The role of intraoperative frozen section analysis in the choice of surgical strategy for ovarian tumors
Malignant ovarian tumors are latent, lack specific clinical and diagnostic markers, and rarely detected at early stages resulting in suboptimal treatment outcomes. Gynecologists and oncologists often have different views of the clinical situation when the organ-sparing approach of gynecologists may run counter to the radical strategy of oncologists. Intraoperative frozen section analysis of ovarian tumors is necessary to determine tumor grade and guide surgical decision-making.Nosova Yu.V., Solopova A.E., Asaturova A.V., Tregubova A.V., Kometova V.V., Khabas G.N.
Aim. To determine the sensitivity, specificity, and prognostic significance of intraoperative frozen section analysis (IFSA) in the differential diagnosis of ovarian tumors, its role in the choice of surgical strategy, and the feasibility of determining the histopathologic subtype of cancer.
Materials and methods. This was a descriptive longitudinal study conducted at the Department of Innovative Oncology and Gynecology, V.I. Kulakov NMRC for OG&P. During the 2-year data collection period, 77 intraoperative frozen section analyses were performed.
Results. Among 77 patients with ovarian tumors, frozen section results were reported as benign, malignant, and borderline in 20 (26%), 39 (50.6%), and 18 (23.4%) tumors. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of IFSA for detecting benign tumors were 100%, 93.4%, 77.8%, and 100%, respectively. For the detection of malignant tumors, the sensitivity specificity, PPV, and NPV were 95%, 97.1%, 97.4%, and 94.4%. However, IFSA had a lower sensitivity (76.2%) and PPV (88.9%) for the diagnosis of borderline tumors. In these ovarian neoplasms, specificity and NPV were 96.3% and 91.2%.
Conclusion. IFSA is useful in the differential diagnosis of ovarian tumors and helps guide surgical decision-making.
Keywords
Ovarian tumors are the most commonly diagnosed gynecological malignancy, ranking second only to uterine neoplasms. According to the Royal College of Obstetricians and Gynecologists (RCOG), up to 10% of women will have some form of surgery during their lifetime for an ovarian mass [1, 2]. Approximately 85% of patients are diagnosed with epithelial tumors, among which benign tumors constitute 70–80%, while malignant tumors account for about 20–30% [3, 4].
Factors linked to a higher risk of developing ovarian cancer (OC) include age, family history of ovarian cancer, inherited germline mutations in BRCA1 and BRCA2 genes, obesity, infertility or a history of only one pregnancy, use of hormone replacement therapy, and increased the number of ovulatory cycles during lifetime [5, 6]. Most risk factors demonstrate significant heterogeneity in OC subtypes [7]. Inherited mutations account for 5–15% of ovarian carcinomas. Considering this, it is necessary to have awareness regarding hereditary ovarian cancer in patients with a history of a malignant neoplasm of any location and established predisposing mutations BRCA1, BRCA2, P53, STK11 (Peutz-Jeghers syndrome) [8–12], DNA mismatch repair genes (Lynch syndrome – hereditary non-polyposis colorectal cancer), RAD51C, DICER, etc., as well as a complicated genetic history of breast, ovarian, endometrial, colon, stomach, kidney, lung cancer. Genetic counseling is recommended for these women, including discussing the risks and benefits of prevention (risk reduction surgery, RRSO) [13–15]. Malignant ovarian tumors are latent, lack specific clinical and diagnostic markers, spread by direct implantation or dissemination by hematogenous or lymphatic spread, and rarely detected at early stages resulting in suboptimal treatment outcomes. Five-year survival rates remain below 35%, while the prognosis depends on the stage of cancer at diagnosis [16, 17]. The five-year survival rate for patients with stage I disease is 80–90%, and the rate for patients with stage III–IV disease is 10–20% [18]. Leading scientific societies focused on gynecologic oncology (SGO, ACOG) have proposed standards for patient examination, including 1) medical history taking and clinical examination; 2) CA-125 blood test, 3) transabdominal and transvaginal Doppler color flow mapping.
Differential diagnosis has a crucial role in decision-making for the management of patients with ovarian neoplasms. To date, there is no effective and cost-effective screening. Intraoperative frozen section analysis (IFSA) of ovarian tumors is used, as a rule, in tertiary centers during the operation for a quick diagnosis to decide if further surgical treatment is required during a single procedure. The importance of IFSA remains undisputed since the use of this technique helps avoid repeated surgery or unnecessarily extensive surgical interventions, which affects patients’ quality of life of, fertility, the rate of intraoperative and postoperative complications, the length of hospital stay, and the duration of the rehabilitation. The present study was conducted to investigate the role of IFSA in the choice of surgical strategy, and we also attempted to determine the histotype of tumors at the stage of intraoperative diagnosis.
The present study aimed to determine the sensitivity, specificity, and prognostic significance of IFSA in the differential diagnosis of ovarian tumors, its role in guiding the extent of surgery, and the feasibility of determining the histopathologic subtype of cancer.
Materials and methods
This descriptive longitudinal study was conducted at the Department of Innovative Oncology and Gynecology, V.I. Kulakov NMRC for OG&P. The study analyzed IFSA results of 77 patients who underwent surgery between 2018 and 2020. The number of sections varied from 1 to 4 and was determined based on the type and size of the tumor. Sections (7 to 8 μm thick) were prepared using Leica CM1950 and were stained with hematoxylin and eosin. All sections were examined under a microscope at low and high magnification by two pathologists. If there was a discrepancy between the diagnoses, a consensus decision was made by the council. The turnaround time of frozen section diagnosis ranged from 20–30 minutes. The diagnosis was passed on to the surgical team. The IFSA result was later compared with the final histological diagnosis.
The diagnostic accuracy of this technique (sensitivity, specificity, positive and negative predictive value) was determined by the formula:
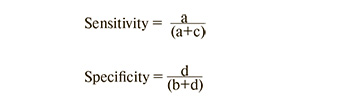
[a – patients identified by the test (true positive); b – healthy patients with a positive test result (false positive); c – patients not identified by the test (false negative); d – healthy individual who have a negative test result (true negative)].
Statistical analysis was performed using SPSS software version 16.0 for Windows. We also analyzed the findings of a standard clinical examination (the level of the CA-125, ultrasound characteristics of neoplasms). Written informed consent was obtained from all patients who participated in this prospective study.
Results
Intraoperative frozen section analysis was performed in 77 patients with ovarian neoplasms during 18 months. The age of the patients in the study group varied from 18 to 71 years, most commonly ranging from 31 to 45. The age distribution ranged from 18 to 30, from 31to 45, from 46 to 60, and from 61 to 75 in 9, 32, 23, and 13 patients, respectively.
Clinical findings, symptoms, menstrual function, family history, and past exposure to risk factors were also assessed. Among 77 patients, the most common symptoms were lower abdominal pain (68.6%), an increase in the abdominal volume, dyspepsia, menstrual irregularities, constipation, and frequent urination with elements of urinary incontinence. The patients underwent pre-operative ultrasound examination with a detailed description of the tumor. All patients with neoplasms with «borderline risk of malignancy» (suspected extra-ovarian invasion, ureteral involvement, hydronephrosis, lymphadenopathy, and/or moderate to massive ascites) were referred for MRI as a second-level follow-up study. The laparoscopic and laparotomic approach was used in 29 and 48 patients, respectively.
Sonographic characteristics
Ascites of varying severity was detected in 28 (36.4%) of patients. Twenty three (57.5%) patients with malignant ovarian neoplasms had severe ascites. In 43/77 (55.8%) patients, the tumor size was more than 10 cm. Most ovarian tumors were unilateral. Of 23 (29.9%) bilateral neoplasms, 17 (73.9%) were malignant. It was noted that 37 (48%) tumors were cystic, but in 40 (52%) cases, there was a solid component.
Tumor marker CA-125
Serum levels of the CA-125 tumor marker were determined in all patients during the preoperative examination. Thirty six (46.8%) patients had serum CA-125 > 35 U/ml. Serum levels of CA-125 > 200 U/ ml were detected in 23.4% of patients (n=18). Thirty-two of 40 patients (80%) with histologically confirmed malignant tumors had increased levels of serum CA-125. After the tumor removal, it was immediately transferred to the pathomorphological laboratory with the patient's clinical data. After careful examination of the tumor, sections were performed at the pathologist's discretion. The number of frozen sections ranged from 1 to 4, which was determined based on the tumor type and size. In all tumors diagnosed as borderline, at least two cryostat sections were examined using a Leica CM1860 cryostat. Cryostat sections (7 to 8 μm thick) were stained with hematoxylin and eosin. All sections were studied under a microscope at low and high magnification by two pathologists to reduce the operator dependence of the technique. If there was a discrepancy between the diagnoses, a consensus decision was made by the council. The diagnosis was passed on to the surgical team. The turnaround time of frozen section diagnosis from receipt in the laboratory to the issue of a report was approximately 20 minutes. The diagnosis was classified as primary ovarian epithelial neoplasm – benign, borderline, or malignant; primary tumor from ovarian germ cells, benign non-neoplastic conditions, and during the study, the histological subtype of the tumor was determined. Based on the IFSA results, the extent of surgical intervention was determined intraoperatively. The IFSA result was later compared with the final histopathologic diagnosis (Figure).
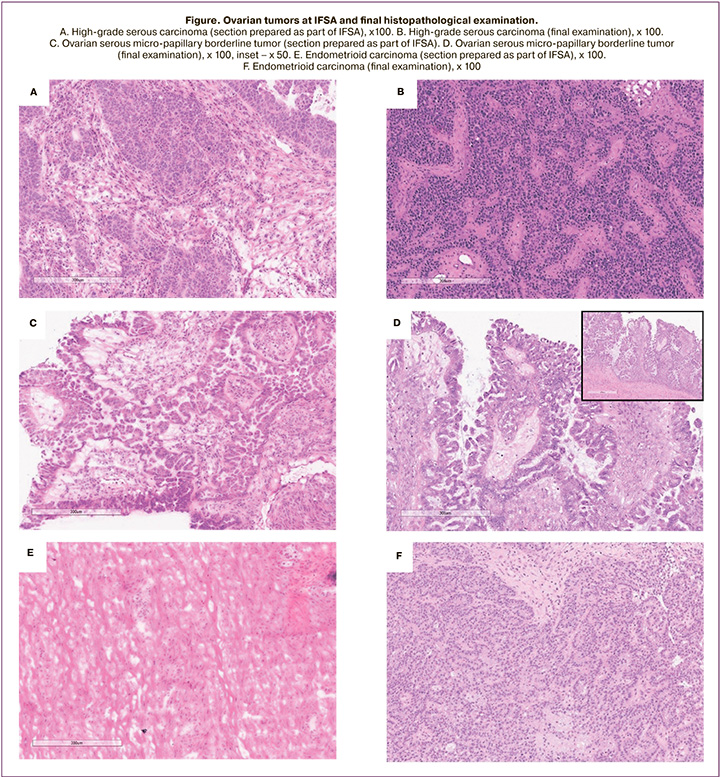
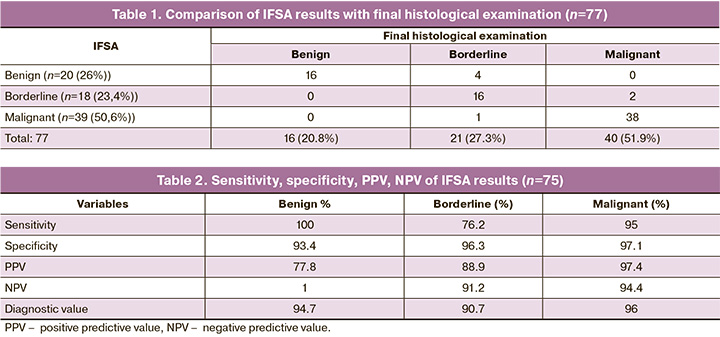
Intraoperative frozen section analysis showed that 20 (26%), 39 (50.6%), and 18 (23.4%) tumors were benign, malignant, and borderline, respectively. The final histopathological diagnosis showed that there were 75 (97.4%) epithelial tumors, one (1.3%) benign Brenner's tumor, and one (1.3%) luteinized ovarian thecoma. Among 75 epithelial tumors, 14 (20.8%) were benign (10 serous and four mucinous), 21 (27.3%) were borderline tumors (15 serous, three serous, and three mucinous), and 40 (51.9%) were malignant (29 high-grade serous carcinomas, five low-grade serous carcinomas, one mucinous carcinoma, three endometrioid and two clear cell carcinomas).
Seven (9.3%) out of 75 cases of EOT had a discrepancy between IFSA results and the final histological findings, while the frequency of coincidences between diagnoses was 90.7% (Table 1). There were two reports where frozen section results and final histological diagnosis were reported as borderline serous cystadenoma and low-grade serous papillary cystadenocarcinoma (LGSC), respectively.
Among 7 cases, four borderline ovarian tumors were misdiagnosed as benign (one mucinous and three serous) by IFSA. And also, one borderline serous cystadenoma was misdiagnosed as high-grade serous cystadenocarcinoma (HGSC).
The IFSA accuracy for detecting benign, borderline, and malignant tumors was 94.7%, 90.7%, and 96, respectively. For benign tumors, IFSA showed 100% sensitivity, 93.44% specificity, 77.8% positive predictive value (PPV), and 100% negative predictive value (NPV). For the detection of malignant tumors, the sensitivity was 95%, specificity 97.1%, PPV 97.44%, and NPV 94.4%. However, IFSA had a lower sensitivity (76.2%) and PPV (88.9%) to diagnose borderline tumors. In these ovarian neoplasms, specificity and NPV were 96.3% and 91.2% (Table 2). Both cases of diagnosis of non-epithelial tumors were correctly identified based on IFSA results. We also attempted to determine the histotype of the studied tumors as part of IFSA.
Discussion
IFSA of ovarian tumors is aimed at intraoperative diagnosis, which is needed due to the lack of preoperative morphological data. Puncture of the neoplasm is associated with a high risk of malignancy dissemination. IFSA is an essential diagnostic tool for determining the nature of ovarian masses. In 54% of cases, a bilateral examination of the ovaries was carried out. IFSA is quite accurate in diagnosing non-neoplastic ovarian masses, such as follicular, luteal, endometrioid cysts, endometriosis, etc. IFSA of true ovarian tumors has some limitations, especially in BOT, and are characterized by some histological features including abnormal stratification of the epithelial layer, an increase in mitotic activity, nuclear atypia in some cases, and have no stromal invasion. Intraoperative frozen section analysis allows timely adjustment of the extent of surgery. In benign and borderline ovarian tumors, as well as in many malignant neoplasms, organ-sparing treatment is possible in women of reproductive age, while malignant epithelial tumors, as a rule, require extensive surgical intervention such as hysterectomy with adnexectomy, omentectomy, surgical staging, as well as pelvic and para-aortic lymphadenectomy. Examination of ovarian tumors requires a careful gross examination of the resected specimen in conjunction with clinical data and imaging data. An analysis of multiple cryostat sections from different parts may be needed. Also, due to the high operator dependence of the technique, two independent specialists should be involved; in case of a discrepancy between the diagnoses, a consensus decision should be made by the council. According to our data, ovarian tumors were underdiagnosed in 7.8%, which is mainly associated with the presence of only micro-foci of the tumor. The effectiveness of IFSA in the differential diagnosis of benign and malignant ovarian tumors increases in the presence of a pronounced solid component. Large tumor size, bilateral involvement, and mucinous nature are also unfavorable factors in determining the malignant potential using IFSA. The main disadvantages of intraoperative frozen section analysis include a limited number of areas used and the lower quality of histological specimens than in the regular histological examination in paraffin blocks, which affects the accuracy of the morphological diagnosis. Ice crystal formation results in artifacts and poor morphological preservation of the tissue, which renders low slide quality and difficulty obtaining a diagnosis.
The age of the patients in the study group varied from 18 to 71 years, most commonly ranging from 31 to 45. The youngest patient in our study was 18 years old, and the oldest 71 years old. The median age was 43.6 years.
Visualization characteristics
In our study, most patients with malignant ovarian tumors had tumors measuring more than 10 cm with a solid component and ascites. These are significant parameters of the malignant potential of the tumor.
Tumor marker CA-125
Serum CA-125 > 35 U/ml were found in 36 (46.8%) cases. Serum CA-125 >200 U/ml were detected in 23.4% of cases (18 patients). In 32 of 40 patients with histologically confirmed malignant tumors (80%) CA-125 level exceeded the normal range.
Diagnostic accuracy of intraoperative frozen section analysis
Of 77 cases of EOT, 7 cases (9.3%) showed a discrepancy between the IFSA results and the final histological findings, while the coincidence rate was 90.7%. There were 2 reports where IFSA showed serous borderline cystadenoma, and in the final histological diagnosis, low grade serous papillary cystadenocarcinoma (LGSC) was verified. Among 7 cases, four borderline ovarian tumors were misdiagnosed as benign (one mucinous and three serous) by IFSA. And also, one borderline serous cystadenoma was misdiagnosed as high-grade serous cystadenocarcinoma (HGSC).
Consistent with previously published studies, our findings showed that the frozen section had high sensitivity, specificity, and predictive values for benign and malignant ovarian neoplasms, but low sensitivity and PPV for borderline tumors. The diagnostic accuracy of cryostat sections can be influenced by various factors, including sampling error, quality of specimens, and pathologist experience. IFSA is quite accurate in diagnosing non-neoplastic ovarian masses, such as follicular, luteal, endometrioid cysts, endometriosis, etc. A mucinous ovarian tumor can sometimes contain benign, borderline, and malignant components, in contrast to serous tumors. About 20% of borderline serous tumors contain small foci of serous cancer, which may not be included in the section during IFSA due to the limited sampling [19, 20]. The possibilities of intraoperative study of borderline mucinous tumors are mainly limited due to the large tumor size and heterogeneity of the structure: benign, borderline, and malignant areas are present in the same tumor. The WHO classification (4th edition, 2014) provides the requirements for preparing material for routine histological examination. For tumors less than 10 cm, at least one section per centimeter largest tumor diameter should be examined, increasing to two blocks per centimeter diameter in tumors >10 cm. Particular attention should be paid to areas of solid structure, which is not feasible under IFSA conditions [21].
The morphological complexity of borderline tumors was found to be the leading cause of inaccurate IFSA results. Another possible reason of the low PPV for borderline tumors in the present study may be an insufficient number of borderline tumors. PPV and NPV are directly related to disease prevalence. In two cases of underdiagnosis, non-radical surgical intervention was performed. The second operation was hysterectomy with adnexectomy, omentectomy, pelvic and lumbar lymphadenectomy (low grade serous papillary cystadenocarcinoma). Given the young age of patients who had borderline benign tumors, the extent of surgery was ultra-conservative, which allowed the patients to preserve fertility and avoid unnecessarily extensive surgery. In the case where borderline serous cystadenoma was misdiagnosed as HGSC, the patient (28 years old, unfulfilled reproductive function) categorically refused a radical operation and underwent organ-sparing surgery. In this case, overdiagnosis could have profound health implications for the young patient. None of the patients underwent chemotherapy based on IFSA. Specific treatments were administered based on final histopathological examination.
Ovarian cancer is a highly heterogeneous disease characterized by multiple histological subtypes that differ in their cellular origin, pathogenesis, molecular genetic characteristics, and prognosis. Ovarian malignant tumors, also known as carcinomas, are composed of five main histotypes: high grade serous ovarian carcinomas (HGSOC; 70%), endometrioid ovarian carcinomas (ENOC; 10%), clear cell ovarian carcinomas (CCOC; 10%), mucinous ovarian carcinomas (MOC; 3%) and low-grade serous ovarian carcinomas (LGSOC; <5%). Within each of these subtypes, although most commonly serous and mucinous, there are tumors of undetermined malignant potential, known as borderline tumors or low-grade tumors, which contain microscopic malignant foci without invasion of the underlying stroma.
The cellular origin and pathogenesis of ovarian cancer are not entirely clear. Morphological and genetic studies have generated a hypothesis of extraovarian origin of epithelial ovarian cancer. According to this hypothesis, serous tumors originate from the fallopian tube epithelium (directly or indirectly through endosalpingiosis), endometrioid and clear-celled tumors are derivatives of the endometrium (indirectly through endometriosis), and mucinous tumors are presumably associated with transcellular transitional epithelium in the mesentery of the Fallopian tube (mesosalpinx).
Serous ovarian cancers are now classified as high-grade serous ovarian carcinoma (HGSOC) and low-grade serous ovarian carcinoma (LGSOC). In addition to different histological and molecular genetic characteristics, these two variants are believed to have different origins and different pathogenesis.
Research suggests that HGSOC originates from the epithelium of the Fallopian tube. Most likely, this type of inclusion cysts is formed when the epithelium of the fallopian tube is implanted onto the surface of the ovary and transforms into serous tubal intraepithelial carcinoma (STIC). Accordingly, in the absence of STIC in women with HGSOC or primary serous carcinoma of the peritoneum, it can be assumed that the initiation of this process did not occur on in the Fallopian tube, but in the ovary (in an inclusive cyst). However, the predecessor of the pathological process, in this case, will still be the epithelium of the Fallopian tube.
In many cases, HGSOCs, as some researchers believe, develop from LGSOCs, but the mechanism of this transformation is not fully understood. The origin of HGSOC from the surface epithelium of the ovary cannot be completely ruled out, especially in the area of the tubo-ovarian and tubo-peritoneal transition zones.
The current concept of the pathogenesis of well-differentiated serous ovarian carcinomas (LGSOC) suggests the sequential transformation of the epithelium of inclusion cysts with a Müllerian phenotype into serous cystadenomas, serous cystadenomas into borderline serous tumors, and the latter into non-invasive LGSOCs with further development of LGSOCs.
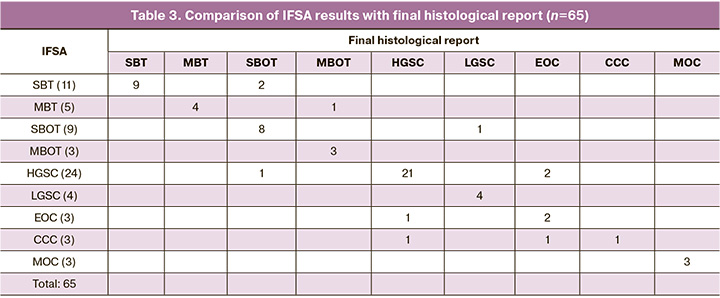
In our study, we attempted to determine the histotype of the tumor at the stage of intraoperative frozen section analysis. Sixty-five samples of ovarian neoplasms were examined. The results presented in Tables 3 and 4 demonstrate high diagnostic accuracy of the intraoperative diagnosis in cases of highly differentiated and poorly differentiated ovarian cancer. However, they were less promising in the differential diagnosis of endometriotic and clear cell types of tumors. Some high-grade serous and endometrioid carcinomas are extremely difficult and sometimes impossible to differentiate by IFSA.
Moreover, the differential diagnosis remained challenging to make by final histopathological examination. In many cases, an additional immunohistochemical study was required. Differential diagnosis by IFSA is problematic in borderline serous tumors and low-grade serous carcinomas. The determining factor in making these diagnoses is the presence of a micro-invasive tumor component, which can sometimes be detected only during the final histopathological examination. And if, in most cases of differential diagnosis of high-grade serous, clear cell, and endometrioid carcinomas, the determination of the histotype is not of fundamental importance (there is enough information that the tumor is a high-grade malignancy). But in the case of differential diagnosis of low-grade serous borderline tumors and serous carcinomas, the histologist’s report of IFSA results helps the surgeon choose a tailored surgical strategy. Therefore, improving the quality of cryostat sections and modifying the algorithms for IFSA play an essential role.
Conclusion
IFSA has demonstrated high sensitivity, specificity, and NPV for detecting benign and malignant ovarian neoplasms. Intraoperative frozen section analysis was found to be accurate and useful for intraoperative assessment of ovarian neoplasms. IFSA results help determine the tumor type and choose a tailored surgical strategy. Of particular note are borderline tumors, low-grade ovarian carcinomas, and mucinous ovarian tumors. The likelihood of overdiagnosis and the limitations of IFSA should be discussed with young patients before surgery that may mistakenly result in loss of fertility. The choice of the appropriate surgical strategy in gynecological cancer patients of reproductive age significantly affects not only the success of anticancer treatment but also the duration and quality of life of patients. The use of organ-sparing surgery in an appropriate clinical situation allows the preservation of sexual and reproductive functions. The informed consent should include discussion of all possible options that may be considered based on IFSA results. Finally, intraoperative communication between surgeons and pathologists is crucial and can significantly minimize errors.
References
- Management of Suspected Ovarian Masses in Premenopausal Women (Green-top Guideline N 62) [Electronic resource]: RCOG/BSGE Joint Guideline /British Society of Gynecological Endoscopy (BSGE). London: RCOG, 2011. URL: https://www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg62.
- Макаров О.В., Борисенко С.А. Профилактика, диагностика, лечение рака яичников. Российский медицинский журнал. 1996; 3: 36-40. [Makarov O.V., Borisenko S.A. Prophylaxis, diagnosis, treatment of ovarian cancer. Medical Journal of the Russian Federation. 1996; 3: 36-40. (in Russian)].
- Гаспаров А.С., Жорданиа К.И., Паяниди Ю.Г., Дубинская Е.Д. Онкогинекологические аспекты кистозных образований яичников. Вестник Российской академии медицинских наук. 2013; 68(8): 9-13. [Gasparov A.S., Zhordania K.I., Payanidi Y.G., Dubinskaya E.D. Oncogynecologycal aspects of adnexal masses. Annals of the Russian Academy of Medical Sciences. 2013;68(8):9-13. (in Russian)].
- Permuth-Wey J., Besharat A., Sellers T. Epidemiology of ovarian cancer: An update. In: Advances in diagnosis and management of ovarian cancer. Farghaly S.A., editor. New York: Springer Science and Business Media; 2014. PMID: None.
- Wentzensen N., Poole E.M., Trabert B. et al. Ovarian Cancer Risk Factors by Histologic Subtype: An Analysis From the Ovarian Cancer Cohort Consortium. J Clin Oncol. 2016;34(24):2888-98. PMID: 27325851. http://dx.doi.org/10.1200/JCO.2016.66.8178.
- Song H., Ramus S.J., Tyrer J. et al. A genome-wide association study identifies a new ovarian cancer susceptibility locus on 9p22.2. Nat Genet. 2009; 41(9): 996-1000. PMID: 19648919. http://dx.doi.org/10.1038/ng.424.
- Pharoah P.D., Tsai Y.Y., Ramus S.J. et al. GWAS meta-analysis and replication identifies three new susceptibility loci for ovarian cancer. Nat Genet. 2013;45(4):362-70, 70e1-2. PMID: 23535730. http://dx.doi.org/10.1038/ng.2564.
- Permuth-Wey J., Lawrenson K., Shen H.C. et al. Identification and molecular characterization of a new ovarian cancer susceptibility locus at 17q21.31. Nat Commun. 2013; 4: 1627. PMID: 23535648. http://dx.doi.org/10.1038/ncomms2613.
- Kuchenbaecker K.B., Ramus S.J., Tyrer J. et al. Identification of six new susceptibility loci for invasive epithelial ovarian cancer. Nat Genet. 2015;47(2):164-71. PMID: 25581431. http://dx.doi.org/10.1038/ng.3185.
- Bolton K.L., Tyrer J., Song H. et al. Common variants at 19p13 are associated with susceptibility to ovarian cancer. Nat Genet. 2010;42(10):880-4. PMID: 20852633. http://dx.doi.org/10.1038/ng.666.
- Bojesen S.E., Pooley K.A., Johnatty S.E. et al. Multiple independent variants at the TERT locus are associated with telomere length and risks of breast and ovarian cancer. Nat Genet. 2013;45(4):371-84, 84e1-2. PMID: 23535731. http://dx.doi.org/10.1038/ng.2566.
- National Institute for Health and Care Excellence (NICE). Clinical guideline [CG164]: Familial breast cancer: classification, care and managing breast cancer and related risks in people with a family history of breast cancer. 2013. PMID: None.
- Daly M.B., Pilarski R., Axilbund J.E. et al. Genetic/Familial High-Risk Assessment: Breast and Ovarian, Version 2.2015. J Natl Compr Canc Netw. 2016;14(2):153-62. PMID: 26850485. http://dx.doi.org/10.6004/jnccn.2016.0018.
- Moyer V.A., Force USPST. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(4):271-81. PMID: 24366376. http://dx.doi.org/10.7326/M13-2747.
- Domchek S.M., Friebel T.M., Singer C.F. et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304(9):967-75. PMID: 20810374. http://dx.doi.org/10.1001/jama.2010.1237.
- Jayson G.C., Kohn E.C., Kitchener H.C., Ledermann J.A. Ovarian cancer. Lancet. 2014;384:1376–1388.
- Chien J., Poole E. Ovarian cancer prevention, screening and early detection: report from the 11th Biennial Ovarian Cancer Research Symposium. Int J Gynecol Cancer. 2018;27:20-22.
- Institute of Medicine, Committee on the State of the Science in Ovarian Cancer Research, Board on Health Care Services, et al. Ovarian Cancers: Evolving Paradigms in Research and Care. Washington (DC): National Academies Press (US); 2016. PMID: 27253000.
- Pavlakis K., Messini I., Vrekoussis T., Yiannou P., Panoskaltsis T., Voulgaris Z. Intraoperative assessment of epithelial and non-epithelial ovarian tumors: a 7-year review. Eur J Gynaecol Oncol. 2009;30(6):657-60.
- Sukumaran R., Somanathan T., Mathews A., Kattor J., Sambasivan S., Nair R.P. Role of frozen section in intraoperative assessment of ovarian masses: a tertiary oncology center experience. Indian J Surg Oncol.2014;5(2):99-103. https://doi.org/10.1007/s13193-014-0311-x.
- Kurman R., Carcangiu M., Herrington C., Young R.H. WHO Classification of tumours of female reproductive organs.Lyon: IARC Press; 2014.
Received 21.07.2020
Accepted 09.09.2020
About the Authors
Julia V. Nosova, Ph.D. Student at the V.I. Kulakov NMRC for OG&P of Minzdrav of Russia. E-mail: yu_nosova@oparina4.ru. 4 Oparin str., 117997, Moscow, Russia.Alina E. Solopova, Dr. Med. Sci., Associate Professor, Leading Researcher at the Radiology Department, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
E-mail: a_solopova@oparina4.ru. 4 Oparin str., 117997, Moscow, Russia.
Aleksandra V. Asaturova, Head of the 1st Department of Anatomic Pathology, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
E-mail: A_asaturova@oparina4.ru. 4 Oparin str., 117997, Moscow, Russia.
Anna V. Tregubova, Junior Researcher at the 1st Department of Anatomic Pathology, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
E-mail: A_tregubova@oparina4.ru. 4 Oparin str., 117997, Moscow, Russia.
Vlada V. Kometova, Head of Oncopathology Department, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia. E-mail: v_kometova@oparina4.ru.
4 Oparin str., 117997, Moscow, Russia.
Grigorii N. Khabas, Ph.D., Head of the Department of Innovative Oncology and Gynecology, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
E-mail: g_khabas@oparina4.ru. 4 Oparin str., 117997, Moscow, Russia.
For citation: Nosova Yu.V., Solopova A.E., Asaturova A.V., Tregubova A.V., Kometova V.V., Khabas G.N. The role of intraoperative frozen section analysis in the choice of surgical strategy for ovarian tumors.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2020; 9: 120-128 (in Russian)
https://dx.doi.org/10.18565/aig.2020.9.120-128



