., Huppertz B. Implantation and extravillous trophoblast invasion: From rare archival specimens to modern biobanking. Placenta. 2017; 56: 19-26. https://doi.org/10.1016/j.placenta.2017.02.007.
- Huang Z., Li S., Fan W., Ma Q. Transforming growth factor β1 promotes invasion of human JEG-3 trophoblast cells via TGF-β/Smad3 signaling pathway. Oncotarget. 2017; 8(20): 33560-70. https://doi.org/10.18632/oncotarget.16826.
- Choi J.H., Lee H.J., Yang T.H., Kim G.J. Effects of hypoxia inducible factors-1α on autophagy and invasion of trophoblasts. Clin. Exp. Reprod. Med. 2012; 39(2): 73-80. https://doi.org/10.5653/cerm.2012.39.2.73.
- Fenton T.R., Kim J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013; 13: 59. https://doi.org/10.1186/1471-2431-13-59.
- Cift T., Uludag S., Aydin Y., Benian A. Effects of amniotic and maternal CD-146, TGF-β1, IL-12, IL-18 and IFN-γ, on adverse pregnancy outcome. J. Matern. Fetal Neonatal Med. 2013; 26(1): 21-5. https://doi.org/10.3109/14767058.2012.722712.
- Rab A., Szentpéterib I., Kornyac L., Börzsönyid B., Demendid C., Valent S. et al. Placental gene expression of transforming growth factor beta 1 (TGF-β1) in small for gestational age newborns. J. Matern. Fetal Neonatal Med. 2015; 28(14): 1701-5. https://doi.org/10.3109/14767058.2014.966673.
- Genbacev O., Zhou Y., Ludlow J.W., Fisher S.J. Regulation of human placental development by oxygen tension. Science. 1997; 277(5332): 1669-72. https://doi.org/10.1126/science.277.5332.1669.
- Caniggia I., Grisaru-Gravnosky S., Kuliszewsky M., Post M., Lye S.J. Inhibition of TGF-beta 3 restores the invasive capability of extravillous trophoblasts in preeclamptic pregnancies. J. Clin. Invest. 1999; 103(12): 1641-50. https://doi.org/10.1172/JCI6380.
- Munaut C., Lorquest S., Pequex C., Blacher S., Berndt S., Frankenne F. et al. Hypoxia is responsible for soluble vascular endothelial growth factor receptor –1 but not for soluble endoglin induction in villous trophoblast. Hum. Reprod. 2008; 23(6): 1407-15. https://doi.org/10.1093/humrep/den114.
- Scheid A., Wenger R.H., Schäffer L., Camenisch I., Distler O., Ferenc A. et al. Physiologically low oxygen concentrations in fetal skin regulate hypoxia-inducible factor 1 and transforming growth factor-β3. FASEB J. 2002; 16(3): 411-3. https://doi.org/10.1096/fj.01-0496fje.
- Schäffer L., Scheid A., Spielmann P., Breymann C., Zimmermann R., Meuli M. et al. Oxygen-regulated expression of TGF-β3, a growth factor involved in trophoblast differentiation. Placenta. 2003; 24(10): 941-50. https://doi.org/10.1016/s0143-4004(03)00166-8.
- Nishi H., Nakada T., Hokamura M., Osakabe Y., Itokazu O., Huang L.E. et al. Hypoxia-inducible factor-1 transactivates transforming growth factor-β3 in trophoblast. Endocrinology. 2004; 145(9): 4113-8. https://doi.org/10.1210/en.2003-1639.
- Yinon Y., Nevo O., Xu J., Many A., Rolfo A., Todros T. et al. Severe intrauterine growth restriction pregnancies have increased placental endoglin levels: hypoxic regulation via transforming growth factor-beta 3. Am. J. Pathol. 2008; 172(1): 77-85. https://doi.org/10.2353/ajpath.2008.070640.
- Ходжаева З.С., Акатьева А.С., Холин А.М., Сафонова А.Д., Вавина О.В., Муминова К.Т. Молекулярные детерминанты развития ранней и поздней преэклампсии. Акушерство и гинекология. 2014; 6: 14-9. [Khodzhaeva Z.S., Akatyeva A.S., Kholin A.M., Safonova A.D., Vavina O.V., Muminova K.T. Molecular determinants of the development of early and late preeclampsia. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2014; 6: 14-19. (in Russian)].
Received 20.05.2019
Accepted 21.06.2019
Zarine V Khachatryan., postgraduate student of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. Tel.: +7(909)656-24-56. E-mail:
z.v.khachatryan@gmail.com
117997 Russia, Moscow, Ac. Oparina, 4 str.
Natalia E. Kan, PhD, MD, professor of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov
Ministry of Health of Russia. Tel.: +7(926)220-86-55. E-mail:
kan-med@mail.ru. Number Researcher ID B-2370-2015. ORCID ID 0000-0001-5087-5946
117997 Russia, Moscow, Ac. Oparina, 4 str.
Valentina V. Vtorushina, PhD, doctor of the highest category of the Clinical Immunology of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. Tel.:+7(916)980-78-95. E-mail:
vtorushina@inbox.ru
117997 Russia, Moscow, Ac. Oparina, 4 str.
Lyubov V Krechetova., MD, Head of the Clinical Immunology Laboratory of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named
after Academician V.I. Kulakov Ministry of Health of Russia (117997, Moscow, Ac. Oparina, 4 str.). Tel.: +7(916)647-39-29. E-mail:
l_krechetova@oparina4.ru .
ID Y-4837-208
117997 Russia, Moscow, Ac. Oparina, 4 str.
Daria K. Kharchenko, postgraduate student of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. Tel.: +7(915)165-87-00. E-mail:
drkharchenko@mail.ru
117997 Russia, Moscow, Ac. Oparina, 4 str.
Diana A Mantrova., postgraduate student of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. Tel.: +7(909)636-72-59. E-mail:
d-mantrova@yandex.ru
117997 Russia, Moscow, Ac. Oparina, 4 str.
Victor L. Tyutyunnik, PhD, MD, Head of the obstetric physiological department of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. Tel.: +7(903)969-50-41. E-mail:
tioutiounnik@mail.ru. ID B-2364-2015.ORCID ID 0000-0002-5830-5099
117997 Russia, Moscow, Ac. Oparina, 4 str.
For citation: Khachatryan Z.V., Kan N.E., Vtorushina V.V., Krechetova L.V., Kharchenko D.K., Mantrova D.A., Tyutyunnik V.L. Role of transforming growth factor-β in the formation of fetal growth restriction.
Akusherstvo i Ginekologiya / Obstetrics and Gynecology. 2019; (11): 107-12. (in Russian).
http://dx.doi.org/10.18565/aig.2019.11.107-112


 С целью определения значимости TGF-β3 в качестве диагностического теста при ЗРП нами был проведен ROC-анализ (рисунок). Согласно полученным данным, TGF-β3 является перспективным маркером для выявления групп беременных с риском развития ЗРП AUC=0,81 (95% ДИ 0,66–0,94) с чувствительностью 61%, специфичностью 85%. Это дает возможность предполагать, что именно изоформа TGF-β3 играет значимую роль в патогенезе ЗРП и может являться предиктором развития данного осложнения.
С целью определения значимости TGF-β3 в качестве диагностического теста при ЗРП нами был проведен ROC-анализ (рисунок). Согласно полученным данным, TGF-β3 является перспективным маркером для выявления групп беременных с риском развития ЗРП AUC=0,81 (95% ДИ 0,66–0,94) с чувствительностью 61%, специфичностью 85%. Это дает возможность предполагать, что именно изоформа TGF-β3 играет значимую роль в патогенезе ЗРП и может являться предиктором развития данного осложнения.


