Уникальность женского репродуктивного тракта заключается в его способности одновременно выполнять функцию защиты от патогенов и обеспечивать иммунологическую толерантность к сперматозоидам и наполовину чужеродному плоду [1].
Система врожденного иммунитета, которая является первой линией иммунной защиты, осуществляет распознавание патогенных микроорганизмов с помощью сигнальных рецепторов. Роль Толл-подобных рецепторов в патогенезе репродуктивных осложнений наиболее изучена [2]. Однако недавно были открыты и другие группы сигнальных рецепторов, среди которых наибольший интерес представляют NOD-подобные рецепторы.
NOD-подобные рецепторы (NOD-like receptors, NLRs) – это семейство рецепторов, состоящее из 23 структурно родственных белков [3], играющих важную роль в индукции иммунного ответа и апоптоза, а также в процессе развития зиготы [4].
NLRs разделяют на группы в зависимости от их структуры и биологических функций в организме человека.
К группе рецепторов, играющих роль в процессах репродукции, относят NLRP 2, 4, 5, 8, 9, 11, 13 и 14 [5]. Показана их важная роль в развитии зиготы на стадии морулы, в патогенезе трофобластической болезни и болезней геномного импринтинга (синдрома Беквита-Видемана и Сильвера-Рассела) [4, 5].
Большая группа NLRs индуцирует воспалительный ответ. NLRP1, NLRC4 и NLRP3 участвуют в образовании инфламмасом [6, 7]. Функции NLRP6, NLRP7 и NLRP12 изучены недостаточно хорошо, однако предполагают, что они способны играть роль в образовании инфламмасом, а также ингибировать NF-kB-зависимую продукцию цитокинов [8–10].
Рецепторы NOD1 и NOD2 не участвуют в образовании инфламмасом, но при связывании с пептидогликаном бактерий и вирусной РНК индуцируют выработку провоспалительных цитокинов, интерферонов, а также активируют процессы аутофагии [5]. Данные рецепторы представлены во всех отделах женского репродуктивного тракта.
Рецепторы NOD1 и NOD2. Рецепторы NOD1 (синонимы: NLRC1, CARD4) и NOD2 (синонимы: NLRC2, CARD15), как и остальные члены семейства NLRs, являются цитоплазменными рецепторами системы врожденного иммунитета [11]. Есть данные, что NOD1 и NOD2 могут перемещаться на клеточную мембрану, к месту бактериальной инвазии [12]. NOD1 экспрессируется, как гемопоэтическими, так и негемопоэтическими клетками, NOD2 – гемопоэтичскими и некоторыми эпителиальными клетками [13, 14].
Активация NOD1 происходит при связывании c диаминопимелиновой кислотой (iE-DAP), являющейся частью пептидогликана всех грамотрицательных и некоторых грамположительных бактерий. NOD2 связывается c мурамилпептидом, который является основной структурной единицей пептидогликана и входит в состав клеточной стенки, как грамположительных, так и грамотрицательных бактерий [12].
Основные сигнальные пути рецепторов NOD1 и NOD2 представлены на рисунке.
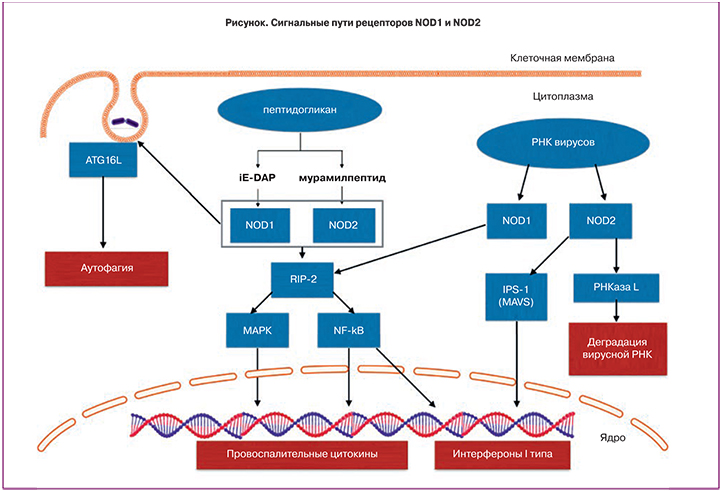
Связывание NOD1 и NOD2 c компонентами пептидогликана приводит к выработке антимикробных пептидов, провоспалительных цитокинов и хемокинов [13].
NOD1 и NOD2 способны распознавать вирусную РНК, что приводит к стимуляции выработки интерферона (IFN)-β. Кроме того, NOD2 активирует РНКазу L, что приводит к деградации вирусной РНК [15].
NOD1 и NOD2 участвуют также в процессах аутофагии. Аутофагия представляет собой процесс элиминации из клетки поврежденных органелл и белков. Этот процесс заключается в формировании аутофагосомы вокруг элиминируемой структуры, ее слиянии c лизосомой и деградации содержимого с последующим высвобождением молекул в цитоплазму клетки. Аутофагия также критически важна для элиминации бактерий, вирусов и простейших [16].
Экспрессия NOD1 и NOD2 в женском репродуктивном тракте и ее гормональная регуляция. Экспрессия NOD1, NOD2 и белка их сигнального пути RIP-2 на уровне мРНК была выявлена в маточных трубах, эндометрии, эндо- и экзоцервиксе [17]. При этом максимальная экспрессия мРНК NOD1 и NOD2 определялась в маточных трубах [18]. Сниженная экспрессия NOD1 и NOD2 в нижних отделах репродуктивного тракта, по-видимому, призвана обеспечить толерантность к присутствующей там нормальной микрофлоре, в то время как основной функцией маточных труб является поддержание стерильности и обеспечение сохранности гамет, а затем – и зиготы.
Экспрессия NOD1 и NOD2 на уровне белка была выявлена методом иммуногистохимического анализа в железистом эпителии эндометрия, эндотелии и клетках стромы. Различий в локализации данных рецепторов в зависимости от фазы менструального цикла выявлено не было. Экспрессия NOD1 и NOD2 на уровне белка также была выявлена в I триместре беременности в децидуализированной строме и железистом эпителии [19], а также, наряду c RIP-2, в клетках цито- и синцитиотрофобласта [20].
Было также установлено, что NOD1 и NOD2 во всех отделах женской репродуктивной системы были функционально активны, а при стимуляции соответствующими компонентами пептидогликана (iE-DAP, мурамилпептидом) продуцировали провоспалительные цитокины [17, 19]. Активация NOD2 мурамилпептидом в клетках трофобласта в I триместре беременности также приводила к активации синтеза провоспалительных цитокинов [20].
Было выявлено, что в культуре клеток стимуляция эпителиоцитов полицитидиловой кислотой (poly (I:С)), являющейся аналогом двухцепочечной вирусной РНК, приводила к увеличению экспрессии мРНК NOD2 и в меньшей степени – NOD1, а также к увеличению синтеза интерлейкина (IL) -8 [18].
Влияние половых гормонов на экспрессию мРНК NOD1 и NOD2 было изучено in vitro и in vivo. Было установлено, что добавление эстрадиола в культуру эпителиальных клеток маточных труб, эндометрия и цервикального канала не влияло на экспрессию мРНК NOD1 и NOD2 [18]. У пациенток репродуктивного возраста экспрессия мРНК NOD1 в эндометрии не изменялась в течение всего менструального цикла и в первом триместре беременности, а также не имела достоверной корреляционной связи c уровнями эстрадиола и прогестерона в сыворотке крови. Экспрессия мРНК NOD2 была максимальной в позднюю секреторную фазу, по сравнению c другими фазами менструального цикла и I триместром беременности. Она имела отрицательную корреляционную связь c уровнем прогестерона в сыворотке крови, в то время как достоверной корреляции c уровнем сывороточного эстрадиола отмечено не было [19].
Роль NOD1 и NOD2 в распознавании патогенных микроорганизмов в женском репродуктивном тракте. Известно, что NOD1 и NOD2 вовлечены в распознавание целого ряда бактериальных и вирусных патогенов [21]. Так, в экспериментах in vivo была установлена их способность связываться c лигандами Clostridium difficile, Escherichia coli, Pseudomonas aeruginosa и Citrobacter [22]. На модели мастита, вызванного Staphylococcus aureus, у крыс было выявлено увеличение экспрессии мРНК NOD2 [23].
В присутствии возбудителя листериоза Listeria monocytogenes происходит активация NOD2. При проникновении в клетку листерия также индуцирует NOD1-опосредованную аутофагию [24]. Однако листерия способна избегать связывания c NOD1, модифицируя структуру пептидогликана путем деацитилирования, что обусловливает ее длительную внутриклеточную персистенцию [25].
NOD1 и NOD2 способны распознавать компоненты пептидогликана Neisseria gonorrhoeae [26]. Известно, что пептидогликан гонококков способен вызывать гибель клеток реснитчатого эпителия маточных труб [27]. Однако гонококк c помощью собственных ферментов (литических транс-гликозилаз) способен расщеплять пептидогликан до небольших мономерных фрагментов, которые являются слабыми активаторами NOD2. Это позволяет Neisseria gonorrhoeae снижать интенсивность иммунного ответа [28]. Интересно, что в культуре эпителиальных клеток цервикального канала добавление убитого штамма Neisseria gonorrhoeae вызывает увеличение экспрессии NOD2 и индукцию выработки IL-8, в то время как в культуре эпителиальных клеток маточных труб увеличения экспрессии мРНК NOD2 и синтеза IL-8 не происходит. Авторы предполагают, что для активации NOD2 в эпителии маточных труб необходимо их связывание c компонентами пептидогликана живых гонококков [18]. Можно также предположить, что NOD2 в эпителиоцитах маточных труб имеет меньшее сродство к компонентам пептидогликана гонококков, по сравнению c эпителиоцитами цервикального канала.
Известно, что Mycoplasma spp. и Ureaplasma spp. не имеют клеточной стенки, содержащей пептидогликан и поэтому не распознаются NOD1 и NOD2 [29, 30]. Однако недавно было установлено, что хламидии способны синтезировать пептидогликан, в том числе его главный компонент – мурамилпептид [31].
Это позволило предположить, что NOD1 и NOD2 способны участвовать в их распознавании. В эпителиальных клетках и фибробластах in vitro рецептор NOD1 распознает Chlamydia trachomatis, что приводит к стимуляции продукции IL-6, IL-8, макрофагального воспалительного протеина (MIP)-2 и IFN-γ.
Однако in vivo на модели мышей было показано, что инфицирование Chlamydia trachomatis приводит к сниженной продукции провоспалительных цитокинов, по сравнению c Chlamydia pneumonia в легких. Возможно, это связано c недостаточным количеством пептидогликана в клеточной стенке Chlamydia trachomatis или различиями в связывании этих двух видов хламидий c NOD-подобными рецепторами [14, 32].
В большом клиническом исследовании, проведенном в Дании, было установлено, что пациентки c аллелем NOD1 +32656GG значительно реже страдали хламидийной инфекцией, чем пациентки, не имевшие данного аллеля. Для NOD2 аллеля c протективным действием выявлено не было. Однако в тех случаях, когда носительницы аллеля NOD1 +32656GG все-таки заражались хламидийной инфекцией, она сопровождалась выраженными симптомами и чаще приводила к спаечному процессу маточных труб. Авторы предполагают, что данный аллель приводит к функциональной неполноценности NOD1, что в конечном итоге способствует снижению NOD1-опосредованного синтеза IL-1β. Это, в свою очередь, приводит к стимуляции специфического CD4+ Т-клеточного ответа и элиминации Chlamydia trachomatis из репродуктивного тракта. Если же элиминировать патоген не удалось, то избыточный иммунный ответ будет способствовать развитию выраженной клинической симптоматики и увеличивает риск развития спаечного процесса [33].
В клетках трофобласта Chlamydia trachomatis способна стимулировать NOD1-опосредованный синтез провоспалительного цитокина IL-1β [34].
NOD1 и NOD2 не распознают вирус простого герпеса и папилломавирус, но способны распознавать двухцепочечную РНК цитомегаловируса [35, 36].
Так как женский репродуктивный тракт является входными воротами для вируса иммунодефицита человека (ВИЧ), была изучена роль NOD1 и NOD2 эпителиоцитов в его распознавании. В культуре эпителиальных клеток эндометрия добавление ВИЧ-1 IIIB (CXCR4/X4-тропный вирус) приводило к увеличению экспрессии мРНК NOD2, в то время как внесение в ту же культуру клеток CCR5/R5-тропного штамма ВИЧ-1 BAL не влияло на экспрессию мРНК NOD1 и NOD2. Так как CCR5/R5-тропный вирус ВИЧ-1 может проходить сквозь эпителиальные клетки путем трансцитоза, а CXCR4/X4-тропный вирус ВИЧ-1 способен реплицироваться в эпителиальных клетках, авторы предполагают, что эпителиоциты имеют механизмы для распознавания последнего и индукции иммунного ответа [18].
Известно, что NOD1 и NOD2 не распознают дрожжеподобные грибы рода Candida [37, 38]. Однако на модели мышей была показана роль NOD2 в распознавании возбудителя протозойной инфекции – Toxoplasma gondii, для которой характерна внутриклеточная персистенция. Было выявлено, что у нокаутированных по NOD2 мышей, зараженных токсоплазмой, происходит слабая дифференцировка CD4+ Т-лимфоцитов в Т-хелперы и снижение синтеза IL-2, что приводит к длительной персистенции патогена [39].
Заключение
Таким образом, NOD1 и NOD2 экспрессируются во всех органах женского репродуктивного тракта, при этом максимальная их экспрессия наблюдается в маточных трубах. Экспрессия мРНК NOD1 в эндометрии не зависит от фазы менструального цикла, в то время как экспрессия NOD2 максимальна в позднюю секреторную фазу.
NOD1 и NOD2 способны распознавать ряд условно-патогенных и патогенных бактерий, а также вирусов и простейших, персистирующих в женском репродуктивном тракте. Некоторые микроорганизмы (Listeria monocytogenes, Neisseria gonorrhoeae) имеют механизмы, позволяющие избежать распознавания, что обеспечивает их длительную внутриклеточную персистенцию.
Дальнейшие исследования сигнальных рецепторов врожденного иммунитета будут способствовать разработке мер профилактики и лечения инфекционно-воспалительных заболеваний женских половых путей.



