Retrospective analysis of telemedicine consultations conducted in pregnant and puerperal women with novel coronavirus infection COVID‑19
Objective: To evaluate the incidence of prescribing antibacterial agents (ABA) and the results of microbiological examination in pregnant and puerperal women with COVID-19 by analyzing the electronic records of telemedicine consultations (TMC) in patients from different regions of the Russian Federation.Shabanova N.E., Pyregov A.V., Nikolaeva A.V., Bembeeva B.O., Skorobogaty A.V., Chubarov V.V., Denisov P.A., Klimov V.A., Pekarev O.G., Polibin R.V., Priputnevich T.V.
Materials and methods: A retrospective analysis was done on 2500 pregnant and puerperal women from the database of patients with COVID-19 who received TMC between January and July 2021. The analysis included age, gestational age, medical history, chest computed tomography (chest CT), respiratory support, laboratory test results, frequency of microbiological studies, and ABA administration.
Results: This analysis revealed that 78.8%, 49.6%, and 55.8% of pregnant and puerperal COVID-19 patients were prescribed antibiotics, antifungals, and glucocorticoids, respectively. In microbiological testing, Candida albicans was the most frequently isolated microorganism (30.4 %). The most common gram-positive microorganisms were Staphylococcus spp. (18.8%), and Streptococcus spp. (15.9%), and Acinetobacter spp. among the gram-negative (14,5%). Acinetobacter baumannii accounted for 10.1% of the isolates. Klebsiella pneumoniae accounted for 11.6% of the cases.
Conclusion: The retrospective analysis showed that although microbiological testing was important, it was performed in only 4% of the cases. ABA were used in 78.8% of the cases, and broad-spectrum and reserve drugs were the most common choice. Antifungals were used in 49.6% of patients. Prescribing occurred immediately upon admission to the hospital without confirmation of a bacterial or fungal infection.
Authors' contributions: Shabanova N.E. – conducting TMC, analysis of relevant literature, manuscript drafting and editing; Pyregov A.V., Nikolaeva A.V., Klimov V.A., Pekarev O.G. – conducting TMC, literature review; Bembeeva B.O.,
Skorobogaty A.V. – data analysis, manuscript drafting; Chubarov V.V. – manuscript drafting; Denisov P.A. – statistical and data analysis; Polibin R.V. – data analysis; Priputnevich T.V. – conception and design of the study, general supervision.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Shabanova N.E., Pyregov A.V., Nikolaeva A.V., Bembeeva B.O., Skorobogaty A.V., Chubarov V.V., Denisov P.A., Klimov V.A., Pekarev O.G., Polibin R.V., Priputnevich T.V.
Retrospective analysis of telemedicine consultations conducted in pregnant and puerperal women with novel coronavirus infection COVID-19.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (4): 50-57 (in Russian)
https://dx.doi.org/10.18565/aig.2022.317
Keywords
In December 2019, the first case of severe acute respiratory syndrome caused by a new coronavirus, SARS-CoV-2, was reported in Wuhan City, Hubei Province, China [1, 2]. The emergence of the COVID-19 pandemic resulted in severe socio-economic consequences and changes in social life and productive activities [3, 4].
The most vulnerable categories for the development of acute respiratory viral infections, influenza, and COVID-19 include not only the elderly but also pregnant women [5–7].
During pregnancy, physiological changes occur in the immune, cardiovascular, and respiratory systems, suggesting that pregnant women are more vulnerable to pathogenic infectious agents, the development of infection, and severe complications. Pneumonia in pregnant women is associated with a higher incidence of preterm and low-birth-weight deliveries and an increased incidence of caesarean sections [8, 9].
Mortality in patients with SARS-CoV-2 can be related, on the one hand, to the presence of severe chronic comorbidities and, on the other hand, to secondary bacterial infection with resistant bacterial strains [10, 11].
Evidence from early studies has shown that antibacterial agents (ABA) are recommended for patients with COVID-19 mainly because of suspected concomitant bacterial infections [11–13]. MacIntyre C.R. et al. found that the proportion of patients with COVID-19 with bacterial co-infection was lower than during previous influenza pandemics, and the empirical use of ABA in a larger proportion of patients diagnosed with COVID-19 remains an open question [14].
Thus, the difference between the prevalence of bacterial infection and prescription of ABA in patients with COVID-19 is associated with a high risk of bacterial resistance to ABA and requires microbiological monitoring in different categories of patients, including pregnant and postpartum women.
This study aimed to the incidence of prescribing ABA and the results of microbiological examination in pregnant and puerperal women with COVID-19 by analyzing the electronic records of telemedicine consultations in patients from different regions of the Russian Federation.
Materials and methods
Based on Russian Ministry of Health Order 171, March 16, 2020. "On the provisional order of organizing the work of medical organizations to implement measures for preventing and reducing the risks of spread of a new coronavirus infection," the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia organized the Federal Remote Consultation Centre for anesthesiology and critical care on the diagnosis and treatment of COVID-19 and pneumonia.
We retrospectively analyzed 2500 pregnant and puerperal women from a database of patients with confirmed COVID-19 in different regions of the Russian Federation who had TMC between January and March 2021.
The analyzed parameters included age, gestational age, medical history (history of gestational diabetes mellitus (GDM), pre-eclampsia, type 2 diabetes, and obesity (body mass index (BMI)≥30 kg/m2)), measurement of lesion size on chest CT, respiratory support (with and without mechanical ventilation (MV)), laboratory indicators of systemic inflammatory response (white blood cell count in the complete blood count, C-reactive protein (CRP) level, procalcitonin (PCT)), microbiological tests, prescription of ABA, antifungal agents, and glucocorticoids (GCs] ).
Chest CT is the main diagnostic radiologic examination for the detection of viral pneumonia, including that associated with COVID-19. A strict clinical indication and minimal radiation exposure are mandatory for radiotherapy in pregnant women. During pregnancy, chest CT is recommended for patients with severe and extremely severe disease; in all other cases, the decision is made by the medical committee (according to the guidelines "Organization of medical care for pregnant, puerperal women, and newborns with novel coronavirus infection COVID-19", version 3 (21.01.2021)).
The treatment of pregnant and postpartum women with confirmed COVID-19 included supportive pathogenetic and symptomatic therapy. Conservative therapy for comorbidities and complications was carried out in accordance with clinical guidelines and standards of care for a specific nosology (according to the guidelines "Organization of medical care for pregnant, puerperal women, and newborns with novel coronavirus infection COVID-19,” version 1–3).
Thirty-three pregnant and postpartum women who tested negative for SARS-CoV-2 by polymerase chain reaction (PCR) were excluded from the analysis.
Statistical analysis
Data were processed using Python-based software (Pandas, Numpy libraries). Statistical analysis and plotting were performed using Origin 2016 (OriginLab Corp.) and Microsoft Excel.
Results
A total of 2467 pregnant and puerperal women were analyzed. All patients tested positive for SARS-CoV-2 in a PCR test. The patients' ages ranged from 20 to 49 years, and the mean gestational age at admission was 31 weeks. Pregnant women included in the study were predominantly in the third trimester (53.4%), 29.3% in the second trimester, 7.2% in the first trimester, and 10.1% in the postpartum period (Table 1).
Given the changes that occur during the physiological course of pregnancy and the increased risk of preeclampsia and GDM, the presence of comorbidities in pregnant and postpartum women was assessed (Table 1).
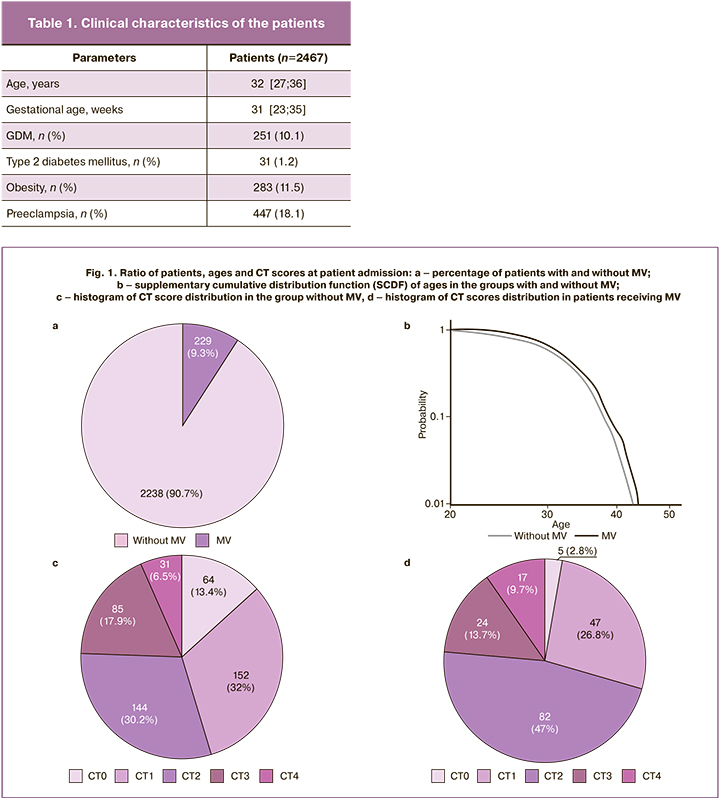
Chest CT on admission was performed in 19.3% of patients (n=476), including 229 patients (9.3%) on MV (Figure 1a), and chest CT was performed in 23.6% (n=54) of patients, due to the presence of clinical indications for chest CT (according to the guidelines "Organization of medical care for pregnant, puerperal women, and newborns with novel coronavirus infection COVID-19,” version 3 (21/01/2021)). When comparing the pattern of lung injury by chest CT in pregnant and puerperal women, distinctive features were noted: CT-1 occurred in 32% of cases without MV and in 26.8% undergoing MV; the number of women with CT-2 increased significantly from 30.2% to 47%, and CT-3 in 17.9% and 13.7%, respectively. The most severe CT-4 lung injury was more frequently reported with MV (9.7 %) than without (6.5 %). In 13.4% of the pregnant and postpartum women, no chest CT changes were detected, but they all received antibiotic therapy (Fig. 1).
There were statistically significant differences in age among patients with and without MV (without MV: mean=31.43 years, SE=0.12; with MV: mean=32.75 years, SE=0.35), p<0.05 was considered statistically significant) (Fig. 1b).
Previous studies have shown that obese patients (BMI≥30 kg/m2) are at risk of severe complications of COVID-19 [15]. In our study, 22.6% of pregnant and postpartum women with a BMI≥30 kg/m2 underwent MV, compared with 10.4% without MV. In addition, the incidence of GDM in overweight patients was higher (25.7%) than that in women without obesity (7.9%). Thus, being overweight in combination with GSD complicates the course of COVID-19, with the development of respiratory distress requiring MV.
Total blood count findings showed that a normal leukocyte count was the most common in pregnant and puerperal women, and leukocytosis was detected in 839 patients, corresponding to 34% of the total number of study participants. Elevation of CRP compared to the reference value was found in 71% of patients, which correlates with the severity of the disease course and inflammatory lung tissue infiltration. PCT levels were more frequently detected in patients on MV and were elevated in 26.6% of the cases. PCT was within the reference range in COVID-19 against the background of inflammatory lung damage of a viral nature and increased with the accession of bacterial infection, as well as correlated with the severity of the disease course.
ABA and GCs are the most frequently used conservative therapies. Among pregnant and postpartum women with COVID-19, 78.8% received ABA, 49.6% received antifungals and 55.8% received GCs (Table 2).
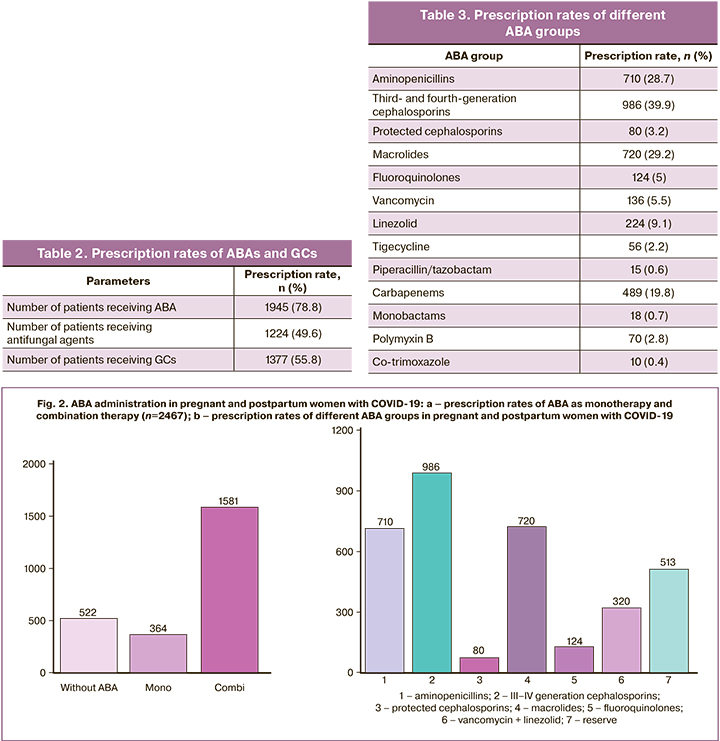
GCs and antifungals were administered to pregnant and postpartum women concurrently with ongoing antibiotic therapy. Immunosuppressive effects of GCs are associated with an increased risk of secondary bacterial and fungal infections, which may explain the detection of Candida spp. fungi in 30.4% of the protocols analyzed.
ABA monotherapy was administered in 18.7% (n=364) of the patients, the remaining patients received two or more ABA from different groups (Fig. 2a). Third- and fourth-generation cephalosporins were prescribed in 50.7% (n=986); aminopenicillins (inhibitor-protected aminopenicillins: amoxicillin/clavulanic acid, ampicillin/sulbactam), and macrolides (azithromycin) were used with equal prescription rates (37 and 36.5%, respectively). Fluoroquinolones (levofloxacin, moxifloxacin) were used in the postpartum period and protected cephalosporins (cefoperazone/sulbactam, cefepime/sulbactam, ceftazidime/avibactam, ceftolosan/tazobactam) were used in 6.4 and 4.1% of cases, respectively (Fig. 2b).
Reserve group ABAs (carbapenems, polymyxin B, tigecycline, piperacillin/tazobactam, monobactams, and co-trimoxazole) in combination with drugs affecting gram-positive microflora (vancomycin and linezolid) were used as second- or third-line therapies in 42.8% of patients. Among the reserve group ABAs, carbapenems (meropenem and imipenem/cilastatin) were used most frequently (n=489) (Table 3).
Despite the widespread use of ABAs, microbiological testing in pregnant and postpartum women with COVID-19 was registered in the TMC protocols in only 4% (n=98) of all patients; of these, 10 (5.3%) had severe to extremely severe disease. In some cases, the results of microbiological tests were not recorded in the TMC protocols. Samples for microbiological examination were collected from different sites, including sputum (34.1%), central venous catheter (CVC) (11.4%), peripheral venous blood (3.8%), tracheobronchial aspirate (3%), and other locations (47.7%) (abdominal aspirate, postoperative wound contents, vaginal and cervical discharge, etc.) (Fig. 3a).
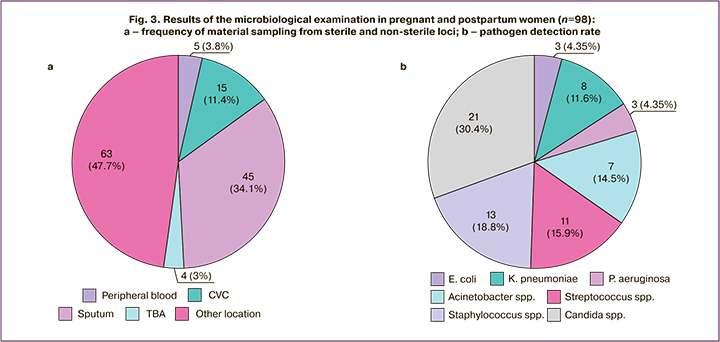
According to the results of microbiological studies presented in the TMC protocols, the most frequently isolated microorganism was Candida albicans (30.4%). Among gram-positive microorganisms, Staphylococcus spp. (18.8%), and Streptococcus spp. (15.9%). Among gram-negative microorganisms, it was Acinetobacter spp. (4.5%), of which A. baumannii accounted for 10.1% and K. pneumoniae accounted for 11.6%, respectively (Fig. 3b).
Discussion
Antibiotic use during the pandemic reached catastrophic proportions; antibiotics were prescribed in 90% of cases, although only 10% of patients who developed bacterial complications actually required them. Antibiotics were given to all patients with COVID-19, starting with mild disease on an outpatient basis in all febrile patients with mono- or combination therapy. As a result, the patients received ABA at every stage, including outpatients, on admission to a hospital in the usual specialist departments and in the intensive care unit, irrespective of the indication.
Our findings on the use of ABA in pregnant and postpartum women with COVID-19 are consistent with those of a meta-analysis of 154 studies. A total of 30 623 patients (general population) were included in the analysis; of these, 74.6% received antibiotic therapy (95% CI 68.3–80.0%) [16], and our data were 78.8%.
According to our study, the choice of antibiotic therapy showed prioritization of the three main groups of drugs, including third- and fourth-generation cephalosporins, macrolides, and protected aminopenicillin inhibitors. This differs from the results obtained in the study by Karoly N.A. et al., in which macrolides were most commonly prescribed, followed by fluoroquinolones (only in the postpartum period) and third-generation cephalosporins, but concurs with the use of cephalosporins and macrolides as the leading antibiotic therapy [17].
Evidence from a systematic review that analyzed case series studies comprising 104 pregnant women with COVID-19 suggests a slightly different approach to the prescription and selection of antibiotic therapy. Antibiotics, antivirals, and hydroxychloroquine were administered as treatments. ABAs were administered in 25.9% of cases, compared to 78.8% of patients receiving ABAs according to our results. Third-generation cephalosporins and ceftriaxone were the most frequently used, which was consistent with our findings, followed by vancomycin and azithromycin [18].
A multicenter, prospective, cohort study of the microbiological diagnosis of infections in patients with COVID-19 was conducted at the UK Viral Research Center. Microbiological diagnosis was performed in 8,649 (17.7%) of the 48,902 patients who were admitted to hospital, and only 1,107 patients (12.7%, of the total number of studies) had clinically significant culture results [19].
At the beginning of the pandemic, given the lack of knowledge about the course of the new COVID-19 infection, antibiotic therapy was administered immediately, even in the presence of mild CT-1 lesions, and in many patients, it was started simultaneously with antiviral therapy. Later on, the view changed, and since version 9 of the Interim guidelines "Prevention, diagnosis and treatment of new-onset coronavirus infection (COVID-19" 26.10.2022), antibiotic therapy is recommended for patients with obvious signs of recurrent bacterial infection (increase in PCT over 0.5 ng/mL, leukocytosis >10×109/l, occurrence of purulent sputum). At the same time, in the guidelines "Organization of Medical Care for Pregnant, Parturient, and Puerperal Women and Newborns during new coronavirus infection COVID-19 pandemic, version 5 (29.12. 2021), empirical antibiotics should be used only if a bacterial infection with characteristic symptoms (neutrophilic shift, lobular thickening on CT or X-ray, etc.) is suspected, which may explain the extensive use of antibiotics when a grade 1 chest CT lung lesion is obtained. The choice of ABA and its route of administration are based on the severity of the patient's condition, taking into account stratification, risk factors for polyresistant pathogens (presence of concomitant diseases, previous antibiotic use, etc.), and microbiological findings.
Conclusion
The findings of our study showed that the TMC protocols included microbiological data in only 4% of the cases, with ABA being used in 78.8% and the most common choice being broad-spectrum and reserve drugs. ADA was immediately administered on admission to the hospital without confirmation of bacterial infection. Changing antimicrobial therapy was most often empirical, which reduces its efficacy and increases therapy duration and hospital stay, contributing to more frequent ABA changes.
The overuse of ABA potentiates both the occurrence of side effects and the risk of multidrug-resistant strains, thus increasing the incidence of adverse clinical outcomes. Microbiological studies play an important role in the selection of a rational antimicrobial therapy and help curb the growth of resistance in bacteria and fungi.
These findings can help clinicians to provide better treatment and rehabilitation approaches, since the most important factor in preventing infectious diseases is the timely diagnosis and treatment of the most susceptible populations. Based on these findings, we believe it is necessary to develop and implement clear criteria for prescribing ABA for COVID-19, as well as to update the guidelines ("Organization of medical care for pregnant, parturient, puerperal women and newborns with novel coronavirus infection COVID-19", version 5 (29.12.2021)).
References
1. Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020; 382(18): 1708‑20. https://dx.doi.org/10.1056/NEJMoa2002032.
2. Никифоров В.В., Суранова Т.Г., Чернобровкина Т.Я., Янковская Я.Д., Бурова С.В. Новая коронавирусная инфекция (COVID‑19): клинико‑эпидемиологические аспекты. Архивъ внутренней медицины. 2020; 10(2): 87‑93. [Nikiforov V.V., Suranova T.G., Chernobrovkina T.Ya., Yankovskaya Ya.D., Burova S.V. New coronavirus infection (Covid‑19): clinical and epidemiological aspects. The Russian Archives of Internal Medicine. 2020; 10(2): 87‑93. (in Russian)]. https://dx.doi.org/10.20514/ 2226‑6704‑2020‑10‑2‑87‑93.
3. Брико Н.И., Каграманян И.Н., Никифоров В.В., Суранова Т.Г., Чернявская О.П., Полежаева Н.А. Пандемия COVID‑19. Меры борьбы с ее распространением в Российской Федерации. Эпидемиология и вакцинопрофилактика. 2020; 19(2): 4‑12. [Briko N.I., Kagramanyan I.N., Nikiforov V.V., Suranova T.G. Chernyavskaya O.P., Polezhaeva N.A. Pandemic COVID‑19. Prevention measures in the Russian Federation. Epidemiology and Vaccinal Prevention. 2020; 19(2): 4‑12. (in Russian)]. https://dx.doi.org/10.31631/2073‑3046‑2020‑19‑2‑4‑12.
4. Щелканов М.Ю., Колобухина Л.В., Бургасова О.А., Кружкова И.С., Малеев В.В. COVID‑19: этиология, клиника, лечение. Инфекция и иммунитет. 2020; 10(3): 421‑45. [Shchelkanov M.Yu., Kolobukhina L.V., Burgasova O.A., Kruzhkova I.S., Maleev V.V. COVID‑19: etiology, clinical picture, treatment. Russian Journal of Infection and Immunity. 2020; 10(3): 421‑45. (in Russian)]. https://dx.doi.org/10.15789/2220‑7619‑CEC‑1473.
5. Kister G.S. Morphology and mechanisms of prenatal and perinatal viral infections. EURO Rep. Stud. 1985; 93: 3‑16.
6. Skehel J.J., Wiley D.C. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu Rev. Biochem. 2000; 69: 531‑69. https://dx.doi.org/10.1146/annurev.biochem.69.1.531.
7. Li Z., Ren A., Liu J., Pei L., Zhang L., Guo Z. Maternal flu or fever, medication use, and neural tube defects a population‑based case‑control study in Northern China. Birth Defect Res. A Clin. Mol. Teatol. 2007; 79(4): 295‑300. https://dx.doi.org/10.1002/bdra.20342.
8. Wenling Y., Junchao Q., Xiao Z., Ouyang S. Pregnancy and COVID‑19: management and challenges. Rev. Inst. Med. Trop. de Sao Paulo. 2020; 62: e62. https://dx.doi.org/10.1590/S1678‑9946202062062.
9. Припутневич Т.В., Гордеев А.Б., Любасовская Л.А., Шабанова Н.Е. Новый коронавирус SARS‑CoV‑2 и беременность: обзор литературы. Акушерство и гинекология. 2020; 5: 6‑12. [Priputnevich T.V., Gordeev A.B., Lyubasovskaya L.A., Shabanova N.E. The novel coronavirus SARS‑CoV‑2 and pregnancy: literature review. Obstetrics and Gynecology. 2020; (5): 6‑12. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.5.6‑12.
10. Sharifipour E., Shams S., Esmkhani M., Khodadadi J., Fotouhi-Ardakani R., Koohpaei A. et al. Evaluation of bacterial co‑infections of the respiratory tract in COVID‑19 patients admitted to ICU. BMC Infect. Dis. 2020; 20(1): 646. https://dx.doi.org/10.1186/s12879‑020‑05374‑z.
11. Lansbury L., Lim B., Baskaran V., Lim W.S. Co‑infections in people with COVID‑19: a systematic review and meta‑analysis. J. Infect. 2020; 81: 266‑75. https://dx.doi.org/10.1016/j.jinf.2020.05.046.
12. Rawson T.M., Moore L.S.P., Zhu N., Ranganathan N., Skolimowska K., Gilchrist M. et al Clin. Infect. Dis. 2020; 71(9): 2459‑68. https://dx.doi.org/10.1093/cid/ciaa530.
13. Langford B.J., So M., Raybardhan S., Leung V., Westwood D., MacFadden D.R. et al. Bacterial co‑infection and secondary infection in patients with COVID‑19: a living rapid review and meta‑analysis. Clin. Microbiol. Infect. 2020; 26(12): 1622‑9. https://dx.doi.org/10.1016/j.cmi.2020.07.016.
14. MacIntyre C.R., Chughtai A.A., Barnes M., Ridda I., Seale H., Toms R. The role of pneumonia and secondary bacterial infection in fatal and serious outcomes of pandemic influenza a(H1N1)pdm09. BMC Infect. Dis. 2018; 18(1): 637. https://dx.doi.org/10.1186/s12879‑018‑3548‑0.
15. Zhou Y., Chi J., Lv W., Wang Y. Obesity and diabetes as high‑risk factors for severe coronavirus disease 2019 (Covid‑19). Diabetes Metab. Res. Rev. 2021; 37(2): e3377. https://dx.doi.org/10.1002/dmrr.3377.
16. Langford B.J., So M., Raybardhan S., Leung V., Soucy J.R., Westwood D. et al. Antibiotic prescribing in patients with COVID‑19: rapid review and meta‑analysis. Clin. Microbiol. Infect. 2021; 27(4): 520‑31. https://dx.doi.org/10.1016/j.cmi.2020.12.018.
17. Кароли Н.А., Апаркина А.В., Григорьева Е.В., Магдеева Н.А., Никитина Н.М., Смирнова Н.Д., Ребров А.П. Антибактериальная терапия пациентов с COVID‑19 на амбулаторном и стационарном этапах. Антибиотики и химиотерапия. 2022; 67(1‑2): 24‑31. [Karoli N.A., Aparkina A.V., Grigoryeva E.V., Magdeeva N.A., Nikitina N.M., Smirnova N.D., Rebrov A.P. Antibacterial Therapy of Patients With COVID‑19 During The Outpatient and Hospital Stages. Antibiotics and Chemotherapy. 2022; 67(1‑2): 24‑31. (in Russian)]. https://dx.doi.org/10.37489/0235‑2990‑2022‑67‑1‑2‑24‑31.
18. Abou Ghayda R., Li H., Lee K.H., Lee H.W., Hong S.H., Kwak M. et al. COVID‑19 and adverse pregnancy outcome: a systematic review of 104 cases. J. Clin. Med. 2020; 9(11): 3441. https://dx.doi.org/10.3390/jcm9113441.
19. Russell C.D., Fairfield C.J., Drake T.M., Turtle L., Seaton R.A., Wootton D.G.; ISARIC4C investigators. Co‑infections, secondary infections, and antimicrobial use in patients hospitalised with COVID‑19 during the first pandemic wave from the ISARIC WHO CCP‑UK study: a multicentre, prospective cohort study. Lancet Microbe. 2021; 2(8): e354‑65. https://dx.doi.org/10.1016/ S2666‑5247(21)00090‑2.
Received 02.02.2023
Accepted 31.03.2023
About the Authors
Natalia E. Shabanova, PhD, Associate Professor, Head of the Unit of Clinical Pharmacology, Institute of Microbiology, Antimicrobial Therapy and Epidemiology, AcademicianV.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(985)097-58-27, n_shabanova@oparina4.ru, https://orcid.org/0000-0001-6838-3616, 4, Oparin str., Moscow, 117997, Russia.
Alexey V. Pyregov, Dr. Med. Sci., Professor, Director of the Institute of Anesthesiology-Resuscitation and Transfusiology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, a_pyregov@oparina4.ru, 4, Oparina str., Moscow, 117997, Russia.
Anastasia V. Nikolaeva, PhD, Chief Physician, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, a_nikolaeva@oparina4.ru, 4, Oparina str., Moscow, 117997, Russia.
Bayr O. Bembeeva, bacteriologist at the Laboratory of Medical Microbiology, Institute of Microbiology, Antimicrobial Therapy and Epidemiology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, b_bembeeva@oparina4.ru,
https://orcid.org/0000-0003-1813-6718, 4, Oparina str., Moscow, 117997, Russia.
Alexey V. Skorobogaty, Junior Researcher at the Unit of Clinical Pharmacology, Institute of Microbiology, Antimicrobial Therapy and Epidemiology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, a_skorobogatiy@oparina4.ru,
https://orcid.org/0000-0003-2137-6421, 4, Oparina str., Moscow, 117997, Russia.
Valery V. Chubarov, Head of Epidemiological Surveillance Department, Institute of Microbiology, Antimicrobial Therapy and Epidemiology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, v_chubarov@oparina4.ru, 4, Oparina str., Moscow, 117997, Russia.
Pavel A. Denisov, Researcher at the Laboratory of Bioinformatic Analysis, Institute of Microbiology, Antimicrobial Therapy and Epidemiology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, pa_denisov@oparina4.ru,
https://orcid.org/0000-0003-1813-6718, 4, Oparina str., Moscow, 117997, Russia.
Vladimir A. Klimov, PhD, Head of the Service for Organization of Medical Care and Information Service, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, va_klimov@oparina4.ru, https://orcid.org/0000-0002-4699-7614,
4, Oparina str., Moscow, 117997, Russia.
Oleg G. Pekarev, Dr. Med. Sci., Professor, Deputy Director of the Institute of Obstetrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, o_pekarev@oparina4.ru, https://orcid.org/0000-0001-7122-6830, 4, Oparina str., Moscow, 117997, Russia.
Roman V. Polibin, PhD, Associate Professor, Associate Professor at the Department of Epidemiology and Evidence-Based Medicine, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia (Sechenov University), https://orcid.org/0000-0003-4146-4787, 8-2 Trubetskaya str. Moscow, 119991, Russia.
Tatiana V. Priputnevich, Dr. Med. Sci., Corresponding Member of the RAS, Director of the Institute of Microbiology, Antimicrobial Therapy and Epidemiology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(910)414-56-16, priput1@gmail.com, http://orcid.org/0000-0002-4126-9730, 4, Oparina str., Moscow, 117997, Russia.
Corresponding author: Natalia E. Shabanova, n_shabanova@oparina4.ru



