The role of polyamines in noninvasive diagnosis of placenta-associated pregnancy complications
Gasanbekova A.P., Frankevich N.A., Chagovets V.V., Tokareva A.O., Kachikowski Yu.N., Novoselova A.V., Karapetyan T.E.
Relevance: Polyamines are organic molecules involved in regulating systemic inflammatory responses, cell growth, and cell division. Noninvasive diagnosis of fetal growth restriction (FGR) and preeclampsia (PE) with determination of urinary polyamine levels is a promising method for the prediction, early detection, and monitoring of obstetric complications.
Objective: To investigate the characteristics of urine polyamine profiles in pregnant women with FGR and PE in order to identify biomarkers with prognostic and diagnostic potential.
Materials and methods: This study analyzed urine polyamines obtained from 73 pregnant women. The PE group included 22 pregnant women, the FGR group included 30 women, and the control group included 21 healthy women without pregnancy-related complications. Polyamine levels were determined by liquid chromatography with mass spectrometric detection.
Results: The study identified significant multidirectional changes in the metabolites studied, depending on obstetric complications (PE or FGR). For putrescine and its ketone derivative N-acetylputrescine, the change was statistically significant; however, while putrescine levels were higher in both the PE and FGR groups than in the control group, N-acetylputrescine levels were lower in both pathology groups, with slightly higher concentrations in the PE group (p=0.05). Compared to the controls, the level of 1,7-diaminoheptane was increased in patients with FGR and decreased in patients with PE (p = 0.04). For the timely verification of placenta-associated pregnancy complications based on the established levels of urine polyamines, predictive models were built with a high sensitivity of 1 (1; 1) and specificity of 1 (1; 1), with an area under the AUC curve of 1 and a threshold value of 0.5.
Conclusion: Noninvasive determination of the level of urine polyamines during FGR and PE over time may become an important marker of the severity of these pregnancy complications and help in choosing the management strategy and timing of delivery in this cohort of patients.
Authors’ contributions: Frankevich N.A. – analysis of clinical data, systematic analysis, drafting of the manuscript;
Gasanbekova A.P. – collection and preparation of biological media, drafting of the manuscript; Chagovets V.V. – conducting metabolomic analysis using mass spectrometry, statistical analysis, editing the manuscript; Tokareva A.O. – statistical analysis of the obtained data, editing of the manuscript; Kachikowski Yu.N. – assistance in creating an electronic database; Novoselova A.V. – conducting metabolomic analysis using mass spectrometry, processing mass spectrometric data; Karapetyan T.E. – analysis of clinical data.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: This study was supported by the state assignment from the Ministry of Health of the Russian Federation [State registration number 121040600408-4].
Acknowledgment: We express special gratitude to Doctor of Physical and Mathematical Sciences, Head of the Department of Systems Biology of the V.I. Kulakov NMRC for OG&P to Vladimir Evgenievich Frankevich for assistance in preparing the study, systematic analysis and editing of the manuscript.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors’ Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Gasanbekova A.P., Frankevich N.A., Chagovets V.V., Tokareva A.O., Kachikowski Yu.N., Novoselova A.V., Karapetyan T.E. The role of polyamines in noninvasive diagnosis of placenta-associated pregnancy complications.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (2): 52-61 (in Russian)
https://dx.doi.org/10.18565/aig.2023.265
Keywords
The noninvasive diagnosis of fetal growth restriction (FGR) and preeclampsia (PE) through the determination of urinary metabolites is a promising method that may help in the early detection and monitoring of these obstetric conditions.
FGR and PE are two devastating obstetric complications that can negatively impact pregnancy outcomes and the health of both the mother and the fetus. Prediction and early accurate diagnosis of these conditions are essential for providing timely medical care. Determination of metabolites in urine can provide valuable information for diagnosing and monitoring pregnancy [1].
Promising subjects for study using modern analytical methods include metabolites of amino acids, lipids, antioxidants, and their intermediate products. Amino acids and lipids play important roles in fetal development and metabolic processes in the body. Some studies have suggested that changes in the amino acid profile may be associated with FGR and PE [2]. For example, increased levels of the amino acid homocysteine and decreased levels of arginine may be associated with the risk of PE [3], and changes in the levels of lipoproteins of different densities may be associated with FGR [4]. Antioxidants play a crucial role in protecting the cells from oxidative stress. Changes in antioxidant metabolites may indicate disturbances in the body and are associated with PE [5].
Several studies have considered metabolomic analysis of urine to develop diagnostics for pregnant women with FGR and PE [6]. Urine collection is a noninvasive and relatively simple procedure that can be performed regularly during pregnancy. The collected urine samples were examined for various metabolites using modern analytical methods such as mass spectrometry and NMR spectroscopy. The results of the metabolite analysis can be compared with normal reference values, and the detected changes can serve as warning markers for FGR and PE. Early detection and monitoring of FGR and PE allows timely medical intervention and improved pregnancy outcomes.
Studying the level of polyamines in the urine can also be an important aspect in the diagnosis and monitoring of various conditions, including FGR and PE. Polyamines are small organic molecules that consist of saturated hydrocarbon chains with several amino groups. Polyamines, such as spermine, spermidine, and cadaverine, play an important role in cellular metabolism and can be associated with various physiological and pathological processes [7]. Detectable levels of polyamines in urine are associated with a disorder or physiological change in their metabolism. Research suggests that polyamine levels may be altered by systemic inflammatory responses and oxidative stress [8], cell growth, and division, and may accurately reflect disturbances in cellular processes associated with FGR and PE. Therefore, changes in urinary polyamine levels may serve as potential biomarkers for diagnosis and monitoring of FGR and PE.
Further research in this area may lead to the development of more accurate and effective methods for diagnosing and treating FGR and PE, helping to improve the health of pregnant women and their fetuses.
This study aimed to investigate the characteristics of urine polyamine profiles in pregnant women with FGR and PE to identify biomarkers with prognostic and diagnostic potential.
Materials and methods
The study included 73 pregnant women who were admitted and delivered at Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation. The PE and FGR groups consisted of 22 and 30 patients with confirmed diagnoses, respectively. The control group consisted of 21 healthy pregnant women without any complications. All patients signed an informed consent form to participate in this study. The study complies with the requirements of the Declaration of Helsinki, the International Conference on Harmonization (ICF), the Standards of Good Clinical Practice (GCP), the Federal Law “On the Fundamentals of Protecting the Health of Citizens in the Russian Federation” No. 323-FZ of November 21, 2011, and was approved by the local ethics committee of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation. The inclusion criteria for the study were pregnant women aged 18–35 years, singleton pregnancy at 24–40 weeks of gestation, and early PE and FGR. The exclusion criteria were Rh and ABO isoimmunization, chromosomal abnormalities, genetic mutations, congenital malformations in the fetus, presence of severe extragenital pathology in the mother, chronic kidney disease, large uterine fibroids, and acute infectious diseases. For the analysis, urine was collected from women at various stages of pregnancy.
Method of polyamine analysis
The optimized procedure for preparing urine samples for polyamine analysis involves the following steps: Place 400 µl of urine in a 2 ml microcentrifuge tube; add 1000 µl methanol; stir for 5 minutes; centrifuge for 10 minutes at 13000 g; withdraw 1200 µl of the supernatant into a clean 2 ml microcentrifuge tube; dry in a stream of nitrogen at 50°C; add 600 μl of a solution of dansyl chloride (10 mg/ml) in an acetonitrile carbonate buffer solution pH=9.7 (50/50 v/v); stir 1 minute; centrifuge for 1 minute at 13000 g; thermostat at 60°C for 90 minutes; centrifuge for 1 minute at 13000 g; add 1000 µl ethyl acetate; stir for 10 minutes; centrifuge for 10 minutes at 13000 g; remove 1000 µl of the top layer into a clean microcentrifuge tube with a capacity of 2 ml; add 1000 µl ethyl acetate; vortex for 10 minutes; centrifuge for 10 minutes at a speed of 13.4 g; select 1000 µl of the top layer and combine with the previous selection; dry in a stream of nitrogen at 50°C; add 200 μl of acetonitrile; stir for 5 minutes; centrifuge for 10 minutes at 13000 g; transfer 170 µl into a vial with insert for further analysis.
Polyamines were analyzed by liquid chromatography with mass spectrometric detection (LC-MS) on a system consisting of an ABSciex QTrap 5500 triple quadrupole mass spectrometric detector (ABSciex, Canada) equipped with an electrospray ionization source and an Agilent 1260 Infinity liquid chromatograph (Agilent, USA). An Agilent Zorbax Eclipse Plus C18 column (50×3 mm, 1.8 µm, Agilent, USA) was used to separate the samples. For the analysis of organic acids, 20 μl of the sample was injected, and a 0.1% solution of formic acid in water was used as eluent A; eluent B was a 0.1% solution of formic acid in acetonitrile. The flow rate was 650 μL/min and the column temperature was maintained at 30°C. The composition of the mobile phase during the analysis was changed as follows: 0–0.3 minutes – 20% B, up to 5.3 minutes the volume fraction of eluent B increased to 95%, maintained the value until 8.3 minutes and returned to a value of 20%. The mass spectrometer settings were as follows: peripheral gas pressure – 1.4 bar, nebulizer gas pressure – 3.4 bar, source temperature, 500°C; and capillary voltage, 4500 V.
In this study, the following nine polyamines were analyzed: putrescine, ethylenediamine, 1,3-diaminopropane, cadaverine, 1,7-diaminoheptane, 1,6-diaminohexane, N-acetylputrescine, N1-acetylspermine, and spermidine.
Statistical analysis
Statistical analysis was performed using scripts written in R [R Core Team (2018). R: Language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/] in RStudio [RStudio Team (2016). RStudio: Integrated Development for R. RStudio, Inc., Boston, MA URL http://www.rstudio.com/].
Quantitative variables are expressed as median (M) with interquartile range (Q1; Q3). The significance threshold was set at p<0.05. Differences between groups for continuous variables were assessed using the Mann–Whitney test; differences were considered statistically significant at p<0.05. If the p-value was less than 0.001, it was reported in the format p<0.001. The Bonferroni correction was used for multiple comparisons.
Logistic regression models were developed to assess the ability of patients to be classified into groups based on the parameters studied. Of all developed models, four with the largest area under the ROC curve (AUC) were selected. The quality of the developed models was determined by constructing an ROC curve, determining the area under the ROC curve, and calculating the sensitivity and specificity.
Results
In this retrospective case-control study, pregnant women were included by referral based on the diagnosis of FGR or PE. It should be especially emphasized that at the time of urine collection, all patients had moderate PE with stable blood pressure, slight proteinuria with normal 24h diuresis and the absence of impaired fetal-placental blood flow and fetal distress; FGR based on fetometry data (3rd percentile or less) without Doppler signs of fetal distress. None of the patients had regular labor or a threat of preterm birth. At the time of delivery, which occurred on average 3 weeks after urine collection, one-third of the patients were diagnosed with severe PE, and more than half had critical fetal-placental blood flow compromise, which was an indication for emergency cesarean delivery. In the control group, urine was collected at a gestational age comparable to that in patients with PE and FGR. Subsequently, the course of pregnancy and delivery was monitored and was not complicated.
The age and weight of the patients with PE were comparable to those of the control group, since advanced reproductive age and obesity could become serious confounding factors when assessing the level of urine polyamines in this group. The body mass index (BMI) of pregnant women in the FGR group was significantly lower than that of women in the control and PE groups (p=0.005 and p=0.0045, respectively).
The results of the analysis of clinical parameters of the study groups are presented in Table 1.
Considering the need for accelerated delivery for obstetric indications, in conjunction with the implementation of decompensated PE and FGR, the time of delivery for these groups was significantly different from that of the control group (p<0.001). For the PE and FGR cases, the mean birth weights were 2041.0 g and 1828.7 g.
It should be noted that the newborn's Apgar score in the PE group was significantly lower in the first minute of life (p=0.04), while in the FGR group it was significantly lower in the fifth minute (p=0.05), compared with the control group. This may be explained by a violation of adaptation in the early neonatal period in neonates who remain under conditions of chronic hypoxia and growth restriction for long periods of time.
Urine polyamines were analyzed by liquid LC-MS.
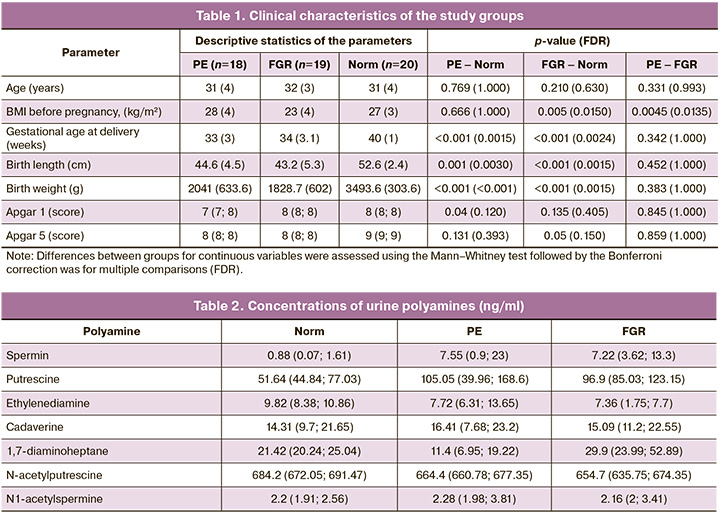
In the first stage of the study, the concentration of urine polyamines was assessed in the three groups and their intergroup comparisons. Seven polyamines were identified in the urine of the pregnant women: spermine, putrescine, ethylenediamine, cadaverine, 1,7-diaminoheptane, N-acetylputrescine, and N1-acetylspermine. In an intergroup comparison, significant differences were found for three polyamines: putrescine, 1,7-diaminoheptane, and N-acetylputrescine (Fig. 1).
Depending on the nature of placenta-associated complications (FGR or PE), the concentrations of urine polyamines showed multidirectional changes relative to the control group. Putrescine and its ketone derivative N-acetylputrescine in groups represented by pregnant women with placenta-associated complications were significantly different from those in the control group. Putrescine was increased in PE and FGR, with the highest concentration in PE, while N-acetylputrescine was decreased in PE and FGR groups compared to normal, but its concentration was higher in PE than in FGR. The concentration of 1,7-diaminoheptane was lower in patients with PE and higher in patients with FGR than in the control group (p=0.04) (Fig. 1, Table 2)
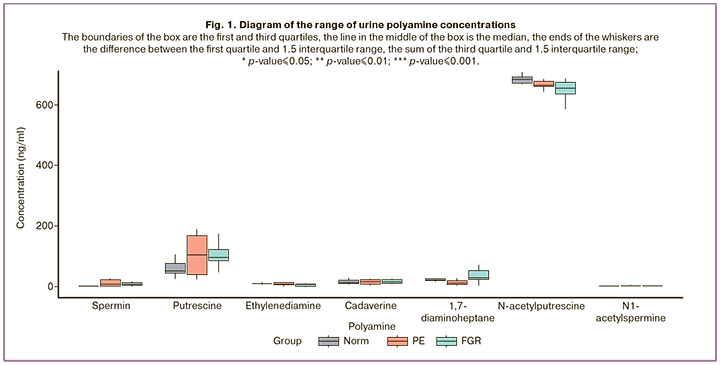
In the second stage of data analysis, logistic regression models were developed based on polyamine concentrations in urine to classify patients belonging to the PE and FGR groups relative to the control group, and the ROC curves of these models were obtained.
Four models were developed with high sensitivity and specificity to classify patients into control or PE groups. The best indicators were obtained for a model built on the concentrations of spermine, ethylenediamine, 1,7-diaminoheptane, and N-acetylputrescine, with a sensitivity of 1 (1; 1), specificity of 0.9 (0.7; 1), and area under the curve AUC= 0.94 (Fig. 2, Table 3).
To classify patients between the control group and the FGR group, 4 models were also developed, which had the highest sensitivity and specificity 1 (1; 1).
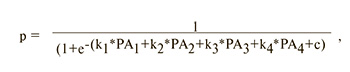
where c, k1, k2, k3, k4 are logistic regression coefficients, PA1, PA2, PA3, PA4 are polyamine levels, p is the dependent variable.
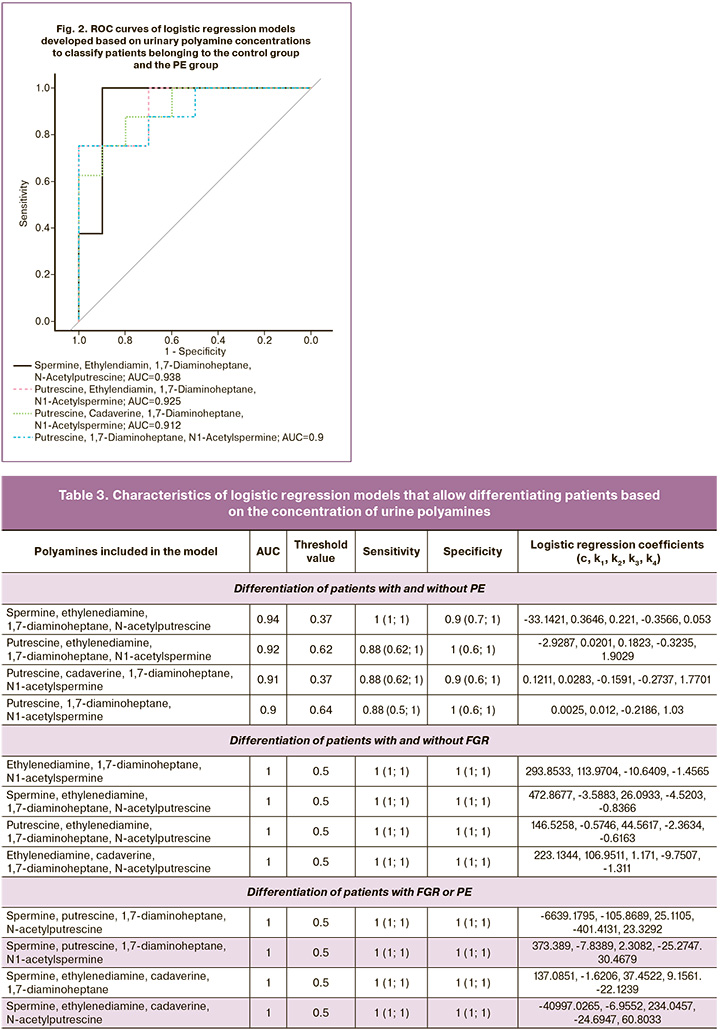
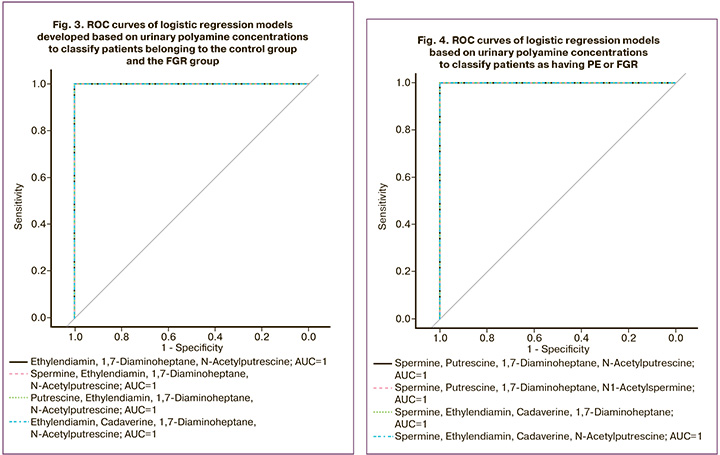
The following polyamines were used as independent variables in these models: ethylenediamine, 1,7-diaminoheptane, N-acetylputrescine, spermine, ethylenediamine, 1,7-diaminoheptane, N-acetylputrescine, putrescine, ethylenediamine, 1,7-diaminoheptane, N-acetylputrescine, ethylenediamine, cadaverine, 1,7-diaminoheptane, and N-acetylputrescine. These models are characterized by a sensitivity and specificity of 1 (1; 1) and area under the curve (AUC) = 1 at a threshold value of 0.5 (Fig. 3, Table 3).
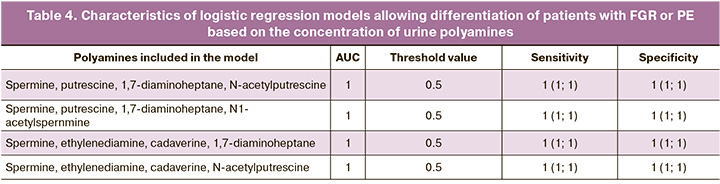
Considering the encouraging results of data analysis on polyamine concentrations in urine for the prospect of using polyamines in non-invasive prediction of PE and FGR, at the third stage of the study we analyzed polyamine concentrations in isolated models for predicting placenta-associated complications. Logistic regression models were generated based on urinary polyamine concentrations to classify patients into the PE or FGR group. All four models had the highest sensitivity of 1 (1; 1) and specificity of 1 (1; 1), with an area under the AUC curve of 1 and a threshold value of 0.5 (Fig. 4, Table 4).
Discussion
The medical use of polyamines in disease diagnosis is an area of active research, and although it is still in its infancy, polyamines may prove useful in several areas.
Cancer diagnosis: Polyamine levels in tissues and body fluids may be abnormally high in cancerous tumors [9]. Research is underway to utilize polyamines as biomarkers for cancer diagnosis, where measuring polyamine levels in biological samples can help detect the presence of cancer and determine its stage [10].
Neurodegenerative diseases: Research is also exploring the connection between polyamines and neurodegenerative diseases, such as Parkinson's and Alzheimer's. Changes in polyamine levels in the nervous tissue may be associated with pathological processes in these diseases, opening opportunities for developing diagnostic methods and monitoring disease progression [11].
Immunodiagnostics: Polyamine levels are associated with specific pathological processes and are used as biomarkers for diagnosing infectious diseases and autoimmune disorders [12].
Polyamines are a group of molecules playing crucial roles in various aspects of biology and medicine. Their biological significance includes regulation of cell growth and development, participation in the synthesis of proteins and nucleic acids, antitoxic effects, and regulation of gene expression [13].
The medical applications of polyamines in disease diagnosis are of great interest because they can serve as important biomarkers for various pathological conditions. In obstetrics, these factors may be associated with various aspects of pregnancy and childbirth. Studying the effects of polyamines on fetal and placental development is crucial for understanding molecular processes during pregnancy, particularly in cellular growth and differentiation, which are essential for fetal organ and system development [14].
Our data demonstrated a significant relationship between changes in polyamine levels in urine and placenta-associated pregnancy complications. The concentration of polyamines in the urine showed multidirectional changes depending on obstetric complications (FGR or PE). For polyamines, such as spermine, putrescine, and cadaverine, increased concentrations were observed in groups represented by pregnant women with placenta-associated complications, with the highest concentrations in PE for all three polyamines. Putrescine showed a statistically significant increase (p=0.05). In contrast, ethylenediamine and N-acetylputrescine exhibited decreased concentrations in the pathology groups, with minimal FGR values. Notably, 1,7-diaminoheptane displayed a different pattern with an increase in concentration of FGR, against a decrease in PE, significantly distinguishing these concentrations from the control group (p=0.04). These changes were recorded during the initial clinical manifestations of obstetric syndromes, indicating a high probability of using polyamines to predict the severity of early PE and FGR and to justify the timing of delivery.
PE and FGR, which are recognized to have a common pathogenesis, exhibit impaired angiogenesis and generalized endothelial damage, with associated inflammation as the dominant symptoms. Higher values of markers of impaired angiogenesis, endothelial damage, and inflammatory markers in both the PE and FGR groups suggest general disturbances in the development of these pathologies. The similarity in lesion profiles between patients with pulmonary embolism and FGR can be used to develop general diagnostic criteria [15]. Considering the literature descriptions of polyamine level changes in ischemic and hemorrhagic brain lesions [16] and their connection with damage to the cardiovascular system and kidneys [17], assessing the degree of nervous tissue damage in fetuses and pregnant women with FGR and PE by measuring polyamine levels dynamically is highly probable.
An experimental study conducted in 2023 by Dasdelen et al. examined the effects of cerebral ischemia-reperfusion (IR) by evaluating several liver enzymes, free radicals, cytokines, oxidatively damaged DNA, and spermidine/spermine-N-1-acetyltransferase (SSAT). A rat experiment revealed that brain IR induced inflammatory and oxidative damage, resulting in elevated levels of nuclear factor-κB, interleukin-6, and DNA damage in the liver samples. The administration of putrescine via gavage reduces liver damage, demonstrating anti-inflammatory and antioxidant effects [16]. Consequently, the significant increase in putrescine concentration in the urine of pregnant women with placenta-associated complications, particularly preeclampsia (PE), may be attributed to physiological compensation for inflammation and oxidative damage processes. In the case of fetal growth restriction (FGR), a notable increase in 1,7-diaminoheptane was observed. In 2002, a group of Chinese researchers described the unusual amino acid hypusine [nepsilon-(4-amino-2-hydroxybutyl)lysine], which is post-translationally formed in a cellular protein, eukaryotic translation initiation factor 5A (eIF5A), under the influence of deoxyhypusine synthase and deoxyhypusine hydroxylase [18]. Although the essential nature of eIF5A and its hypusine modification for eukaryotic cell viability are acknowledged, its true physiological function remains unknown. The authors of the study investigated the effects of N1-guanyl-1,7-diaminoheptane (GC7), a potent deoxyhypusine synthase inhibitor, on endothelial cell proliferation, differentiation, and apoptosis. Treatment of human umbilical vein endothelial cells (HUVECs) with GC7 resulted in dose-dependent inhibition of hypusine formation and cell proliferation. At a concentration of 10 μM, GC7 almost completely inhibited cellular hypusine synthesis and caused cytostasis in HUVECs. Moreover, pretreatment of HUVECs with up to 50 μM GC7 for 4 days had a minimal impact on cell attachment and differentiation on Matrigel and did not induce apoptosis. Instead, pretreatment with GC7 (96 h at 5–50 μM) conferred protective effects against the apoptotic death of HUVECs induced by serum starvation. These findings suggest that eIF-5A may play a role in the expression of proteins necessary for endothelial cell apoptosis as well as the proteins required for cell proliferation. This observation corroborates the significance of the observed association between increased concentrations of 1,7-diaminoheptane in urine during FGR, which may indirectly indicate a dose-dependent inhibition of cell proliferation and serve as a potential biomarker of FGR, with levels increasing in accordance with the severity of the fetal condition.
Of great interest is the development of methods for diagnosing and monitoring polyamine levels in pregnant women, serving as tools to predict the risk of obstetric syndrome. Our analysis demonstrated the role of polyamines in predicting placenta-associated complications using logistic regression models with the highest sensitivity and specificity, AUC curve equal to 1, and threshold value of 0.5. These promising results suggest potential applications after in-depth validation and control at several time-points.
Conclusion
Studying the role of polyamines in obstetrics and their potential for diagnosing and predicting obstetric syndromes is an important area of research that may lead to the development of new screening and treatment methods for pregnant women, ultimately improving maternal and fetal health during pregnancy.
References
- Clinton C.M., Bain J.R., Muehlbauer M.J., Li Y., Li L., O'Neal S.K. et al. Non-targeted urinary metabolomics in pregnancy and associations with fetal growth restriction. Sci. Rep. 2020; 10(1): 5307. https://dx.doi.org/10.1038/s41598-020-62131-7.
- Погорелова Т.Н., Гунько В.О., Авруцкая В.В., Каушанская Л.В., Дурницына О.А. Нарушение плацентарного обменах аминокислот при задержке роста плода. Биомедицинская химия. 2017; 63(3): 266-71. [Pogorelova T.N., Gunko V.O., Avrutskaya V.V., Kaushanskaya L.V., Durnitsyna O.A. Impairments of placental amino acid metabolism in fetal growth restriction. Biomed. Khim. 2017; 63(3): 266-71. (in Russian)]. https://dx.doi.org/10.18097/PBMC20176303266.
- Dai C., Fei Y., Li J., Shi Y., Yang X. A novel review of homocysteine and pregnancy complications. Biomed. Res. Int. 2021; 2021: 6652231. https://dx.doi.org/10.1155/2021/6652231.
- Kramer M.S., Kahn S.R., Dahhou M., Otvos J., Genest J., Platt R.W., Evans R.W. Maternal lipids and small for gestational age birth at term. J. Pediatr. 2013; 163(4): 983-8. https://dx.doi.org/10.1016/j.jpeds.2013.05.014.
- Cohen J.M., Beddaoui M., Kramer M.S., Platt R.W., Basso O., Kahn S.R. Maternal antioxidant levels in pregnancy and risk of preeclampsia and small for gestational age birth: a systematic review and meta-analysis. PLoS One. 2015; 10(8): e0135192. https://dx.doi.org/10.1371/journal.pone.0135192.
- Thévenot E.A., Roux A., Xu Y., Ezan E., Junot C. Analysis of the human adult urinary metabolome variations with age, body mass index, and gender by implementing a comprehensive vorkflow for univariate and OPLS statistical analyses. J. Proteome Res. 2015; 14(8): 3322-35. https://dx.doi.org/10.1021/acs.jproteome.5b00354.
- Gugliucci A. Polyamines as clinical laboratory tools. Clinica Chimica Acta. 2004; 344(1-2): 23-35. https://dx.doi.org/10.1016/j.cccn.2004.02.022.
- Zhang M., Wang H., Tracey K.J. Regulation of macrophage activation and inflammation by spermine: a new chapter in an old story. Crit. Care Med. 2000; (4, Suppl.): N60-6. https://dx.doi.org/10.1097/00003246-200004001-00007.
- Holbert C.E., Cullen M.T., Casero R.A. Jr, Stewart T.M. Polyamines in cancer: integrating organismal metabolism and antitumour immunity. Nat. Rev. Cancer. 2022; 22(8): 467-80. https://dx.doi.org/10.1038/s41568-022-00473-2.
- Jänne J., Pösö H., Raina A. Polyamines in rapid growth and cancer. Biochim. Biophys. Acta. 1978; 473(3-4): 241-93. https://dx.doi.org/10.1016/0304-419x(78)90015-x.
- Miller-Fleming L., Olin-Sandoval V., Campbell K., Ralser M. Remaining mysteries of molecular biology: the role of polyamines in the cell. J. Mol. Biol. 2015; 427(21): 3389-406. https://dx.doi.org/10.1016/J.JMB.2015.06.020.
- Handa A.K., Fatima T., Mattoo A.K. Polyamines: bio- molecules with diverse functions in plant and human health and disease. Front. Chem. 2018; 6: 10. https://dx.doi.org/10.3389/FCHEM.2018.00010.
- Raina A., Jänne J. Physiology of the natural polyamines putrescine, spermidine and spermine. Med. Biol. 1975; 53(3): 121-47.
- Rider J.E., Hacker A., Mackintosh C.A., Pegg A.E., Woster P.M., Casero R.A. Jr. Spermine and spermidine mediate protection against oxidative damage caused by hydrogen peroxide. Amino Acids. 2007; 33(2): 231-40. https://dx.doi.org/10.1007/S00726-007-0513-4.
- Kwiatkowski S., Dołegowska B., Kwiatkowska E., Rzepka R., Marczuk N., Loj B., Torbè A. Maternal endothelial damage as a disorder shared by early preeclampsia, late preeclampsia and intrauterine growth restriction J. Perinat. Med. 2017; 45(7): 793-802. https://dx.doi.org/10.1515/jpm-2016-0178.
- Dasdelen D., Cetin N., Menevse E., Baltaci A.K., Mogulkoc R. Effects of putrescine on oxidative stress, spermidine/spermine-N(1)-acetyltransferase, inflammation and energy levels in liver and serum in rats with brain ischemia-reperfusion. Physiol. Int. 2023; 17; 110(1): 34-45. https://dx.doi.org/10.1556/2060.2022.00138.
- Johnsson I.W., Naessen T., Ahlsson F., Gustafsson J. High birth weight was associated with increased radial artery intima thickness but not with other investigated cardiovascular risk factors in adulthood. Acta Paediatrica. 2018; 107(12): 2152-7. https://dx.doi.org/10.1111/apa.14414.
- Lee Y., Kim H.K., Park H.E., Park M.H., Joe Y.A. Effect of N1-guanyl-1,7-diaminoheptane, an inhibitor of deoxyhypusine synthase, on endothelial cell growth, differentiation and apoptosis. Mol. Cell. Biochem. 2002; 237(1-2): 69-76. https://dx.doi.org/10.1023/a:1016535217038.
Received 20.11.2023
Accepted 24.01.2024
About the Authors
Aida P. Gasanbekova, Postgraduate Student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, aidoxxa21@mail.ru, https://orcid.org/0000-0002-2882-8163Natalia A. Frankevich, PhD, Senior Researcher, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, natasha-lomova@yandex.ru, https://orcid.org/0000-0002-6090-586X
Vitaliy V. Chagovets, PhD in Physical and Mathematical Sciences, Senior Researcher at the Laboratory of Proteomics and Metobolomics of the Human Reproduction, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow,
Ac. Oparin str., 4, vvchagovets@gmail.com, https://orcid.org/0000-0002-5120-376X
Alisa O. Tokareva, Specialist at the Laboratory of Proteomics and Metobolomics of the Human Reproduction, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, alisa.tokareva@phystech.edu
Julia N. Kachikowski, 2nd year Resident in Obstetrics and Gynecology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, https://orcid.org/0009-0008-2324-6106
Anastasia V. Novoselova, PhD, Laboratory of Metabolomics and Bioinformatics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, a_novoselova@oparina4.ru
Tamara E. Karapetyan, Dr. Med. Sci., Senior Researcher at the Obstetrics Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, https://orcid.org/0000-0003-0025-3182
Corresponding author: Natalia A. Frankevich, natasha-lomova@yandex.ru



