The role of cytokines and organic acids production in the blood serum and amniotic fluid in spontaneous preterm labor
Aim: Investigation of cytokines and organic acids production in preterm labor.Krukier I.I., Levkovich M.A., Avrutskaya V.V, Churyukina E.V., Nikashina A.A., Chikina L.G.
Material and methods: Blood and amniotic fluid of 88 patients, including 33 women with preterm labor and 55 women with normal childbirth, were studied for production of cytokine IL-33 and IL-1β using the kits produced by “Bender MedSystems GmbH” (Austria), and antagonist of the IL-1β receptor (IL-1Ra) using ELISA kits (USA). The identification and quantitative assessment of organic acids (citric and succinic) was performed by capillary electrophoresis with an unmodified quartz capillary (“Kapel-105”, “Lumex”, St. Petersburg, Russia).
Results: Increased concentrations of IL-33, an antagonist of the IL-1Ra receptor, and citric acid was found in the blood serum of patients with preterm labor compared to women with normal pregnancy. Amniotic fluid was characterized by increased IL-33, IL-1β production and high level of citric acid.
Conclusion: The obtained results confirm the role of the studied cytokines and organic acids production in the blood serum and amniotic fluid in spontaneous preterm labor.
Keywords
Preterm labor (PL) is one of the main challenges of modern medicine. The high incidence of PL, which, according to the World Health Organization, ranges from 5 to 18% in different countries, causes a significant neonatal morbidity [1–3].
PL is associated with increased uterine activity and is regulated by various bioactive agents, such as cytokines [4–7].
Interleukin 33 (IL-33) is a member of the interleukin 1 (IL-1) family, a group of key regulators of inflammation. In addition to its impact on various leukocyte populations, IL-33 also increases activation and proliferation of endothelial cells. IL-33 is a key mediator of the immune response that mainly releases proinflammatory cytokines [8].
The organic acids are known to be derivatives of numerous metabolic processes in the body of a pregnant woman. These acids are also cofactors of redox reactions that produce ATP. They ensure the metabolism and development of fetal tissues during pregnancy, and also have an impact on immune processes and the development of tolerance in the mother-fetal system. The study of the role of succinic and citric acids in the genesis of premature birth is a relevant issue. Organic acids are also used in the treatment of various complications of pregnancy, especially in the presence of fetal hypoxia and hypotrophy.
The antihypoxic effect of succinic acid is known for improvement of tissue respiration, transportation of oxygen from blood to cells, its utilization, and also for its action on free radical oxidation. This mechanism of action of succinate is prolonged by its effect on the activity of HIF-1α (hypoxia-inducible factor), which plays a major role in the response to hypoxia. HIF-1α is an oxygen-sensitive complex that triggers the expression of a number of cytokines, including interleukins and growth factors. Succinic acid has a selective effect on the body, eliminates the negative symptoms of early gestosis and normalizes the hormone levels in a pregnant woman. Citric acid helps to speed up the elimination of harmful substances and toxins and stimulate cellular immunity. These acids activate the delivery of nutrients for normal growth and development of the fetus.
There is practically no data on the role of cytokines, key regulators of inflammation at the systemic and local level (amniotic fluid) and on the role of organic acids in the pathogenesis of preterm labor.
The aim of this study was to investigate the production of cytokines and organic acids in preterm labor.
Materials and methods
The study was performed at the Maternity Department of the Research Institute of Obstetrics and Pediatrics of the Rostov State Medical University of the Ministry of Health of the Russian Federation from 2018 to 2020.
The study included clinical, biochemical, ultrasound, and Doppler ultrasound examination of pregnant women. All patient included in the study had signed informed consent. The study was approved by the local Ethics Committee of the Rostov State Medical University of the Ministry of Health of the Russian Federation.
The study included 67 pregnant women randomly selected by simple randomization. A prospective study was performed. The first group (main) included 32 patients with spontaneous preterm labor (32–35 weeks), and the control group included 35 women with normal course of pregnancy and delivery at term (38–40 weeks).
The criterion for inclusion in the main group was pregnancy complicated by spontaneous preterm labor without premature rupture of membranes (PROM).
Exclusion criteria were: severe somatic disease, endocrine pathology, infection of the pelvic organs in decompensation, fetal malformations, placental abruption, multiple gestation.
The peripheral blood of pregnant women taken in fasting at 32 weeks of pregnancy and the amniotic fluid after labor were studied. All samples were stored in a freezer (Sanyo, Japan) at a temperature (-80°C).
The content of cytokines (IL-33 and IL-1) was assessed by ELISA using Bender Medsistems kits (Austria), and the content of antagonist of the IL-1 receptor (IL-1Ra) – by ELISA kits (USA).
The quantity of organic acids (citric and succinic) was determined by capillary electrophoresis method with unmodified quartz capillary tubes (Kapel-105, Lumex, Saint Petersburg, Russia). The data were processed using an IBM PC with “Multichrome” software (Ampersand Ltd.).
Statistical analysis
An Excel 2003 and software packages “Megastat” and Statistica 6.0 were used for statistical analysis and database formation. The Mann–Whitney U-test was used for independent groups, and the Wilcoxon signed-rank test was used for dependent groups, the maximum permissible error level was at p=0.05. The Fisher–Irwin test was used to analyze the frequency differences in two independent groups. The Spearman's rank correlation coefficient was used to assess the closeness of the relationship between individual indicators. Data are presented as median (Me) and quartiles Q1 and Q3.
Results and discussion
The women were examined in accordance with the Order No. 572н of the Ministry of Health of the Russian Federation dated November 1, 2012, “On approval of the Procedure for the provision of medical care in the profile of “obstetrics and gynecology” (except for the use of assisted reproductive technologies)”.
In examination of pregnant women standard procedures were performed (blood test, urine test, biochemical blood tests, etc.). Screening for reproductive tract infections was performed to exclude patients with an infectious factor of PL.
The average age of pregnant women in the studied groups was comparable. The onset of menarche in studied groups was mainly at the age of 12 years, the duration of the menstrual cycle ranged from 27 to 30 days. No statistically significant differences between the groups in the duration of menstrual cycle were found.
The outcomes of previous pregnancies in patients of studied groups were as follows: 14/32 (43.7%) women with PL had a history of missed miscarriage at 5–11 weeks (in the control group – 1/35 (2.8%); 11/32 (34.4%) women of group 1 had spontaneous abortion (in the control group – 1/35 (2.8%); antenatal fetal death and complications of abortion and labor were noted in 5/32 (15.6%) women of group 1 (in the control group – 1/35 (2.8%).
The incidence of infections which had occurred in the patients of the studied groups in childhood was comparable and did not differ from the average statistical data in the general population.
Data on gynecological and somatic diseases were recorded from all patients. Data obtained in the control group confirmed the low level of both gynecological and somatic morbidity.
5/32 (15.6%) women with spontaneous preterm labor had undergone pelvic surgery. The incidence of respiratory diseases in women with spontaneous preterm labor was 10/32 (31.3%), which was significantly higher than in the control group – 2/35 (5.7%), (p=0.03), and the incidence of urinary system diseases in this group was 11/32 (34, 3%) women compared to 2/35 (5.7%) women in the control group (p=0.02).
The incidence of endocrine and metabolic diseases in the patients of the studied groups was the following: thyroid diseases were found in the group with PL in 4/32(12.5%) cases, (in the control group – 2/35(5.7%)); obesity was present in the group with PL in 4/32(12.5%) cases (in the control group – 2/35(5.7%)), and hypothalamic syndrome was detected in group with PL in 1/32(3.1%) cases (in the control group – 0%).
Among the gestational complications the miscarriage threat was the most frequent occurring in 32/32(100%) cases, placental insufficiency was noted in 10/32(31.3%) cases.
The content of cytokines in blood serum and amniotic fluid are shown in the Table 1.
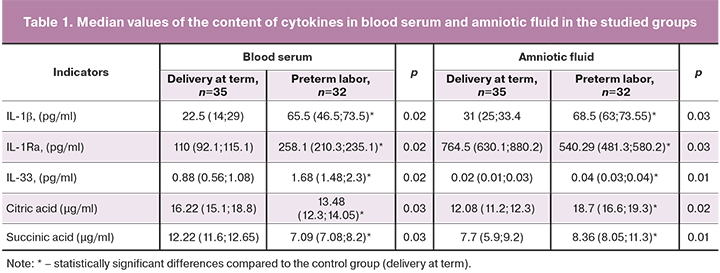
The content of IL-33 in blood serum in patients with preterm labor was 81% higher than in control group (p=0.02), and its level in amniotic fluid was 2 times higher than in the control group (p=0.01).
The expression of IL-33 in decidualized stromal cells is essential during the proinflammatory implantation stage, but its prolonged production may lead to pregnancy loss.
Significant changes were also found in the level of IL-1; its content in the amniotic fluid in patients with preterm labor was 2 times higher than in the control group (p=0.03), and its level in blood serum was 58% higher than in the control group (p=0.02).
In the group of women with PL, the content of the IL-1 receptor antagonist (IL-1Rа) in blood serum was 2.3 times higher than in the control group (p=0.03), and in the amniotic fluid it was 57% lower than in the control group (p=0.02).
Decreased production of the IL-1Ra receptor antagonist in amniotic fluid may enhance the effect of IL-1 in myometrium, cervix, and membranes, indicating an increased inflammatory response in the tissues before labor [9], which may lead to preterm birth. Increased production of IL-1Ra receptor antagonist in blood serum, on the contrary, leads to suppression of proinflammatory mediators such as IL-1 in uterus, placenta, membranes, and amniotic fluid. Moreover, IL-1Ra inhibits stress-associated factors such as JNK, p38, etc. in smooth muscle cells of the endometrium, which prevents the onset of preterm labor associated with adverse outcomes for the newborn [10].
 In contrast, the level of the organic acids (citric acid, succinic acid) in preterm labor was reduced by 98% (p=0.03) and 79% (p=0.03), respectively, compared with the control group, and their content in amniotic fluid was increased by 83% (p=0.02) and 125% (p=0.01), respectively, compared with the control group (Fig. 1).
In contrast, the level of the organic acids (citric acid, succinic acid) in preterm labor was reduced by 98% (p=0.03) and 79% (p=0.03), respectively, compared with the control group, and their content in amniotic fluid was increased by 83% (p=0.02) and 125% (p=0.01), respectively, compared with the control group (Fig. 1).
Abnormal course of implantation and placentation processes, regulated with participation of cytokines and organic acids (citric and succinic acids), leads to various complications of pregnancy, including preterm labor [11].
Different extent of production of the studied cytokines and some organic acids, and sometimes its inverted character in early pregnancy loss, apparently, affects the nature of regulatory processes and compensatory mechanisms, determining the outcome of pregnancy.
According to some evidence, preterm labor is the result of both past obstetric complications (infections) and early activation of inflammatory mediators, which in turn result in activation and contraction of the myometrium.
Since our study did not include patients with pelvic infections, the inflammatory response characterized by increased production of proinflammatory cytokines (IL-1 and IL-33) was clearly nonspecific.
Thus, the bioactive regulators of uterine contractile activity (cytokines and organic acids) appear to be one of the factors in the development of preterm labor.
To elucidate a possible relationship, we performed a correlation analysis of the studied parameters in blood serum and amniotic fluid sampled from women with full-term pregnancy and with preterm labor.
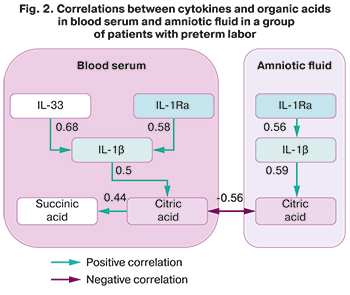 An analysis of the correlations in the group of women with preterm labor (Fig. 2) showed that in this pattern, the strongest relationship was found in blood serum between IL-1 and IL-33 (r=0.68, CI (0.59; 0.75)). A positive relationship was found between IL-1 and IL-1Ra, both in blood serum (r=0.58, CI (0.47; 0.67)) and in amniotic fluid (r=0.56, CI (0.45; 0.66)).
An analysis of the correlations in the group of women with preterm labor (Fig. 2) showed that in this pattern, the strongest relationship was found in blood serum between IL-1 and IL-33 (r=0.68, CI (0.59; 0.75)). A positive relationship was found between IL-1 and IL-1Ra, both in blood serum (r=0.58, CI (0.47; 0.67)) and in amniotic fluid (r=0.56, CI (0.45; 0.66)).
IL-33 is a member of the proinflammatory IL-1 family and, alike the latter, has immunoregulatory functions [12].
IL-33 activates eosinophils, basophils, mast cells, and invariant natural killer T cells (iNKT cells). Activated neutrophils and iNKT cells cause activation of NF-κB and MAP kinases and production of various cytokines and chemokines. Early activation of this proinflammatory pathway can lead to abnormal course of pregnancy and preterm labor [13]. We have developed a “Method for predicting preterm labor in patients with the threat of miscarriage”, which includes the analysis of blood serum of pregnant women by ELISA and assessment of the level of IL-33 (Patent RU 2710244 C 108.08.2019.C.7).
The proposed method is easy to use, highly informative, sensitive, and tested in a sufficient number of cases, which confirms its efficiency and the possibility of using in clinical practice.
The revealed relationship, leading to an imbalance of regulatory processes and compensatory mechanisms, determines the outcome of pregnancy and health of the newborn.
Conclusion
The results of study confirm the role of the production of the studied cytokines and organic acids in blood serum and amniotic fluid in the onset of spontaneous preterm labor. Preterm labor can subsequently influence the development of diseases in the newborn. Successful pregnancy depends on homeostatic balance in a complex cytokine network in the process of placental development. Immunomodulatory factors are the main regulators of trophoblast function; moreover, the abnormal levels of cytokines and organic acids are associated with the development of severe complications of pregnancy.
Metabolic profiles of amniotic fluid and maternal serum provide valuable information about fetal development and may be useful in the diagnosis of complications of pregnancy.
These findings may contribute to elaboration of new predictive and therapeutic approaches to prevent preterm birth.
References
- Ходжаева З.С., Федотовская О.И., Донников А.Е. Клинико-анамнестические особенности женщин с идиопатическими преждевременными родами на примере славянской популяции. Акушерство и гинекология. 2014; 3: 28-32. [Khodzhaeva Z.S., Fedotovskaya O.I., Donnikov A.E. Clinical and anamnestic characteristics of women with idiopathic preterm labor in case of a slavic population. Akusherstvo i Ginekologiya/ Obstetrics and Gynecology. 2014; 3: 28-32. (in Russian)].
- Артымук Н.В., Елизарова Н.Н. Факторы риска преждевременного разрыва плодных оболочек у женщин с преждевременными родами в Кемеровской области. Фундаментальная и клиническая медицина. 2016; 1(2): 6-11. [Artymuk N.V., Elizarova N.N. Risk factors of premature rupture of membranes in women with preterm birth in the Kemerovo region. Fundamental'naya i klinicheskaya meditsina/Fundamental and clinical medicine. 2016; 1(2): 6-11. (in Russian)].
- Радзинский В.Е., Костин И.Н., Оленев А.С., Гагаевич Ч.Г., Парыгина А.Н ., Гаврилова А.А., Гагаев Д.Ч., Дамирова К.Ф., Кузнецова О.А., Смирнова Т.В. Преждевременные роды – нерешенная мировая проблема. Акушерство и гинекология: новости, мнения, обучение. 2018; Suppl. 3: 55-64. [Radzinsky V.E., Kostin I.N., Olenev A.S., Gagaev Ch.G., Parygina A.N., Gavrilova A.A. et al.Preterm delivery is an unsettled world problem. Akusherstvo i ginekologiya: novosti, mneniya, obucheniye/Obstetrics and gynecology: News, Opinions, Training. 2018; S3: 55-64. (in Russian)]. https://dx.doi.org/10.24411/2303-9698-2018-13909.
- Малышкина А.И., Сотникова Н.Ю., Крошкина Н.В., Таланова И.Е., Куст А.В., Козелкова Е.В. Особенности содержания цитокинов периферической крови у беременных женщин с привычным невынашиванием беременности. Клиническая лабораторная диагностика. 2020; 65(5): 299-303. [Malyshkina A.I., Sotnikova N.Y., Kroshkina N.V., Talanova I.E., Kust A.V., Kozelkova E.V. Peculiarities of the content of peripheral blood cytokines in pregnant women with a habitualm is carriage. Klinicheskaya Laboratornaya Diagnostika/ Russian Clinical Laboratory Diagnostics. 2020; 65(5): 299-303. (in Russian)]. https://dx.doi.org/10.18821/0869-2084-2020-65-5-299-303
- Назарова А.О., Малышкина А.И., Назаров С.Б., Бойко Е.Л. Факторы риска угрожающих преждевременных родов: результаты клинико-эпидемиологического исследования. Акушерство и гинекология. 2020; 6: 43-8. [Nazarova A.O., Malyshkina A.I., Nazarov S.B., Boyko E.L. Risk factors for threatened preterm labor: a clinical and epidemiological study. Akusherstvo i Ginekologiya/ Obstetrics and Gynecology. 2020; 6: 43-8. (in Russian)] https://dx.doi.org/10.18565/aig.2020.6.43-48.
- Сухих Г.Т., Серов В.Н., Адамян Л.В., Филиппов О.С., Баев О.Р., Клименченко Н.И., Тетруашвили Н.К., Тютюнник В.Л., Ходжаева З.С., Холин А.М. Преждевременные роды. Клинические рекомендации (протокол). Министерство здравоохранения Российской Федерации; 2014. 35с. [Sukhikh G.T., Serov V.N., Adamyan L.V., Filippov O.S., Baev O.R., Klimenchenko N.I. et al. Premature birth. Clinical guidelines (protocol). M.: Ministry of Health of the Russian Federation; 2014. 35p. (in Russian)].
- Сотникова Н.Ю., Малышкина А.И., Крошкина Н.В., Батрак Н.В. Особенности регуляции FAS-зависимого апоптоза при привычном невынашивании беременности ранних сроков. Российский иммунологический журнал. 2017; 11(3): 510-2. [Sotnikova N.Yu., Malyshkina A.I., Kroshkina N.V., Batrak N.V. The regulation of fas-dependent apoptosis in rsa pregnancy. Rossiyskiy immunologicheskiy zhurnal/ Russian Journal of Immunology. 2017; 11(3): 510-2. (in Russian)].
- Romero R., Grivel J.-C., Tarca A.L. Evidence of perturbations of the cytokine network in preterm labor. Am. J. Obstet. Gynecol. 2015; 213(6): 836. https://dx.doi.org/10.1016/j.ajog.2015.07.037.
- Nadeau-Vallée M., Quiniou C., Palacios J., Hou X., Erfani A., Madaan A. et al. Novel noncompetitive IL-1 receptor-biased ligand prevents nfection- and inflammation-induced preterm birth. J. Immunol. 2015; 195(7): 3402-15. https://dx.doi.org/10.4049/jimmunol.1500758.
- Nadeau-Vallee M., Obari D., Beaudry-Richard A., Sierra E.M., BeaulacA., Maurice N. et al. Preterm birth and neonatal injuries: importance of interleukin-1 and potential of interleukin-1 receptor antagonists. Curr. Pharm. Des. 2017; 23(40): 6132-41. https://dx.doi.org/10.2174/1381612823666170825145114.
- Крукиер И.И., Авруцкая В.В., Левкович М.А., Нарежная Е.В., Смолянинов Г.В., Ерджанян Л.Л., Никашина А.А. Особенности изменения биорегуляторов и органических кислот в сыворотке крови и амниотической жидкости женщин со спонтанными преждевременными родами. Вестник Российской академии медицинских наук. 2018; 73(6): 361-7. [Krukier I.I., Avrutskaya V.V., Levkovich M.A., Narezhnaya E.V., Smolyaninov G.V., Yerdzhanyan L.L. et al. Peculiarities of Changing Bioregulators and Organic Acids in the Serum of Blood and Amniothic Fluid of Women with Spontaneous Preterm Labor. Vestnik Rossiyskoy akademii meditsinskikh nauk/Annals of the Russian Academy of Medical Sciences. 2018; 73(6): 361-7. (in Russian)]. https://dx.doi.org/10.15690/vramn1017.
- Левкович М.А., Афонин А.А., Левкович А.Ю., Кравченко Л.В., Куценко И.И., Бердичевская Е.М., Цатурян Л.Д. Оценка цитокинового баланса околоплодных вод беременных с плацентарной недостаточностью при ранней и отсроченной манифестации церебральной патологии у их новорожденных. Российский иммунологический журнал. 2017; 11(3): 408-10. [Levkovich M.A., Afonin A.A., Levkovich A.Yu., Kravchenko L.V., Kutsenko I.I., Berdichevskaya E.M. et al. Evaluation of cytokine balance of amniotic fluid in pregnant women with placental insufficiency in early and delayed manifestation of cerebral pathology in their newborns. Rossiyskiy immunologicheskiy zhurnal/ Russian Journal of Immunology. 2017; 11(3): 408-10. (in Russian)].
- Topping V., Than N.G., Tarca A.L., Xu Z., Kim S.Y. Interleukin-33 in the human placenta. J. Matern. Fetal Neonatal Med. 2013; 26(4): 327-38. https://dx.doi.org/10.3109/14767058.2012.735724.
Received 04.05.2021
Accepted 14.07.2021
About the Authors
Irina I. Krukier, Dr. Sci. (Bio), Leading Researcher, Department of Obstetrics and Gynecology, Research Institute of Obstetrics and Pediatrics,Rostov State Medical University, Ministry of Health of Russia, biochem@rniiap.ru, https://orcid.org/0000-0003-4570-6405, 344012, Russia, Rostov-on-Don, Mechnikov str., 43.
Marina A. Levkovich, Dr. Med. Sci., Associate Professor, Leading Researcher, Department of Allergic and Autoimmune Diseases in Pediatrics, Research Institute
of Obstetrics and Pediatrics, Rostov State Medical University, Ministry of Health of Russia, xlma@mail.ru, https://orcid.org/0000-0001-8047-7148,
344012, Russia, Rostov-on-Don, Mechnikov str., 43.
Valeriya V. Avrutskaya, Dr. Med. Sci., Associate Professor, Chief Researcher, Department of Obstetrics and Gynecology, Research Institute of Obstetrics and Pediatrics,
Rostov State Medical University, Ministry of Health of Russia, v.avrutskaya@rniiap.ru, https://orcid.org/0000-0001-6399-5007,
344012, Russia, Rostov-on-Don, Mechnikov str., 43.
Ella V. Churyukina, MD, Leading Researcher, Head of Department; Department of Allergic and Autoimmune Diseases in Pediatrics, Research Institute of Obstetrics and Pediatrics, Rostov State Medical University, Ministry of Health of Russia, echuryukina@mail.ru, https://orcid.org/0000-0001-6407-6117,
344012, Russia, Rostov-on-Don, Mechnikov str., 43.
Anastasia A. Nikashina, PhD, Researcher, Department of Obstetrics and Gynecology, Research Institute of Obstetrics and Pediatrics, Rostov State Medical University, Ministry of Health of Russia, laigash@yandex.ru, https://orcid.org/0000-0001-8099-9093, 344012, Russia, Rostov-on-Don, Mechnikov str., 43.
Lubov G. Chikina, Dr. Sci. (Physics and Mathematics), Professor, Institute of Mathematics, Mechanics, and Computer Science, Southern Federal University,
lchikina@sfedu.ru, https://orcid.org/0000-0002-2935-5839, 344090, Russia, Rostov-on-Don, Stachki ave., 200/1.
Corresponding author: Irina I. Krukier, biochem@rniiap.ru
Authors’ contributions: Krukier I.I., Nikashina A.A. – the concept and design of the study; Levkovich M.A., Avrutskaya V.V. – material collection and processing; Chikina L.G. – statistical data processing; Krukier I.I., Levkovich M.A., Avrutskaya V.V. – writing the text of the article; Churyukina E.V., Nikashina A.A. – editing the article.
Conflicts of interest: The authors declare no conflicts of interest.
Funding: The study was carried out as a part of the research "Determination of clinical and diagnostic markers of complicated pregnancy" (2017–2020) and was funded from budget resources of the State Assignment No. 056-00144-18-00.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Krukier I.I., Levkovich M.A., Avrutskaya V.V, Churyukina E.V., Nikashina A.A., Chikina L.G. The role of cytokines and organic acids production
in the blood serum and amniotic fluid in spontaneous preterm labor.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 9: 60-65 (in Russian)
https://dx.doi.org/10.18565/aig.2021.9.60-65



