Впервые в 1991 г. в обзоре Labrie et al. было сделано смелое заявление о том, что «интракринное образование эстрогенов в периферических тканях у женщин составляет порядка 75% в репродуктивном периоде и почти 100% после менопаузы» [1]. Благодаря широкому внедрению и увеличению применения жидкостной хроматографии и масс-спектрометрии (ЖХ-МС/МС), в последние годы возросло количество исследований концентраций тканеспецифичных стероидов [2]. Растет научный интерес к исследованию роли местных (интракринных) стероидов в этиологии эстрогензависимых заболеваний, таких как эндометриоз, рак молочной железы и рак эндометрия [3], а также в регуляции репродуктивной функции [4].
Эндометрий является одной из удивительных систем женского организма, характеризующийся высоким регенеративным потенциалом, обеспечивающим восстановление, адаптацию и реагирование с целью имплантации и трансформации чужеродных белков эмбриона, что позволяет в будущем прогрессировать беременности. Эндометрий – это комплекс клеток с функцией рецепторной восприимчивости в течение фертильного цикла. Тканевой ответ к стероидным гормонам включает регенерацию, ангиогенез, дифференцировку и локальный воспалительный ответ. При отсутствии раздражителя в виде эмбриона возникает отторжение эндометрия – менструация.
В ответ на колебания концентраций эстрогенов и прогестерона, циркулирующих в крови, ткань эндометрия испытывает циклические эпизоды пролиферации и секреции, а при отсутствии беременности в норме- отторжение функционального слоя и регенерация. Функциональные изменения эндометрия характеризуются перестройкой желез и дифференцировкой стромальных клеток (децидуализацией) [5]. Они сопровождаются изменениями как количества, так и популяции резидентных иммунных клеток, которые играют ключевую роль в регулировании дифференцировки сосудистой сети при подготовке к имплантации и репарации эндометрия во время менструации [6]. По мнению Gibson D.A. et al. и Mueller J.W. et al. менструация является воспалительным процессом, учитывая повышенные концентрации простагландинов, которые также могут быть вызваны интракринными механизмами, включая локальную экспрессию ферментов, а также повышенный синтез провоспалительных цитокинов, хемокинов и матриксных металлопротеиназ [7].
Существуют эстрогеновые рецепторы ЭРα и ЭРβ, кодируемые отдельными генами – ESR1 и ESR2, соответственно, обладающие высоким сродством к эстрогенам. Удивительным является то, что эстрадиол (Е2) обладает более высоким сродством к ЭРα и ЭРβ в отличие от эстрона (Е1), имеющего более высокое сродство только к ЭРβ [8]. Основным регулятором эстроген-зависимой пролиферации в матке является ЭРα [9], тогда как экспрессия ЭРβ необходима для секреторной трансформации эндометрия и поддержания беременности.
Необходимо отметить наличие единственного гена рецептора андрогенов (АР), расположенного на Х-хромосоме, состоящего из 3 основных доменов: вариабельный N-концевой домен, выступающий в роли «якоря» для сохранения ядерного рецептора, ДНК-связывающий домен, состоящий из двух «цинковых пальцев», и стабильный С-концевой домен [10].
Эстрогены и андрогены вызывают изменения функции клеток через афинность к ЭР и АР соответственно. Эти «негеномные» сигнальные реакции инициируются путем взаимодействия с трансмембранными рецепторами, связанными с гормонально-зависимыми G-белками.
Gibson D.A. et al. при исследовании экспрессии ранее не изученного ЭР46 в эндометрии, обнаружили его локализацию на мембране лейкоцитов –естественных киллеров (uNK) матки, участвующих в ремоделировании сосудов, что обеспечивает быстрый ответ на эстрогены [11].
Анализ биоптатов эндометрия мышей после овариэктомии при введении 0,25 мкг 17β-эстрадиола (E2) показал, что эпителиальный ЭРα необходим для пролиферации эндометрия [12]. Неоднократные исследования на мышах продемонстрировали важную роль ЭРα в паракринной регуляции децидуализации эндометрия и роль эндогенных эстрогенов как определяющего фактора при взаимодействии между различными клетками эндометрия, особенно в период имплантационной подготовки.
Также в эндометрии синтезируются ферменты, участвующие в метаболизме и конверсии андрогенов в эстрогены. Конверсия стероидов зависит от фазы менструального цикла, так, например, во время секреторной фазы наблюдается повышенное превращение ДГЭА в тестостерон [13].
Poutanen et al. сравнили концентрации эстрона и эстрадиола в крови и в биоптатах эндометрия у женщин методом жидкостной хроматографии/масс-спектрометрии. Так в ответ на децидуализацию в ткани эндометрия повышается экспрессия ферментов ароматазы и 17β-гидроксистероиддегидрогеназы [14]. При определении концентрации андрогенов и прогестинов в крови и в эндометрии в секреторную фазу менструального цикла было обнаружено, что тканевые концентрации ДГЭА в эндометрии были значительно выше по сравнению с его концентрациями в сыворотке крови. Напротив, концентрации андростендиона и тестостерона были значительно ниже в ткани эндометрия, чем в сыворотке крови независимо от фазы менструального цикла [15].
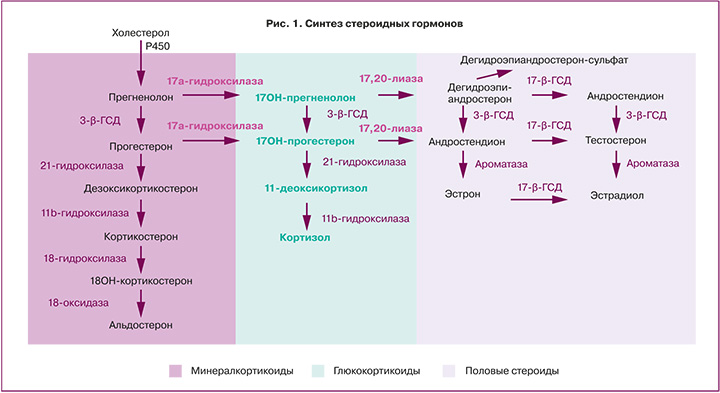
Биосинтез андрогенов в тканях осуществляется в результате стероидогенеза de novo, например, в яичниках, или путем преобразования предшественников андрогенов экстрагонадно. Для биосинтеза стероидов de novo требуется холестерин, который перемещается к внутренней митохондриальной мембране с помощью стероидогенного регуляторного белка (StAR), где он подвергается расщеплению боковой цепи посредством CYP11A1 (фермент расщепления боковой цепи холестерина) с образованием прегненолона. Прегненолон подвергается двум ферментативным превращениям, опосредованным CYP17A1, сначала в 17-гидроксипрегненолон при помощи 17-гидроксилазы, а затем под действием лиазы-17,20 в ДГЭА [16].
Андрогены и эстрогены могут метаболизироваться из общего предшественника ДГЭА под действием стероидсульфатазы, которая десульфатирует ДГЭА-сульфат в ДГЭА. Стероидсульфатаза экспрессируется как в здоровом эндометрии, так и при злокачественном поражении эндометрия. Экспрессия стероидсульфатазы повышается во время децидуализации стромальных клеток эндометрия in vitro [17].
Сульфатирование является одним из ключевых механизмом деактивации стероидов и осуществляется изоформами фермента сульфотрансферазы совместно с 30-фосфоаденозин-50-фосфосульфатсинтазой [18]. Основная эстрогенсульфотрансфераза – SULT1E1 экспрессируется в эпителиальных клетках эндометрия. Ее экспрессия увеличивается в ответ на прогестерон [19], что обеспечивает баланс между активацией и инактивацией стероидов в течение менструального цикла.
ДГЭА и его сульфатная форма (ДГЭАС) являются предшественниками стероидных гормонов в эндометрии [20]. После преобразования ДГЭА в андростендион при помощи 3-гидроксистероиддегидрогеназы активация агонистов андрогенов тестостерона и дигидротестостерона контролируется ферментом альдокеторедуктазой 1 (рис. 1). Преобразование андростендиона в тестостерон и выработка дигидротестостерона зависит от фазы менструального цикла, что было выявлено Catalano et al при оценке гомогенатов цельной ткани эндометрия. Так экспрессия альдокеторедуктазы 1 увеличивается в секреторную фазу с пиком концентрации в среднюю секреторную фазу [21]. В отличие от альдокеторедуктазы, экспрессия 5-альфа-редуктазы, которая превращает тестостерон в более активный и неароматизирующийся дигидротестостерон, снижается в клетках эндометрия по мере прогрессирования децидуализации [22].
Локальный биосинтез андрогенов – тестостерона/дигидротестостерона в стромальных клетках человека регулируется AР и экспрессией факторов рецептивности. Дигидротестостерон ингибирует клеточную миграцию, увеличивает резистентность к апоптозу при селективных модуляторах андрогеновых рецепторов (СМАР), подавляет уровень активности данных функций и экспрессию генов, регулирующих AР. Применение дигидротестостерона увеличивает размеры матки с индукцией пролиферативных эпителиальных клеток, разрастанием железистого эпителия с изменением иммуноэкспрессии AР матки. Открытие СМАР расширило горизонты по изучению АР при репродуктивных неудачах, но побочные эффекты, включая гирсутизм, являющийся ярким симптомом избытка андрогенов при СПКЯ, возможны при длительном применении этой группы препаратов [23].
В исследовании сравнивали влияние дигидротестостерона с даназолом и СМАР нового поколения GTx-024 (остарин, более известный в научной среде как энобосарм) и GTx-007 (андарин) на репродуктивные органы мышей и обнаружили, что и даназол и GTx-024 восстановили вес матки после овариэктомии самок мышей по сравнению с интактными животными, а GTx-007 не оказали подобного эффекта [24]. Селективные модуляторы андрогенных рецепторов использовались в клинических испытаниях лечения РМЖ, но применение их в качестве лечения эндометриоза и злокачественных поражениях эндометрия еще предстоит изучить [25]. Интересно, что введение антагонистов прогестероновых рецепторов или селективных модуляторов прогестероновых рецепторов, таких как улипристал ацетат, с целью лечения обильных менструальных кровотечений приводит к значительному увеличению экспрессии андрогенных рецепторов [26], что отчасти может объяснить их антипролиферативное действие.
Взаимное превращение активных/неактивных андрогенов опосредуется изоферментами 17-гидроксистероиддегидрогеназы. Преобладающей изоформой с окислительной активностью в эндометрии является 17-гидроксистероиддегидрогеназа 2, концентрация которой повышается в секреторную фазу под действием прогестерона и превращает активный тестостерон в андростендион [27].
Ингибиторы 17-гидроксистероиддегидрогеназы были первоначально разработаны для целенаправленного синтеза биоактивного эстрадила при гормонозависимом раке молочной железы [28]. Было предложено использование ингибиторов 17-гидроксистероиддегидрогеназы 1 как инновационный метод лечения эндометриоза [29]. Ферменты 17-гидроксистероиддегидрогеназа 5 и альдокеторедуктаза 1 играют важную роль в метаболизме стероидов и простагландинов, участвуют в возникновении боли, связанной с эндометриозом. Предполагалось достаточно многообещающее исследование с оценкой эффективности различных доз и прорывом в области лечения эндометриоза. В клиническое исследование были включены 121 женщина, но через 8 месяцев в связи с гепатотоксическим действием исследование было прекращено [30]. В недавнем обзоре Rinzer и Penning пришли к выводу, что побочный эффект в виде гепатотоксичности, вероятно, был связан со сложным механизмом взаимодействия этих ферментов, что не исключает дальнейшие исследования и разработку лекарственных препаратов, нацеленных исключительно на альдокеторедуктазу 1 или в сочетании с другими ферментами. [31]
В клетках эндометрия существует два основных пути синтеза эстрогенов: превращение андрогенов, таких как андростендион и тестостерон, в активные эстрогены, эстрон и эстрадиол, соответственно, через действие ферментного комплекса ароматаз, ключевым компонентом которого является белок, кодируемый геном CYP19A1; превращение сульфатированных эстрогенов эстрадиолсульфата или эстронсульфата в их биоактивные метаболиты под действием стероидсульфатазы.
В образцах эндометрия здоровых женщин, экспрессия ароматаз низкая или отсутствует. Однако, в первичных стромальных клетках эндометрия человека было выявлено, что активность ароматазы увеличивается после децидуализации, что приводит к увеличению синтеза эстрона и эстрадиола, необходимых для сохранения беременности, что подтверждено исследованиями Bagchi M.K et al. [32]. В ряде экспериментов они показали, что при введении ингибитора ароматазы in vivo процессы децидуализации и ремоделирования сосудов нарушаются [33].
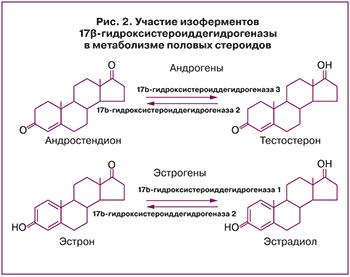 Экспрессия окислительной и восстановительной изоформ 17-гидроксистероиддегидрогеназы также может способствовать образованию эстрадиола из эстрона. Активация эстрона в эстрадиол в матке посредством восстановительной активности 17-гидроксистероиддегидрогеназы в первую очередь опосредуется 17-гидроксистероиддегидрогеназа 1, но также 17-гидроксистероиддегидрогеназа 4 и 7. При патологии эндометрия экспрессия этих ферментов выше, чем в норме (рис. 2).
Экспрессия окислительной и восстановительной изоформ 17-гидроксистероиддегидрогеназы также может способствовать образованию эстрадиола из эстрона. Активация эстрона в эстрадиол в матке посредством восстановительной активности 17-гидроксистероиддегидрогеназы в первую очередь опосредуется 17-гидроксистероиддегидрогеназа 1, но также 17-гидроксистероиддегидрогеназа 4 и 7. При патологии эндометрия экспрессия этих ферментов выше, чем в норме (рис. 2).
В исследованиях, изучающих циклический метаболизм андрогенов, обработка стромальных клеток эндометрия in vitro флутамидом, антагонистом рецепторов андрогенов, снижала секрецию маркеров децидуализации и рецептивность эндометрия [22]. Добавление ДГЭА увеличивает биосинтез тестостерона и дигидротестостерона и связано с дозозависимым увеличением экспрессии маркеров децидуализации белка 1, связывающего инсулиноподобный фактор роста (IGFBP1), пролактина, а также маркера рецептивности эндометрия остеопонтина SPP1. Эти исследования предполагают, что тканевой метаболизм андрогенов зависит от фазы менструального цикла и является значимым фактором, обеспечивающим готовность эндометрия к беременности [34], поскольку циркулирующие концентрации предшественников андрогенов, таких как ДГЭА и андростендиона, а также тестостерона и дигидротестостерона снижаются с возрастом [35].
Влияние тканевого метаболизма половых стероидов на патогенез неопролиферативных процессов в эндометрии
Исследование эктопических эндометриоидных поражений показало, что они характеризуются высокими уровнями экспрессии ароматазы и дефицитом 17b-гидроксистероиддегидрогеназы 2, фермента, ответственного за синтез эстрона из эстрадиола [36]. Поскольку ароматаза экспрессируется в очагах эндометриоза, ингибиторы ароматазы применяются в качестве средств для лечения болевого синдрома.
В Кокрановском обзоре (2014) был сделан вывод, что для женщин с болевым синдромом при эндометриозе подавление менструальных циклов с помощью аналогов гонадотропин-рилизинг-гормона, внутриматочной системы с левоноргестрелом или даназола сопровождалось улучшением самочувствия [37]. Однако, для планирующих реализацию репродуктивной функции подавление функции яичников нежелательно, так как наряду с сохранением тканевого метаболизма могут быть серьезные побочные эффекты медикаментозной аменореи.
Предполагается, что локальный синтез эстрогенов, сопровождаемый интракринной и паракринной передачей сигналов через ЭР β в эндометриоидных гетеротопиях поддерживает воспалительное состояние и пролиферацию клеток в очагах эндометриоза [38]. Перитонеальная жидкость у женщин с эндометриозом содержит высокие концентрации провоспалительных цитокинов, таких как ФНО-α и интерлейкин-1 [39], которые обладают способностью стимулировать экспрессию фермента синтеза простагландинов ЦОГ-2 и увеличивать секрецию простагландина E2 эндометриоидными клетками и перитонеальными макрофагами [40]. Простагландин Е2, в свою очередь, стимулирует выработку циклического аденозинмонофосфата и стероидогенного фактора-1, экспрессия которого повышена в очагах эндометриоза [41].
Huhtinen et al. сообщили, что в пролиферативную фазу менструального цикла тканевые концентрации эстрадиола в эндометрии и очагах эндометриоза были значительно выше по сравнению с концентрациями в сыворотке крови, тогда как в секреторную фазу наблюдалась противоположная картина. Более того, экспрессия 17-гидроксистероиддегидрогеназы 2 была значительно ниже в очагах поражения по сравнению с тканью нормального эндометрия, тогда как экспрессия 17-гидроксистероиддегидрогеназы 6 и CYP19A1 была значительно выше. Ингибирование активности этих ферментов открыло возможности для разработки новых специфических лекарственных средств для лечения эндометриоза и рака эндометрия [42].
Следует отметить, что существует явная разница как в местных концентрациях стероидов, так и в экспрессии ферментов, метаболизирующих стероиды при разных типах поражений. Например, в пролиферативную фазу концентрации эстрадиола в эндометриоидных кистах яичников составляли приблизительно 3430 пг/мл, в то время как концентрация эстрадиола в очагах эндометриоза брюшины составляла 238 пг/мл. Это показывает неоднородность интракринного действия стероидов при различной локализации и распространении эндометриоза, что в случае поражений яичников может быть вследствие интимного расположения эндометриоидных клеток с фолликулами. Это объясняет успешность использования агонистов ГНРГ в циклах ВРТ и их эффективность при бесплодии, обусловленном эндометриозом различной локализации [43]. Для изучения этой проблемы необходимы дальнейшие исследования.
Bulun и его коллеги обнаружили, что при раке молочной железы, эндометрия и яичников экспрессия гена CYP19A1 сопровождается повышенной активностью промоторной области I.3/II, которая может регулироваться простагландинами, такими как ПГЕ2, обеспечивая связь между избыточной экспрессией простагландинов при раке эндометрия и интракринном биосинтезе эстрогенов [44]. Sasano et al. [45] представили новые антитела и сообщили об увеличении иммуноэкспрессии ароматазы, STS и 17-гидроксистероиддегидрогеназы при гиперплазии и раке эндометрия, что выявляется повышенной биодоступностью эстрона и эстрадиола при злокачественной трансформации тканей. Примечательно, что экспрессия ароматазы и 17-гидроксистероиддегидрогеназы при раке эндометрия коррелирует с неблагоприятным прогнозом [46].
В недавнем исследовании Kamal et al. [47] сообщили, что экспрессия андрогенного рецептора снижена при раке эндометрия высокой степени злокачественности, но увеличена при метастазах, что может использоваться для терапии. Ito et al. обобщили эпидемиологические данные, подтверждающие связь между повышенным содержанием циркулирующих андрогенов и риском развития рака эндометрия [48]. Было выявлено в этой популяции 8-кратное локальное повышение дигидротестостерона при раке эндометрия по сравнению с неизмененным эндометрием [49].
Заключение
При всей ясности роли андрогенов и эстрогенов в репродуктивной функции, их дефицит приводит к бесплодию, тогда как избыток также формирует патологию не только репродуктивной системы, в том числе эндометрия, но и различных органов и тканей.
При сниженной функции яичников меняется экспрессия половых гормонов, включая андрогены, чья метаболическая активность продолжает изучаться. До настоящего времени нет точных данных о том, какой из андрогенов является ранним маркером снижения функции яичников и, соответственно, – изменения рецептивности эндометрия. Известно, что при низких концентрациях половых стероидов, в том числе андрогенов, формируются изменения рецептивности эндометрия.
Сбалансированная регуляция действия половых стероидов необходима для правильного функционирования эндометрия и его репродуктивной состоятельности. Избирательно действующие на стероидные рецепторы препараты, такие как СМЭР и СМАР, являются многообещающей инновационной терапией, но нуждаются в изучении для детального понимания их механизма действия, безопасности, эффективности и экономической рентабельности при длительном применении.
Осознавая роль интракринного метаболизма половых стероидов – эстрогенов и андрогенов, как одного из важных регуляторов функции эндометрия, проведение дальнейших исследований роли андрогенов как предшественников метаболизма эстрогенов с основательным изучением особенностей внутриклеточного метаболизма андрогенов и андрогенных рецепторов является обоснованным для формирования новой концепции ведения женщин с синдромом андрогендефицита, включая низкий овариальный резерв и бедный ответ.



