The results of the examination of cervical-vaginal microbiota in pregnant women with threatened preterm birth using a real-time polymerase chain reaction
Objective.To investigate the prevalence of urogenital infections and status of the cervical-vaginal microbiota in pregnant women with threatened preterm birth using real-time polymerase chain reaction (PCR). Material and methods. We conducted a cross-sectional study of 300 pregnant women at 27 to 32 weeks’ gestation, of which 118 were diagnosed with threatened preterm labor, and 182 had a late uncomplicated pregnancy (control group). We analyzed the results of antenatal screening for urogenital infections, which included real-time PCR detection of etiologically significant pathogens associated with bacterial vaginosis, vulvovaginal candidiasis, aerobic vaginitis, and the causative agents of four sexually transmitted infections (Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium, Trichomonas vaginalis). All study participants attended antenatal clinics. Results Among pregnant women with threatened preterm birth and women in the control group, the prevalence of mixed infections, bacterial vaginosis, aerobic vaginitis, vulvovaginal candidiasis, and infection caused by Mycoplasma genitalium was 14% and 4% (p <0.01), 17% and 7% (p <0.05), 12% and 3% (p < 0.05), 16% and 9% (p = 0.08), and 2% and 1% (p> 0.05), respectively. No women in the control group had chlamydial infection and gonorrhea, while they were found in 3% (p < 0.05) and 0.8% (n = 1) (p < 0.05) among patients with threatened preterm labor, respectively. The vaginal concentration of Lactobacillus spp. was significantly higher in the women in the control group compared with the patients with threatened preterm birth (8.24 ± 0.67 and 7.38 ± 1.27 GE/ml; p <0.001). Conclusion Real-time PCR screening for female genital tract infections revealed significantly higher rates of mixed infections, bacterial vaginosis, aerobic vaginitis, chlamydial infection among pregnant women with threatened preterm birth.Dobrokhotova Yu.E., Bondarenko K.R., Gushchin A.E., Rumyantseva T.A., Dolgova T.V., Kuznetsov P.A., Dzhokhadze L.S.
Keywords
Preterm birth is defined as childbirth between the 20th and 37th weeks from the first day of the mother’s last normal menstrual period with a regular menstrual cycle. Two-thirds of preterm births occur after the spontaneous onset of labor, whereas the remainder is medically indicated due to maternal or fetal life-threatening complications [1].
Across 184 countries, the rate of preterm birth ranges from 5% to 18% of babies born, averaging 9.6% [2]. Every year, an estimated 15 million babies are born preterm [3], and approximately 1 million children die each year due to complications of preterm birth [4]. Preterm births account for 75% of perinatal mortality [5]. Globally, prematurity is the leading cause of death in children under the age of 5 years, and three-quarters of these deaths are considered conditionally preventable [2, 6]. Effective prevention of preterm birth can be implemented by reducing or eliminating so-called “modifiable risk factors” that include adolescent pregnancy, short time gaps between births, pre-pregnancy underweight or obesity, chronic disease (e.g., diabetes), tobacco and alcohol abuse, poor psychological health and infectious diseases [7–9].
It has been previously shown that 40–50% of spontaneous preterm births are due to various infectious factors. In these cases, bacterial infection plays a leading role in the etiology of preterm births. It is important to note that infectious causes are associated with very early and early preterm births (from the 22nd to the 32nd weeks’ gestation) [10].
The vaginal biotope is considered the most likely source and/or reservoir of bacterial pathogens [11]. The association between the term of delivery and the status of the cervical-vaginal microbiota has been shown in a study reporting that women with abnormalities of the vaginal flora in the first trimester have a 75% higher risk of delivery before 35 weeks compared with women with normal vaginal flora [12]. At the same time, most of the published studies of the vaginal microbiota in the second half of pregnancy have not revealed any interrelations between the microbial composition of the lower female genital tract and obstetric outcomes. Owing to a lack of conclusive scientific evidence for the impact of the genital tract infections on obstetric outcomes [13–15], a population study of genital tract infections in asymptomatic pregnant women in the later stages of gestation is warranted. In accordance with current regulatory documents, the assessment of the state of the vaginal biotope during pregnancy is carried out twice (at the initial presentation and at 30 weeks) using microscopic and bacteriological examination; women with complicated pregnancies undergo polymerase chain reaction (PCR) to rule out chlamydial infection by detecting C. trachomatis DNA in the cylindrical epithelium of the cervical canal (order of the Ministry of Health of the Russian Federation of 01.11.2012, No. 572n). Obviously, the screening for urogenital infections regulated by this order is limited both by the spectrum of the detected microorganisms and by the diagnostic methods, which, in particular, do not allow isolation from the female genital tract a number of anaerobes and any sexually transmitted infection (STI) agents that are clinically significant for gestation.
Fragmentary evidence supporting the role of reproductive organ infections occurring in the second half of pregnancy in the preterm birth and conflicting data on the prevalence of urogenital infections diagnosed using laboratory tests with low diagnostic efficacy (bacterioscopy, vaginal flora culture) reduce the effectiveness of interventions aimed at preventing perinatal morbidity and mortality.
This study aimed to investigate the status of the cervical-vaginal microbiota in pregnant women with threatened preterm birth using PCR.
Material and methods
A total of 300 pregnant women at 27 to 32 weeks’ gestation underwent prenatal infectious disease screening. All women included in the study participated in antenatal care and underwent a clinical and laboratory examination as provided by the order of the Ministry of Health of the Russian Federation on November 1, 2012, No. 572n. The study group comprised 118 women who were hospitalized in maternity hospitals No. 1, No. 10, No. 24 in Moscow due to threatened preterm labor. The patients were enrolled in the study group under the following inclusion criteria: informed consent prior to their inclusion in the study; age between 17 and 45 years; singleton pregnancy; diagnosis of threatened preterm labor defined as regular contractions of the uterus (at least 4 in 20 min) between 22 and 316 weeks’ gestation, cervical length of < 25 mm on transvaginal ultrasound and/or dynamic changes of the uterine cervix (shortening and smoothing), given that the opening of the internal cervical os was less than 30 mm and amniotic membranes were intact.
The exclusion criteria for the study were as follows: pregnancy occurring as a result of assisted reproductive technologies; severe fetal malformations; neoplasms and severe non-obstetric comorbidities (obesity, autoimmune diseases, diabetes mellitus, arterial hypertension); HIV infection, syphilis, infectious hepatitis; mental disorders, chronic alcoholism, drug addiction; antibiotic therapy three weeks before examination and patient selection.
Among the 118 pregnant women in the study group, 69% (n = 81) had threatened preterm labor, and in 31% (n = 37) of hospitalized patients, pregnancy was complicated both by threatened preterm labor and preeclampsia and/or fetal growth restriction.
The control group comprised 182 women with the physiological course of the second half of gestation who had no obstetric complications and had an uneventful delivery. A sampling of biological material from pregnant women in the control group was performed in outpatient settings during a routine examination at 27 to 32 weeks’ gestation.
The two groups were comparable regarding the age (30.45 ± 4.79 and 30.69 ± 4.92 years; p> 0.05) and did not differ in socioeconomic status, non-obstetric comorbidities, body mass index, and parity. Fifty two percent (n = 61) of the patients in the study and 54% (n = 98) in the control groups were primiparous (p> 0.05). Prenatal infectious disease screening was conducted at 30 ± 2.7 and 30 ± 1.5 weeks’ gestation in the study and control groups, respectively (p> 0.05).
For laboratory verification of the etiology of “bacterial vaginosis” (BV), “aerobic vaginitis” (AV), “candidal vulvovaginitis” (CVV), “chlamydial infection” (CI), “gonorrhea”, “trichomoniasis”, and “infection caused by M. genitalium” was performed using cervical-vaginal smear microscopy and a multiplex real-time polymerase chain reaction test that offers sensitivity and specificity comparable with international clinical and laboratory criteria for the diagnosis of these infections.
Diagnosis of the reproductive tract infections was confirmed using the following clinical and laboratory criteria:
- “BV” was diagnosed according 3 of 4 Amsel’s composite criteria [16] and real-time PCR, which was based on calculating ratios of logarithms of DNA concentrations of the detected Lactobacillus spp., total bacterial DNA and G.vaginalis, A.vaginae DNA in the vagina.
- “CVV” was diagnosed based on pathognomonic subjective symptoms (itching, burning, “cheesy” vaginal discharge) and objective signs (hyperemia and edema of the vulva, vagina, change in the nature of the discharge), microscopic confirmation of the inflammatory reaction (increase in the number of leukocytes more than 10 per HPF and the presence of yeast-like fungi or their fragments (spores, mycelium). Along with microscopy, “CVV” was diagnosed using real-time PCR by detecting DNA from one of 5 species of Candida fungi at a concentration above 102 GE/ml.
- “AV” was diagnosed by the presence of subjective manifestations (itching, burning, discharge from the genital tract), objective symptoms (hyperemia and edema) in combination with microscopic signs of an inflammatory reaction (leukocytosis more than 10per HPF), a decrease in lactomorphotypes and upon real-time PCR detection of DNA of aerobic microorganisms (Enterobacteriaceae, Streptococcus spp., Staphylococcus spp.) in concentrations exceeding that of Lactobacillus spp. DNA.
- “CI”, “gonorrhea”, “trichomoniasis”, “infection caused by M. genitalium” were diagnosed by detecting DNA of C. trachomatis, N.gonorrhoeae, T. vaginalis, M. genitalium.
Vaginal discharge for real-time PCR analysis was collected from the posterior vaginal fornix with a urogenital probe, the working part of which was left in a test tube with Transport Medium with Mucolytic Agent (Russia). The material was transported in accordance with the manufacturer’s instructions.
Real-time PCR examination consisted of the following steps:
- DNA extraction using sorbent-based DNA-Sorb-AM reagent kit (Russia);
- Real-time DNA amplification with hybridization-fluorescence detection in a Rotor-Gene 6000 instrument (Corbett Research, Australia) by the manufacturer’s instructions;
- Analysis and interpretation of results.
Identification of the main pathogens of reproductive organ infections by multiplex PCR with hybridization-fluorescent detection of amplification products in DNA samples obtained by extraction from scrape samples was performed using the following PCR kits:
- “AmpliSens Florocenosis/BV-FL” designed to calculate DNA amount ratios for Lactobacillus spp., G.vaginalis, A.vaginae [17];
- “AmpliSens Floracenosis /Aerobes-FL” designed to determine the amount of DNA of Enterobacteriaceae, Staphylococcus spp., Streptococcus spp. [18];
- “AmpliSens Florocenosis/Candida FL” designed to determine the amount of DNA of 5 species of Candida spp.: C.albicans, C.glabrata, C.krusei, C.parapsilosis and C.tropicalis [19];
- “AmpliSens Florocenosis/Mycoplasma-FL” designed to determine the amount of DNA of U.parvum / urealyticum and M.hominis [20];
- “AmpliSens N.gonorrhoeae/ C.trachomatis/ M.genitalium/ T.vaginalis MULTIPIME-FL” intended for the isolation of DNA of N.gonorrhoeae, C.trachomatis, M.genitalium and T.vaginalis.
The obtained concentrations of microbial DNA were expressed as genomic equivalents per milliliter (GE/ml). The data were processed using Florocenosis (version 1.0) MS Excel based software.
Statistical analysis was carried out using the modern statistical software. The normality of the distribution was tested by the Shapiro-Wilk and Kolmogorov-Smirnov tests. Variables showing normal distribution were presented as a mean (M) and the standard error of the mean (m) and analyzed by Student’s t-test and Pearson’s linear correlation coefficient (r). The structure was reported by frequencies and percentages (%). Categorical variables were compared by χ2 test, for small groups by χ2 with the Yeats correction or Fisher’s exact test. The odds ratios (OR) and 95% confidence intervals (CI) were calculated using contingency tables. Differences were considered statistically significant at p <0.05.
Results
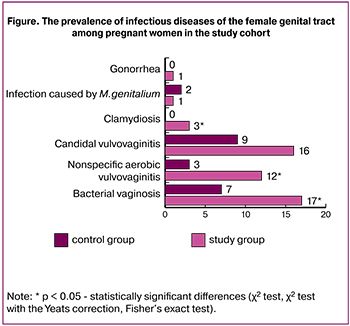 Screening for infectious diseases revealed (figure) that among the patients of the study group the incidence of BV (n = 20; 17%) was more than twice higher than among women in the control group (n = 12; 7%); (p <0.01). In the whole study cohort, 32 out of 300 (11%) women had BV. Among patients with threatened preterm labor and healthy subjects, AV was observed in 12% (n = 14) and 3% (n = 5) women, respectively (p <0.01), which regarding the whole study cohort was 6% (19 out of 300). CVV was the most frequently detected infection (n = 35; 12%). CVV was detected in 16% (n = 19) and 9% (n = 16) of women in the study and the control group, respectively; the difference was not statistically significant (p = 0.08). Detection of BV and AB in the second half of pregnancy was associated with a 3-fold (OR 2.89; 95% CI 1.36–6.17; p <0.05) and almost 5-fold (OR 4.77; 95% CI 1.67–13.61; p <0.05) increase in the risk of threatened preterm birth, respectively. No association was found between threatened preterm labor and HBV (OR 1.99; 95% CI 0.98–4.05; p> 0.05).
Screening for infectious diseases revealed (figure) that among the patients of the study group the incidence of BV (n = 20; 17%) was more than twice higher than among women in the control group (n = 12; 7%); (p <0.01). In the whole study cohort, 32 out of 300 (11%) women had BV. Among patients with threatened preterm labor and healthy subjects, AV was observed in 12% (n = 14) and 3% (n = 5) women, respectively (p <0.01), which regarding the whole study cohort was 6% (19 out of 300). CVV was the most frequently detected infection (n = 35; 12%). CVV was detected in 16% (n = 19) and 9% (n = 16) of women in the study and the control group, respectively; the difference was not statistically significant (p = 0.08). Detection of BV and AB in the second half of pregnancy was associated with a 3-fold (OR 2.89; 95% CI 1.36–6.17; p <0.05) and almost 5-fold (OR 4.77; 95% CI 1.67–13.61; p <0.05) increase in the risk of threatened preterm birth, respectively. No association was found between threatened preterm labor and HBV (OR 1.99; 95% CI 0.98–4.05; p> 0.05).
Of four bacterial STI pathogens detectable by the test system, three were detected, including CI in three patients, and gonorrhea and infection associated with M. genitalium, each in one patient. Of obligate pathogens, only M.genitalium was isolated in 2% (n = 3) of women in the control group. The groups did not differ in the incidence of bacterial STIs which were detected in 4% (n = 5) and 2% (n = 3) patients; (p> 0.05). The intergroup differences were found in the frequency of detection of C. trachomatis DNA (p <0.05).
In the whole study cohort, 22% (67 out of 300) of women were found to have mono- or mixed urogenital infections. Furthermore, pregnant women in the study group were twice more likely to have infections of the reproductive organs (n = 39; 33%) than women in the control group (n = 28; 15%); p <0.001).
As shown in Table 1, mixed infections of the reproductive tract (two or more distinct syndromes/diseases) were detected in 14% (n = 17) women in the study group and only in 4% (n = 8) women in the control (p <0.01), which corresponds to a greater risk of preterm birth in pregnant women with mixed infections of the lower genital tract (OR 3.88; 95% CI 1.63–9.24; p <0, 05). The most common mixed infections in both groups were combinations of CVV with BV and CVV with AV, and the second combination was significantly more often observed in the study group (n = 9; 7%) than among controls (n = 2; 1%); (p <0.01). BV was a leading infection among mono-infections in the study group. The patients in the study group were 3 times more likely to have BV (n = 13; 11%) than the patients in the control group (n = 7; 4%); (p <0.05). The detection rates of other mono-infections (AV, CVV, CI, gonorrhea, an infection caused by M.genitalium) did not differ significantly between the groups. The proportion of women with mono-infections in the study group (19%; n = 22) did not differ significantly from that in the control group and (11%; n = 20); (p = 0.06).

A comparison of cervical-vaginal microbiota examined by a real-time multiplex PCR showed a relatively greater diversity of species colonizing the reproductive tract during the development of threatened preterm birth. In our study, among the vaginal conditionally pathogenic microorganisms, the mean number of families (species) per pregnant woman the study group (4.3 ± 2.1) was significantly greater than in the control group (3.5 ± 1.6); (p <0.001).
As can be seen in table 2, the frequency of isolation of the majority of conditionally pathogenic microorganisms was significantly higher in the study group, except A.vaginae and Streptococcus spp.
Lactobacillus spp. were detected in 100% of the samples obtained from 300 pregnant women. The development of preterm labor was accompanied by a decrease in vaginal colonization by Lactobacillus spp. compared with the control group (7.38 ± 1.27 and 8.24 ± 0.67 GE/ml, respectively; p <0.001). Although the mean DNA level of Lactobacillus spp. was within the normal range in both groups, levels of Lactobacillus spp. in the vagina below 7 lg GE/ml were significantly more frequently detected in the patients of the study group (n = 26; 22%) than in the control group (n = 4; 2%) (p <0.001). Vaginal concentrations of Lactobacillus spp. below 7 lg GE/ml were associated with a 12-fold increase in the risk of threatened preterm birth (OR 12.58; 95% CI 4.26–37.12; p <0.001). Fluctuations of the vaginal DNA concentration of Lactobacillus spp. in the study group (from 1.09 lg to 8.85 lg GE/ml) were slightly lower than in the control group (from 3.00 lg GE/ml to 9.64 lg GE/ml). The DNA concentrations of G.vaginalis and A.vaginae in samples obtained from women with threatened preterm labor (5.50 ± 2.07 GE/ml and 5.64 ± 2.12 GE/ml) (Table 3) were significantly higher than that in women with normal pregnancy (4.77 ± 1.97 GE/ml and 4.37 ± 2.00 GE/ml) (p <0.05 and p <0.01 for DNA concentrations of G.vaginalis and A.vaginae). There was a strong positive correlation between BV-associated bacteria (G.vaginalis and A.vaginae) (rs = 0.86, p <0.001), and between G.vaginalis and M.hominis (rs = 0.71, p <0.001).
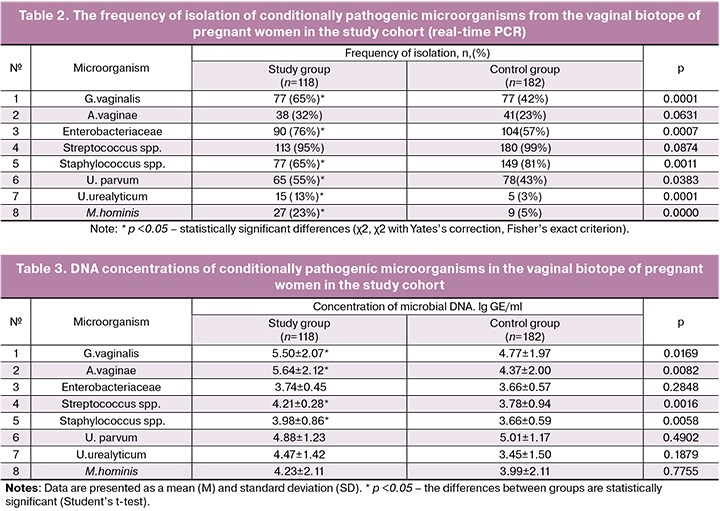
Patients in the study group had increased vaginal colonization by conditional pathogens of AV namely Staphylococcus spp. and Streptococcus spp. compared with the control group (p <0.01 and p <0.01, respectively).
Discussion
As is known, the prolongation of pregnancy closer to a term delivery is provided by a complex of various anatomical, biochemical, immunological, neuroendocrine, metabolic rearrangements in the maternal organism. Recent evidence suggests a fundamental role of microbial communities and individual microorganisms colonizing the intestinal, oral, vaginal, placental and other mother biotopes in maintaining homeostasis in the mother-placenta-fetus system [21, 22]. However, the significance of the quantitative and qualitative composition of cervical-vaginal microbiota in ensuring the physiological gestation and in the pathogenesis of preterm birth is not fully understood. Previous studies have suggested that the imbalance of the microflora of the birth canal may be associated with late obstetric complications and affect the biotope composition in the newborn [21]. In this regard, it is important to timely diagnose cervical-vaginal infections and administer, if necessary, etiotropic therapy, despite the results of a meta-analysis that showed no better obstetric outcomes after the correction of vaginal microflora disorders occurring in the second half of pregnancy [15]. According to R.F. Lamont [8], the conclusions of this meta-analysis regarding antibiotic therapy to prevent preterm labor cannot be considered completely objective and definitive, since antibacterial treatment during pregnancy can be effective if we identify etiologically significant pathogens triggering premature labor, determine their antibiotic sensitivity, and use antibiotics in the early stages of exposure to infection before the formation of irreversible bacterial-induced inflammatory cascades [8].
Our results suggest a higher prevalence of the total number of urogenital infections, BV, AV, CI among women with threatened preterm labor, which indirectly indicates the role of obligate and opportunistic vaginal microorganisms in the development of preterm birth. However, this cross-sectional study cannot provide definite information about cause-and-effect relationships between the imbalance of cervical-vaginal microbiota and the onset of preterm labor. According to the results of the screening, women with threatened preterm birth were more than twice likely to have BV compared with pregnant women who gave term birth, which does not contradict the previously described effect of BV on gestation associated with a 2-fold increase in the risk of preterm birth; when BV is diagnosed before 16 weeks and before 20 weeks, the risk increases 7-fold and 4-fold, respectively [23]. However, the inflammatory reaction mediated by inflammatory mediators accompanying AV is believed to be more consistent with the induction of preterm labor, chorioamnionitis, and rupture of membranes than BV [24]. Indeed, according to our results, AV was 4 times more often associated with threatened preterm birth.
The results of studies studying the relationship of CVV and preterm labor are mixed. Early studies reported that Candida colonization is not associated with low birth weight or preterm delivery [25]. At the same time, more recent studies, by contrast, indicate the role of CVV in the induction of preterm labor [26, 27], especially in the second half of pregnancy [28]. Our data suggest that despite the relatively high frequency of CVV occurrence in the study group, the differences between the groups were not statistically significant. At the same time, marginal p values may indicate a tendency for an increase in the incidence of CVV among pregnant women with threatened preterm birth.
As for STI pathogens, three women in the control group had an infection caused by M. genitalium; currently, there is no evidence for its role in the development of preterm labor [29]. Differences in the detection rates of STI pathogens between women with full-term and preterm birth were not significant, but C. trachomatis and N. gonorrhea were found more often among women with threatened preterm birth compared with the controls; previously these infections in pregnancy were found important in predicting pregnancy outcomes [30]. Chlamydia has been shown to be associated with a 4-fold greater risk of early preterm labor and a 2.5-fold greater risk of delivery before 35 weeks’ gestation [31], although a meta-analysis suggests insufficient evidence to recommend screening for C.trachomatis in pregnancy to improve obstetric outcomes [14]. Although the cross-sectional design of this study did not allow an unequivocal conclusion about the etiological significance of BV and AV in the development of threatened preterm labor, the direction of the causal relationship between CI and threatened preterm labor is obvious, which allows a conclusion about the involvement of C.trachomatis in the induction of preterm labor. Previous studies have reported that 30% of women with vaginitis have mixed infections due to the simultaneous presence of several vaginal pathogens [32]. Our findings showed that among the study participants with threatened preterm labor mixed infections of the reproductive tract were diagnosed 3.5 times more often than in controls. Probably, the induction of preterm labor can be a consequence of the negative effect of the pathological microbial community formed by mixed infections, the pathogenic potential of which, of course, exceeds that of mono-infections. Of particular interest are our findings that the groups were comparable in the number of pregnant women with mono-infections of the reproductive system; however, intergroup differences were observed only in the percentage of pregnant women with BV mono-infection, although, as indicated earlier, the samples differed in the prevalence rates of BV, AV, and CI. Perhaps the realization of the pathogenic properties of vaginal microorganisms becomes more clinically significant for the course of pregnancy in the settings of microbial consortia with higher species diversity.
Indeed, in our study, the mean number of species (families) - associates isolated from the vaginal biotope of pregnant women with threatened preterm births was greater than that in women with normal pregnancy. Our data are consistent with the findings of [13] that the normal course of pregnancy is accompanied by the depletion of vaginal species diversity, which is probably associated with a phylogenetically formed protective mechanism aimed at minimizing the potential risks associated with conditionally pathogenic microorganisms. Strong positive correlations found in our study between the concentrations of the BV-associated bacteria G.vaginalis and A.vaginae are consistent with the concepts of the mechanisms of BV development through the formation of microbial biofilms comprised of these microorganisms [33]. Besides, positive correlations between vaginal contents of G. vaginalis and M. hominis may imply that M. hominis are species associated with BV, rather than an independent bacterial pathogen initiating premature birth, which corresponds to the literature data [34]. The drop in the vaginal level of Lactobacillus spp. below 7 lg GE/ml was associated with a multiple increase in the risk of premature birth, which can be explained by the protective role of lactoflora in maintaining the gestational process. Decreased concentration of Lactobacillus spp. in the vaginal biotope is the key pathogenic event in the development of BV and AV [35]. The literature is lacking data regarding the relationship of the degree of colonization of the reproductive tract by Lactobacillus spp. in the second half of pregnancy and the risk of preterm birth; but it was previously shown that the absence of Lactobacillus spp. in the vagina in the first trimester correlates with a 2.4-fold increase in the risk of premature birth [12]. Insufficient effectiveness of the currently existing screening for infectious diseases of the reproductive tract among pregnant women is evidenced by the fact that all women, including those with endogenous (opportunistic) urogenital infections and STIs, were followed at the antenatal clinic and before hospitalization underwent a full clinical and laboratory examination in accordance with the order number 572n with no STIs diagnosed in any case.
Conclusion
Screening for infections among pregnant women with threatened preterm labor and those with the normal course of the second half of gestation showed that the former had a higher prevalence of infections, including mixed infections of the lower female genital tract. Our findings suggest that the detection of chlamydial infection, bacterial vaginosis, and aerobic (non-specific) vaginitis in pregnant women in the second half of gestation is associated with an increased risk of threatened preterm birth. The design of the study does not allow us to establish whether the aforementioned cervical-vaginal infections cause threatened preterm labor or this obstetric complication results from changes in immune homeostasis in pregnant women. Nevertheless, logic dictates that the decrease in the level of protective vaginal Lactobacillus spp. and increasing concentrations of conditionally pathogenic microorganisms in the second half of gestation facilitate the migration of potential pathogens of chorioamnionitis and intrauterine infections to the upper female genital tract, which, combined with changes in the local immunological environment, can initiate the onset of labor. Conversely, the physiological course of the second half of gestation is characterized by a decrease in the species diversity and the number of commensal microorganisms along with an increase in the level of Lactobacillus spp. These features of the transformation of cervical-vaginal microbiota during the physiological course of the second half of gestation and threatened preterm labor can serve as a rationale to recommend routine screening of pregnant women for infections of reproductive organs using molecular-biological diagnostic methods (PCR), which allow a single-step detection of Lactobacillus spp. and major pathogen concentrations. Etiotropic therapy of the detected cervical-vaginal biotope disorders, even in the second half of gestation may reduce the likelihood of developing preterm labor and postpartum maternal infectious-inflammatory complications and neonatal infections, which, however, requires additional research. The implementation of these measures will lower the risk of bacterial-induced preterm birth and may reduce perinatal and infant morbidity and mortality.
References
1. Goldenberg R.L., Culhane J.F., Lams J.D., Romero R. Epidemiology and causes of preterm birth. Lancet. 2008; 371(9606): 75-84. doi: 10.1016/S0140-6736(08)60074-4.
2. Информационный бюллетень ВОЗ №363, ноябрь 2017. Available at: http://www.who.int/mediacentre/factsheets/fs363/ru/ [Organization WH. Preterm birth: Fact sheet N636 Updated, November 2017. Available from: http://www.who.int/mediacentre/factsheets/fs363/en/.].
3. Howson C.P., Kinney M.V., McDougall L., Lawn J.E. Born toon soon: Preterm birth matters. Reprod. Health. 2013; 10(Suppl. 1): S1. doi:10.1186/1742-4755-10-S1-S1.
4. Liu L., Oza S., Hogan D., Chu Y., Perin J., Zhu J., et al. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016; 388(10063): 3027-35. doi: 10.1016/S0140-6736(16)31593-8.
5. Cunningham F.G., Leveno K.J., Bloom S.L., Hauth J.C., Rouse D.J., Spong C.Y., eds. Williams obstetrics. New York: McGraw-Hill Companies; 2010.
6. Howson C.P., Kinney M., Lawn J.E., eds. Born too soon: the global action report on preterm birth. Recommended citation: March of Dimes, PMNCH, Save the Children, WHO. Geneva: World Health Organization; 2012.
7. Gravett M.G., Rubens C.E., Nunes T.M., the GAPPS Review Group. Global report on preterm birth and stillbirth (2 of 7): discovery science. BMC Pregnancy and Childbirth. 2010;10(Suppl 1):S2. doi:10.1186/1471-2393-10-S1-S2.
8. Lamont R.F. Advances in the prevention of infection-related preterm birth. Front. Immunol. 2015; 6: 566. doi:10.3389/fimmu.2015.00566.
9. Козлов П.В., Иванников Н.Ю., Кузнецов П.А., Богаева И.И. Эпидемиология, этиология и патогенез поздних преждевременных родов. Акушерство, гинекология и репродукция. 2015;9(1):68-76. doi: 10.17749/2313-7347.2015.9.1.068-076. [Kozlov P.V., Ivannikov N.Yu., Kuznetsov P.A., Bogaeva I.I. Epidemiology, etiology and pathogenesis of late premature births. Obstetrics, Gynecology and Reproduction/Akusherstvo, ginekologiya i reproduktsiya. 2015; 9(1): 68-76.].
10. Romero R., Dey S.K., Fisher S.J. Preterm labor: one syndrome, many causes. Science. 2014; 345(6198): 760-5. doi:10.1126/science.1251816.
11. Adams Waldorf K.M., McAdams R.M. Influence of infection during pregnancy on fetal development. Reproduction. 2013; 146(5): R151-62. doi:10.1530/REP-13-0232.
12. Donders G.G., Van Calsteren K., Bellen G., Reybrouck R., Van den Bosch T., Riphagen I., Van Lierde S. Predictive value for preterm birth of abnormal vaginal flora, bacterial vaginosis and aerobic vaginitis during the first trimester of pregnancy. BJOG. 2009; 116(10): 1315-24.
13. Romero R., Hassan S.S., Gajer P., Tarca A.L., Fadrosh D.W., Bieda J. et al. The vaginal microbiota of pregnant women who subsequently have spontaneous preterm labor and delivery and those with a normal delivery at term. Microbiome. 2014; 2: 18. doi:10.1186/2049-2618-2-18.
14. Low N., Redmond S., Uusküla A., van Bergen J., Ward H., Andersen B., Götz H. Screening for genital chlamydia infection. Cochrane Database Syst. Rev. 2016; (9): CD010866. doi: 10,1002 / 14651858.CD010866.pub2
15. Brocklehurst P., Gordon A., Heatley E., Milan S.J. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst. Rev. 2013; (1): CD000262. doi: 10.1002/14651858.CD000262.pub4.
16. Amsel R., Totten P.A., Spiegel C.A., Chen K.C., Eschenbach D., Holmes K.K. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am. J. Med. 1983; 74(1): 14-22. doi: 10.1016/0002-9343(83)91137-3.
17. Румянцева Т.А., Гущин А.Е., Дондерс Г. Международная валидация набора реагентов для диагностики бактериального вагиноза. В кн.: Молекулярная диагностика-2014. Сборник трудов VIII Всероссийской научно-практической конференции с международным участием. М.; 2014; т.1: 153. [Rumyanceva T.A., Gushchin A.E., Donders G. International validation of a reagent kit for the diagnosis of bacterial vaginosis. Proceedings Digest of «Molecular Diagnostics-2014» VIIIth All-Russia Research and Practical Conference with international participation. M., 2014;1:153.]
18. Rumyantseva T.A., Bellen G., Savochkina Y.A., Guschin A.E., Donders G.G. Diagnosis of aerobic vaginitis by quantitative real-time PCR. Arch. Gynecol. Obstet. 2016; 294(1): 109-14. doi:10.1007/s00404-015-4007-4.
19. Румянцева Т.А., Савочкина Ю.А., Долгова Т.И., Зайцева С.В., Кахерская М.А., Кудрявцева Л.В., Гущин А.Е. Диагностика вульвовагинального кандидоза: сопоставление информативности клинических данных и результатов лабораторных исследований. Акушерство и гинекология. 2015; 3: 55-61. [Rumyantseva T.A., Savochkina Yu.A., Dolgova T.V., Zaitseva S.V., Kakherskaya M.A., Kudryavtseva L.V., Gushchin A.E. Diagnosis of vuvlovaginal candidiasis: Comparison of the informative value of clinical and laboratory findings. Obstetrics and Gynecology/Akusherstvo i Ginekologiya. 2015; 3: 55-61.].
20. Румянцева Т.А., Варламова А.В., Гущин А.Е., Безруков В.М. Сравнение тестов для количественной оценки Ureaplasma parvum, Ureaplasma urealyticum, Mycoplasma hominis: «Mycoplasma Duo», «Уреаплазма Микротест», «Микоплазма Микротест» и «Флороценоз-Микоплазмы». Клиническая лабораторная диагностика. 2014; 8: 52-7. [ Rumyantseva T.A., Varlamova A.V., Guschin A.E., Bezrukov V.M. Comparison of various kits for the detection of Ureaplasma parvum, Ureaplasma urealyticum, Mycoplasma hominis: «Mycoplasma Duo», «Ureaplasma microtest», «Mycoplasma microtest», «Florocenosis-Mycoplasmas». Clinical Laboratory Diagnostics/ Klinicheskaya Laboratornaya Diagnostika. 2014; 8: 52-57.].
21. Prince A.L., Chu D.M., Seferovic M.D., Antony K.M., Ma J., Aagaard K.M. The Perinatal microbiome and pregnancy: moving beyond the vaginal microbiome. Cold Spring Harb. Perspect. Med. 2015; 5(6): pii: a023051. doi:10.1101/cshperspect.a023051.
22. DiGiulio D.B., Callahan B.J., McMurdie P.J., Costello E.K., Lyell D.J., Robaczewska A. et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc. Natl. Acad. Sci. USA. 2015; 112(35): 11060-5. doi:10.1073/pnas.1502875112.
23. Leitich H., Bodner-Adler B., Brunbauer M., Kaider A., Egarter C., Husslein P. Bacterial vaginosis as a risk factor for preterm delivery: a meta-analysis. Am. J. Obstet. Gynecol. 2003; 189(1): 139-47. doi:10.1067/mob.2003.339.
24. Donders G., Bellen G., Rezeberga D. Aerobic vaginitis in pregnancy. BJOG. 2011; 118(10): 1163-70. doi:10.1111/j.1471-0528.2011.03020.x.
25. Cotch M.F., Hillier S.L., Gibbs R.S., Eschenbach D.A. Epidemiology and outcomes associated with moderate to heavy candida colonization during pregnancy. Vaginal Infections and Prematurity Study Group. Am. J. Obstet. Gynecol. 1998;178(2): 374-80. doi: 10.1016/S0002-9378(98)80028-8.
26. Roberts C.L., Algert C.S., Richard K.L., Morris J.M. Treatment of vaginal candidiasis for prevention of preterm birth: a systematic review and meta-analysis. Syst. Rev. 2015; 4: 31. doi: 10.1186/s13643-015-0018-2.
27. Доброхотова Ю.Э., Боровкова Е.И., Бондаренко К.Р. Кандидозный вульвовагинит: состояние изученности проблемы. Российский вестник акушера-гинеколога. 2017; 17(3): 108-11. [Dobrokhotova Yu.E., Borovkova E.I., Bondarenko K.R. Vulvovaginal candidiasis: The state of knowledge of the problem. Russian Bulletin of Obstetrician-Gynecologist / Rossiiskii Vestnik Akushera-Ginek ologa. 2017; 17 (3):108-111].
28. Holzer I., Farr A., Kiss H., Hagmann M., Petricevic L. The colonization with Candida species is more harmful in the second trimester of pregnancy. Arch. Gynecol. Obstet. 2017; 295(4): 891-5. doi:10.1007/s00404-017-4331-y.
29. Lis R., Rowhani-Rahbar A., Manhart L.E. Mycoplasma genitalium infection and female reproductive tract disease: A meta-analysis. Clin. Infect. Dis. 2015; 61(3): 418-26. doi: 10.1093/cid/civ312.
30. Liu B., Roberts C.L., Clarke M., Jorm L., Hunt J., Ward J. Chlamydia and gonorrhoea infections and the risk of adverse obstetric outcomes: a retrospective cohort study. Sex. Transm. Infect. 2013; 89(8): 672-8. doi: 10.1136/sextrans-2013-051118.
31. Rours G.I., Duijts L., Moll H.A., Arends L.R., de Groot R., Jaddoe V.W. et al. Chlamydia trachomatis infection during pregnancy associated with preterm delivery: a population-based prospective cohort study. Eur. J. Epidemiol. 2011; 26(6): 493-502. doi:10.1007/s10654-011-9586-1.
32. Sobel J.D., Subramanian C., Foxman B., Fairfax M., Gygax S.E. Mixed vaginitis-more than coinfection and with therapeutic implications. Curr. Infect. Dis. Rep. 2013; 15(2): 104-8. doi: 10.1007/s11908-013-0325-5.
33. Swidsinski A., Mendling W., Loening-Baucke V., Ladhoff A., Swidsinski S., Hale L.P., Lochs H. Adherent biofilms in bacterial vaginosis. Obstet. Gynecol. 2005; 106(5, Pt 1): 1013-23. doi: 10.1097/01.AOG.0000183594.45524.d2.
34. Taylor-Robinson D. Mollicutes in vaginal microbiology: Mycoplasma hominis, Ureaplasma urealyticum, Ureaplasma parvum and Mycoplasma genitalium. Res. Microbiol. 2017; 168(9-10): 875-81. doi: 10.1016/j.resmic.2017.02.009.
35. Бондаренко К.Р., Озолиня Л.А., Бондаренко В.М., Шпирко В.О. Особенности влагалищной микроэкосистемы в период гестации (обзор литературы). Вестник Российского государственного медицинского университета. 2014; 4:6-11. [Bondarenko K.R, Ozolinya L.A., Bondarenko V.M., Shpirko V.O. Features of Vaginal Microecosystem in Gestational Period: a Literature Review. Bulletin of Russian State Medical University/Vestnik Rossijskogo gosudarstvennogo medicinskogo universiteta. 2014; 4:6-11.].
Received 26.03.2018
Accepted 20.04.2018
About the Authors
Dobrokhotova, Yuliya E., Dr.Med.Sci., Professor, Head of the Department of Obstetrics and Gynecology, Faculty of Medicine, N.I. Pirogov RNRMU.Address: 117997, Russia, Moscow, Ostrovityanov str. 1. Tel.: 8 (495) 536-92-70. E-mail: pr.dobrohotova@mail.ru.
ORCID ID https://orcid.org/0000-0003-2786-6181. SPIN-code: 2925-9948. AuthorID: 312767
Bondarenko, Karina R., Ph.D., Teaching Assistant at the Department of Obstetrics and Gynecology, Faculty of Medicine, N.I. Pirogov RNRMU.
Address: 117997, Russia, Moscow, Ostrovityanov str. 1. Tel.: 8 (495) 536-92-70. E-mail: karinabond@mail.ru.
ORCID ID https://orcid.org/0000-0002-6984-6763. SPIN-code: 9976-0735. AuthorID: 798660
Gushchin, Aleksandr E., Ph.D. (bio.sci.), Head of the Laboratory for Molecular Diagnostics and Epidemiology of Infections of Reproductive Organs,
CRI of Epidemiology of Rospotrebnadzor. Address: 111123, Russia, Moscow, Novogireevskaya str. 3a. Tel.: 8 (495) 974-96-46, add. 1122, 8 (916) 144-05-56.
E-mail: aguschin1965@pcr.ru. SPIN-code: 3496-6893. AuthorID: 666674
Rumyantseva, Tat’yana A., Researcher at the Laboratory of Molecular Diagnostics and Epidemiology of Infections of Reproductive Organs; Obstetrician-gynecologist
at the Clinical Diagnostics and Research Center, CRI of Epidemiology of Rospotrebnadzor.
Address: 111123, Russia, Moscow, Novogireevskaya str. 3a. Tel.: 8 (495) 974-96-46, add. 1313, 8 (916) 154-70-67. E-mail: Ivanovatatiana86@yandex.ru.
SPIN-code: 4037-5114, AuthorID: 660075
Kuznetsov, Pavel A., Ph.D., Associate Professor at the Department of Obstetrics and Gynecology, Faculty of Medicine, N.I. Pirogov RNRMU.
Address: 117997, Russia, Moscow, Ostrovityanov str. 1. Tel.: 8 (495) 613-45-09. E-mail: poohsmith@mail.ru. ORCID ID https://orcid.org/0000-0003-2492-3910.
SPIN-code: 6683-4300. AuthorID: 661796
Dzhokhadze, Lela S., Ph.D., Teaching Assistant at the Department of Obstetrics and Gynecology, Faculty of Medicine, N.I. Pirogov RNRMU.
Address: 117997, Russia, Moscow, Ostrovityanov str. 1. Tel.: 8 (495) 613-45-09. E-mail: anton-b1@mail.ru. SPIN-code: 3482-8630. AuthorID: 802877
Makhova, Tamara I., Junior Researcher at the Laboratory for Molecular Diagnostics and Epidemiology of Infections of Reproductive Organs, CRI of Epidemiology
of Rospotrebnadzor. Address: 111123, Russia, Moscow, Novogireevskaya str. 3a. Tel.: 8 (495) 974-96-46, add. 1108, 8 (905) 547-12-10. E-mail: tamara.dolgova89@gmail.com. SPIN-code: 2669-2050. AuthorID: 929175
For citation: Dobrokhotova Yu.E., Bondarenko K.R., Gushchin A.E., Rumyantseva T.A., Dolgova T.V., Kuznetsov P.A., Dzhokhadze L.S. The results of the examination of cervical-vaginal microbiota in pregnant women with threatened preterm birth using a real-time polymerase chain reaction. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2018; (11): 50-9. (in Russian):
https://dx.doi.org/10.18565/aig.2018.11.50-59



