Novel coronavirus infection in the third trimester of pregnancy: perinatal and maternal outcomes
Objective: To investigate the characteristic features of the course of pregnancy, labor, and perinatal outcomes in women who had novel coronavirus disease 2019 (COVID-19) in the third trimester of pregnancy and gave birth after COVID-19. Materials and methods: The study group included 313 patients divided into subgroups 1 (n=90), 2 (n=154), and 3 (n=69) who had COVID-19 at 28–32, 32.1–36.6 and ≥37 weeks of gestation, respectively. The comparison group included 216 women who gave birth before the COVID-19 pandemic (2019). Histological examination of the placenta was performed in the study (n=87) and comparison (n=20) groups. Results: COVID-19 at 28–32 weeks increases the risk of fetal growth restriction (relative risk (RR)=5.6; 95% CI 2.4; 13.0; p<0.001), preterm birth (RR=2.7; 95% CI 1.2; 5.8; p=0.01), placental abruption (RR=5.2; 95% CI 1.6; 16.4; p=0.002), fetal distress (RR=4.9; 95% CI 1.7; 13.6; p=0.001), and emergency caesarean section (RR=4.0; 95% CI 1.8; 9.1; p=0.001). The newborns had significantly smaller chest circumferences and lower Apgar scores. The placentas showed marked signs of maternal and fetal vascular malperfusion. COVID-19 at 32.1–36.6 weeks gestation increased the risk of uteroplacental circulation disorders (UPCD) (RR=4.3; 95% CI 1.1; 16.1; p=0.02), preterm birth (RR=2.8; 95% CI 1.4; 5.6; p<0.001), fetal distress (RR=3.9; 95% CI 1.5; 10.3; p=0.003), and emergency caesarean section (RR=3.4; 95% CI 1.6; 7.3, p=0.001). Newborns had a significantly smaller chest circumference and lower Apgar scores. COVID-19 after 37 weeks increased the risk of UPCD (RR=8.0; 95% CI 2.0; 31.9, p=0.001). Vascular abnormalities were most pronounced in the placentas of patients in group 1. Conclusion: COVID-19 in the early and middle third trimesters is associated with the most adverse perinatal outcomes. Authors' contributions: Malgina G.B. – conception and design of the study, manuscript drafting and editing; Dyakova M.M., Grishkina A.A. – data collection and analysis, manuscript drafting; Bychkova S.V. – literature search of Russian and international studies in Russian and international databases, review of the relevant literature, manuscript drafting; Pepelyaeva N.A, Olkov S.S. – material collection; Melkozerova O.A., Bashmakova N.V. – manuscript editing. Conflicts of interest: The authors have no conflicts of interest to declare. Funding: There was no funding for this study. Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the Ural Research Institute of Maternity and Child Care (Ref. No. 12 dated 21.09.2021). Patient Consent for Publication: All patients provided informed consent for the publication of their data. Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator. For citation: Malgina G.B., Dyakova M.M., Bychkova S.V., Grishkina A.A., Melkozerova O.A., Bashmakova N.V., Pepelyaeva N.A., Olkov S.S. Novel coronavirus infection in the third trimester of pregnancy: perinatal and maternal outcomes. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (4): 58-66 (in Russian) https://dx.doi.org/10.18565/aig.2023.58Malgina G.B., Dyakova M.M., Bychkova S.V., Grishkina A.A., Melkozerova O.A., Bashmakova N.V., Pepelyaeva N.A., Olkov S.S.
Keywords
The third trimester of pregnancy is the time of completion of placental development, the beginning of its physiological involution, active growth and development of fetal organs, including the functional formation of its regulatory systems, reorganization of the mother's body to prepare for the upcoming birth, the formation of the birth dominant [1, 2].
Any adverse effects at this important stage of pregnancy may contribute to maladaptation and disturbance of physiological balance, inhibition, or, conversely, acceleration of the genetically programmed processes of maintaining the pregnancy, its premature termination, and the occurrence of maternal and fetal complications. The coronavirus disease (COVID-19) pandemic is now a global emergency. The high prevalence of a new coronavirus infection (COVID-19) and the regular emergence of new strains of the SARS-CoV-2 virus are of increasing interest to obstetricians because of the danger posed not only by the infection itself but also by its long-term consequences for the mother and fetus.
There is still much debate about whether COVID-19 causes pregnancy complications and adverse perinatal outcomes and whether gestational age at the time of maternal diagnosis of COVID-19 is associated with adverse perinatal outcomes.
Numerous studies have reported an increase in pregnancy complications in women after COVID-19 in the first [3, 4], second [5, 6], and third [7, 8] trimesters. At the same time, there are studies that show to the contrary, that SARS-CoV-2 does not cause any adverse effects in pregnancy [9, 10].
More knowledge is needed regarding the course of pregnancy and perinatal outcomes in women with COVID-19 in the third trimester of pregnancy.
This study aimed to investigate the characteristic features of the course of pregnancy, labor, and perinatal outcomes in women who had COVID-19 in the third trimester of pregnancy and who gave birth after COVID-19.
Materials and methods
This was a single-center retrospective cohort study [11] conducted in the COVID hospital of the Ural Research Institute of Maternity and Child Care, Ministry of Health of the Russian Federation. This study included 823 pregnant women with a confirmed diagnosis of COVID-19 during the first, second, and third waves of the pandemic. The patients were treated according to the guidelines for the organization of medical care for pregnant women, women in labor, women in labor, and newborns with a new coronavirus infection COVID-19 [12]. Of these, 414 were at > 28 weeks of gestation (trimester III) at the time of SARS-CoV-2 infection. The study group included 313 patients who gave birth after clinical recovery from COVID-19, with laboratory confirmation of the absence of viral genetic material in nasopharyngeal smears. The study group was divided into 3 subgroups according to gestational age at the time of infection with SARS-CoV-2: patients in subgroup 1 (n=90) had the disease at 28.1–32.0 weeks' gestation, in subgroup 2 (n=154) at 32.1–36.6 weeks' gestation, and in subgroup 3 (n=69) at 37 weeks' gestation or more.
The mean gestational age at the time of infection in the study group patients was 34.9 (3.9) weeks, and it was 29.4 (0.8), 33.9 (1.8), and 38.9 (1.2) weeks in subgroups1, 2, and 3, respectively.
The inclusion criteria for the study group were pregnant women with a confirmed diagnosis of mild to moderate COVID-19 at 28 weeks of gestation or more, signed informed consent to participate in the study, and clinical recovery at delivery. The non-inclusion criteria were gestational age less than 28 weeks at the time of COVID-19 and delivery during the disease without clinical recovery.
After infection, the patients were followed up by local antenatal clinics until delivery; 87 patients were delivered at the Ural Research Institute of Maternity and Child Care, and 226 patients were delivered in the maternity hospitals of Yekaterinburg and the Sverdlovsk Region. Data on the course of pregnancy in the study group were obtained from the medical records of the Ural Research Institute of Maternity and Child Care and the Regional Obstetric Monitoring System.
The comparison group consisted of 216 patients who delivered during the pre-pandemic period (2019) without indications of acute respiratory viral infection (ARVI) during pregnancy and delivery. This group was recruited from the Regional Obstetric Monitoring System database by random selection using a random number table according to GOST Sections 6.1.,6.2. "Random sampling and randomization procedures" [13]. The sample size of the comparison group was calculated using a power calculator [14].
Morphological and functional evaluation was performed in 87 placentas from women who had mild to moderate COVID-19 in different periods of pregnancy in the third trimester: subgroup 1(n=25) at 28.1–32.0 weeks gestation, subgroup 2 (n=39) at 32.1–36.6 weeks, and subgroup 3 (n=32) at full-term pregnancy. After COVID-19, the patients were delivered at the Ural Research Institute of Maternity and Childcare. The comparison group (n=20) comprised placentas of women with healthy pregnancies without any indication of ARVI during gestation.
Morphological examination of the placenta was performed according to international guidelines [15]. Fragments of the umbilical cord, membranes, and sections of maternal and fetal surfaces at the umbilical cord and disc edge were selected for microscopic examination. The material was fixed in 10% buffered formalin and paraffin blocks and 4 µm thick sections were stained with hematoxylin and eosin according to standard procedures.
Statistical analysis
Statistical analysis and plotting were performed using Microsoft Excel (2010) and SPSS Statistics version 22.0 (IBM Microsoft, USA). Categorical variables were compared using the chi-square (χ2) test with two-by-two contingency tables. Qualitative ordinal signs are presented as median and interquartile range (IQR) (Me (Q1:Q3]). Hypothesis testing was performed using the Mann–Whitney U test.
The distribution of continuous variables was tested for normality using the Kolmogorov–Smirnov and Shapiro–Wilk tests. The procedure for testing the equality of variance was performed using the f-criterion in SPSS Statistics version 22.0, which is directly included in the procedure for testing the hypothesis of a difference in the mean. Data are presented as arithmetic means (M) and standard deviations (SD); categorical variables are presented as counts (n) and percentages (%). The relative risk (RR) with 95% confidence interval (CI) was calculated to estimate the strength of the association between risk factors and outcomes. Differences between the groups were considered statistically significant at p<0.017 and subjected to Bonferroni adjustment.
This study was reviewed and approved by the Research Ethics Committee of the Ural Research Institute of Maternity and Child Care (Ref. No. 12 dated 21.09.2021).
The main limitation of this study was the risk of selection bias due to the single-center nature of the study.
Results and discussion
The age of the patients in the study group ranged from 16 to 52 years and averaged 29.5 (6.1) years. It was 29.6 (6.6) 29.6 (5.9) and 29.3 (5.9) years in subgroups 1, 2, and 3, respectively. The age of the patients in the comparison group ranged from 20 to 47 years, with a mean of 29.1 (5.7) years.
The groups were comparable in terms of age, parity, and obstetric and gynecological histories (p>0.05) (Table 1).
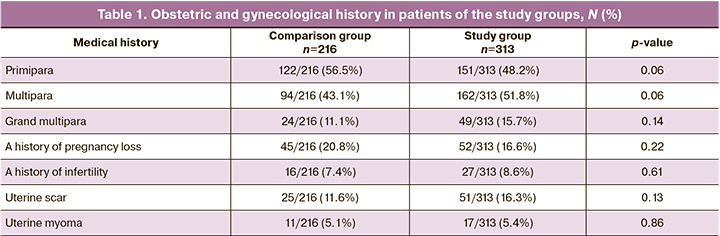
Analysis of the somatic history of the patients in the compared groups showed that pregnant women in the study group were more likely to be overweight and obese and have respiratory diseases (mainly chronic bronchitis and bronchial asthma). It has now been confirmed that these pathologies are among the diseases that worsen the course of COVID-19 [16]. Patients in the comparison group had more frequent diseases of the digestive organs (mainly gastritis and cholecystitis) and urinary system (chronic pyelonephritis).
The most common gestational comorbidities in patients infected in the third trimester with SARS-CoV-2 were gestational diabetes mellitus (GDM) (detected before COVID-19 in every fifth patient, 63/313 (20.1%)) and anemia (detected before COVID-19 in every third patient of the core group, 96/313 (30.7%)). In the post-COVID period, GDM and anemia not detected before the disease were additionally recorded in 16/313 (5.1%) and 13/313 (4.2%) patients in the study group (subgroups 1 and 2, respectively). This suggests an effect of infection on glucose and iron metabolism. Iron deficiency and impaired glucose metabolism in pregnant women create conditions for the rapid development of hypoxic changes, leading to a reduced immune response to infection [17] and may contribute to perinatal complications.
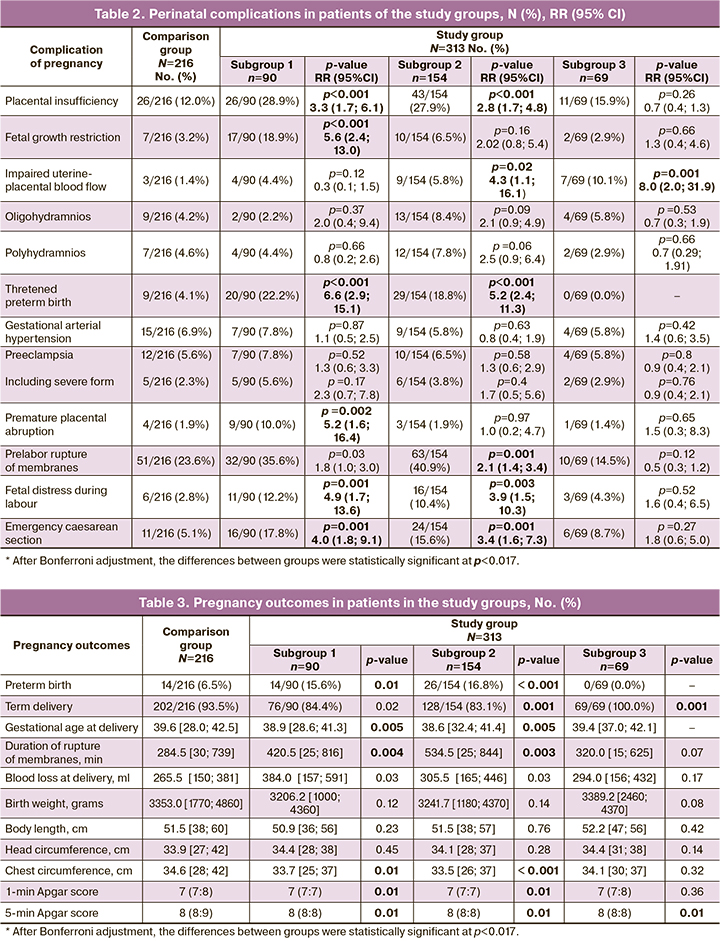
A detailed analysis of the course and outcome of pregnancy in patients with COVID-19 at different stages of the third trimester, compared with those who were pregnant and delivered in the pre-pandemic period, is presented in Tables 2 and 3.
The third trimester of pregnancy places the greatest demand on the adaptive reserves of the pregnant woman and the fetus. On the one hand, the functions of the organs and systems of pregnant women are being restructured in preparation for the forthcoming birth; on the other hand, fetal organ systems are being formed in preparation for life in the extrauterine environment. Therefore, additional pathological effects are associated with the most serious and sometimes unpredictable, consequences [18].
In the early third trimester of a healthy pregnancy, the placenta acquires structural signs of maturity with maximum endocrine function (production of placental lactogens and steroid hormones), blood supply, and oxygenation capacity of the fetus. By 32.0 weeks of gestation, the placenta completes the formation of the villous tree, which is one of the main structural entities. Its terminal villi with syncytocapillary membranes are responsible for the fetoplacental metabolism [1, 2]. By the 28th week of pregnancy, the fetus develops all organ systems that mature in the third trimester. Between 28.0–32.0 weeks, type I and type II alveolar cell differentiation and surfactant production begin [19] and the basic mechanisms of central respiratory regulation are completed [20].
In our study, in women in subgroup 1, who had COVID-19 between 28.1 and 32.0 weeks, the course of pregnancy was complicated by chronic placental insufficiency leading to fetal growth restriction (17/90 (18.9%), p<0.001) and threatened preterm birth (20/90 (22.2%), p<0.001).
Preterm birth occurred in 14/90 (15.6%), twice as often as in the comparison group (14/216 (6.5%), p=0.01). Consequently, the mean time to delivery for patients in subgroup 1 was significantly lower (38.9 [28.6;41.3]) than that in the preterm group (39.6 [28.0;42.5], p=0.005).
Preterm birth in patients in this subgroup occurred on average 1 month after the onset of the disease (34.5 [25.0;44.0] days), and after clinical recovery, preterm birth occurred on average three weeks later (22.5 [15.0;30.0] days).
The patients who experienced COVID-19 early in the third trimester had a longer duration of ruptured membranes than the comparison group, 7.0 (0.9) h versus 4.7 (0.5) h, respectively (p=0.004).
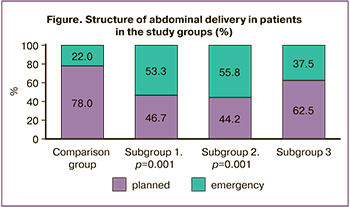
Emergency caesarean section was the most common abdominal delivery procedure (Figure) and was indicated by fetal distress in 11/90 (12.2%) patients (p=0.001) and preterm placental abruption in 9/90 (10.0%) patients (p=0.002).
According to the literature, the number of terminal placental villi increases by the middle of the third trimester, the volume of the intervillous space increases, and the ability to exchange substances between the maternal and fetal blood is maximized [1, 2]. Between 32.0- and 36.6-weeks’ gestation, fetal growth is rapid (average fetal weight increases by 25 g/day), fetal brain development is accelerated, and myelination of nerve fibers in the central nervous system is complete [20].
In our study of patients in subgroup 2 who had COVID-19 between 32.1 and 36.6 weeks, threatened preterm birth was observed in one in five patients (29/154 (18.8%), p<0.001), similar to that in subgroup 1.
The mean delivery time of patients in subgroup 2 differed significantly from that of the comparison group towards a decrease (38.6 [32.4;41.4], p=0.005), which was associated with a higher preterm birth rate (26/154 (16.8%)). Preterm births occurred on average three weeks after onset (23.8 [14.0;30.0] days) and two weeks after clinical recovery (13.5 [7.0;22.0] days).
Women with COVID-19 in the mid-III trimester were significantly more likely to have a prolonged duration of ruptured membranes averaging 8.9 (1.3) h; in the comparison group, the duration of ruptured membranes averaged 4.7 (0.5) h.
Caesarean section was performed in 27.9% of women in this subgroup, which was not significantly different from the comparison group. However, emergency surgery predominated (Figure), 24/43 (55.8%), which was twice as frequent as that in the control group (11/50 (22.0%), p=0.001). The most common indication for emergency caesarean section was fetal distress, in one of ten cases (16/154(10.4%), p=0.003).
The results of our study confirm the reports of domestic and international researchers reporting cases of placental abruption [21] and fetal growth restriction [22] in pregnant women with COVID-19. Systematic reviews reported an increased preterm birth rate of 13.8 to 25.0% among women with COVID-19 [23, 24] compared with a general population rate of 6.1% in the Russian Federation [25]. The authors explained the pathogenetic mechanisms of obstetric pathology in COVID-19 patients. Thus, impaired hemodynamic parameters in the uteroplacental system are due to the interaction of the virus with endothelial cells, which causes hypercoagulation with the formation of micro-clots in the maternal-placenta-fetal unit [26]. COVID-19 triggers preterm birth and is associated with increased production of proinflammatory cytokines in the uteroplacental complex and activation of the maternal and fetal hypothalamic-pituitary system [27].
Anthropometric parameters, such as chest circumference and Apgar score at 1 and 5 min, were significantly lower in infants from patients who had COVID-19 in the early and middle third trimesters than in those born in the pre-pandemic era (Table 3). This may be due to intrauterine hypoxia with prolonged placental insufficiency, resulting in delayed morphological and functional organ maturation, particularly delayed lung maturation [19].
In patients in subgroup 3 who had COVID-19 at the end of pregnancy and delivered after clinical recovery, uterine-placental blood flow failure was found significantly more frequently (7/69 (10.1%), p=0.001), similar to that in patients in subgroup 2. The newborns of the women in subgroup 3 had a significantly lower Apgar score at minute 5, indicating impaired early postnatal adaptation. This indicates that when a mother has COVID-19 in a full-term pregnancy, the fetus remains sufficiently sensitive and vulnerable.
No significant differences were found between the patients in the study and control groups in terms of changes in the duration of labor and frequency of labor anomalies (p>0.05).
All the above characteristics of the course of pregnancy and perinatal outcomes were confirmed by histological examination of the placentas (Table 4).
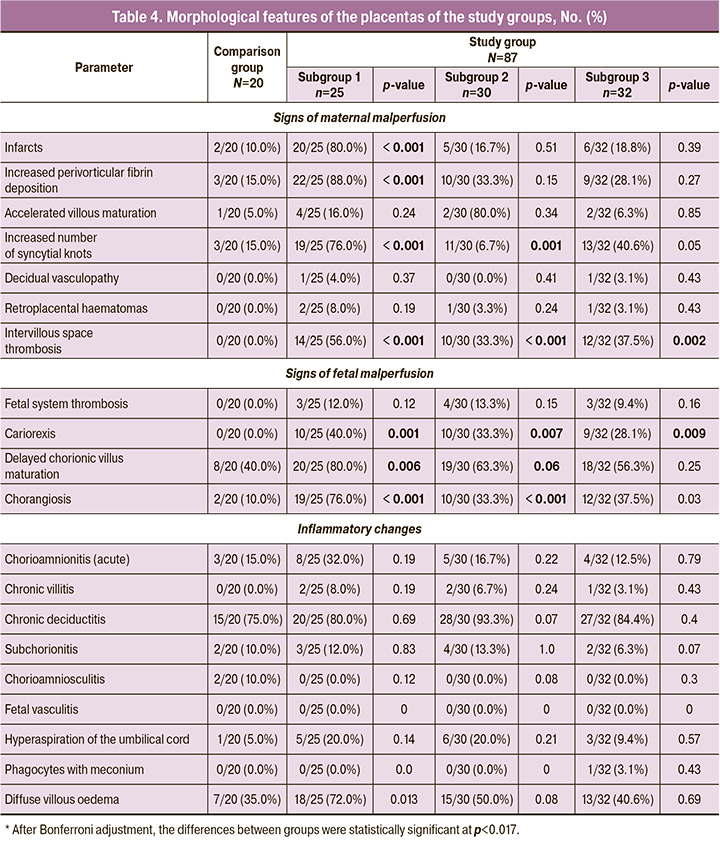
The placentas of patients in subgroup 1 were significantly more likely (80%) to have infarcts and increased fibrinoid deposition with the formation of extensive functional zones (88%). In this regard, there was an increased number of syncytial knots (Tenney–Parker changes), which is a sign of maternal vascular malperfusion. More than half of the placentas had thrombosis in the intervillous space. According to the literature, such placental changes are associated with chronic placental insufficiency, resulting in fetal growth restriction and preterm birth [28]. In addition to maternal vascular insufficiency, signs of fetal malperfusion were significantly more common in the placentas of patients in subgroup 1, including delayed maturation of chorionic villi (80%), increased number of capillaries in the terminal villi (chorangiosis) (76%), and karyorrhexis (40%).
In the placentas of patients in subgroups 2 and 3, maternal and fetal vascular abnormalities were less pronounced than in subgroup 1. However, maternal vascular insufficiency was significantly more common in the placentas, manifested by an increase in the number of syncytial buds and thrombosis of the intervillous space. In placentas, signs of fetal circulatory disorders were significantly more often marked by delayed maturation of chorionic villi and chorangiosis.
In contrast to the placentas of patients in the study group, signs of maternal vascular malperfusion were less frequent, smaller in size, and less likely to extend throughout the placenta. Excessive fibrinoid deposition with the formation of functional zones also occurred in only 15% of cases, despite a later delivery date. Delayed villous maturation was seen in 40% of cases, almost twice as often as in women who had COVID before 32 weeks, that is, before complete formation of terminal villi. Inflammatory changes were isolated and showed no specific features.
It is possible that all of the above morphological changes in the placenta are related to the presence of receptors for coronaviruses in its cells, including angiotensin-converting enzyme-2, serine protease TMPRSS2, and Cd147. However, we did not analyze the presence or absence of SARS-CoV-2 in placentas. In addition, it may be associated with thrombosis, which is characteristic of this disease [28].
Conclusion
The results of this study demonstrate the effect of mild-to-moderate COVID-19 in the third trimester of pregnancy on the development of perinatal complications in the post-COVID period.
The most serious effects were observed in patients who had COVID-19 between 28.1 and 32.0 weeks. They had a 6-fold increased risk of developing chronic placental insufficiency followed by fetal growth restriction, 3-fold increased risk of preterm delivery, 5-fold increased risk of preterm placental abruption, and 5-fold increased risk of fetal distress during labor. At the same time, the prolonged rupture of membranes during labor is significantly increased. There was a 4-fold increase in the risk of emergency cesarean sections.
The most pronounced signs of maternal and fetal malperfusion were found in the placentas of patients who had COVID-19 early in the third trimester compared with the placentas of patients who experienced COVID-19 later in pregnancy and had less pronounced maternal and fetal vascular disorders.
References
1. Радзинский В.Е., Милованов А.П. Экстраэмбриональные и околоплодные структуры при нормальной и осложненной беременности. М.: МИА; 2004. 393с. [Radzinsky V.E., Milovanov A.P. Extraembryonic and amniotic structures in normal and complicated pregnancy. Moscow: MIA; 2004. 393p. (in Russian)].
2. Сидорова И.С., Макаров И.О. Течение и ведение беременности по триместрам. М.: МИА; 2009. 304с. [Sidorova I.S., Makarov I.O. The course and management of pregnancy by trimester. Moscow: MIA; 2009. 304 p. (in Russian)].
3. Мальгина Г.Б., Дьякова М.М., Бычкова С.В., Гришкина А.А., Пепеляева Н.А., Ольков С.С., Мелкозерова О.А., Башмакова Н.В., Давыденко Н.Б. Новая коронавирусная инфекция в I триместре беременности: перинатальные и материнские последствия. Акушерство и гинекология. 2022; 12: 90‑9. [Malgina G.B., Dyakova M.M., Bychkova S.V., Grishkina A.A., Pepelyaeva N.A., Olkov S.S., Melkozerova O.A., Bashmakova N.V., Davydenko N.B. Novel coronavirus infection in the first trimester of pregnancy: perinatal and maternal outcomes. Obstetrics and Gynecology. 2022; (12): 90‑9. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.212.
4. la Cour Freiesleben N., Egerup P., Hviid K.V.R., Severinsen E.R., Kolte A.M., Westergaard D. et al. SARS‑CoV‑2 in first trimester pregnancy: a cohort study. Hum. Reprod. 2021; 36(1): 40‑7. https://dx.doi.org/10.1093/humrep/deaa311.
5. Baud D., Greub G., Favre G., Gengler C., Jaton K., Dubruc E., Pomar L. Second‑trimester miscarriage in a pregnant woman with SARS‑CoV‑2infection. JAMA. 2020; 23(21): 2198‑200. https://dx.doi.org/10.1001/jama.2020.7233.
6. Turgut E., Sakcak B., Uyan Hendem D., Oluklu D., Goncu Ayhan S., Sahin D. Decreased fetal cardiac output in pregnant women with severe SARS‑Cov‑2 infection. Echocardiography. 2022; 39(6): 803‑10. https://dx.doi.org/10.1111/ echo.15367.
7. Zhang L., Dong L., Ming L., Wei M., Li J., Hu R., Yang J. Severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2) infection during late pregnancy: a report of 18 patients from Wuhan, China. Pregnancy Childbirth. 2020; 20(1): 394. https://dx.doi.org/10.1186/s12884‑020‑03026‑3.
8. Piekos S.N., Roper R.T., Hwang Y.M., Sorensen T., Price N.D., Hood L., Hadlock J.J. The effect of maternal SARS‑CoV‑2 infection timing on birth outcomes: a retrospective multicentre cohort study. Lancet Digit. Health. 2022; 4(2): e95‑e104. https://dx.doi.org/10.1016/S2589‑7500(21)00250‑8.
9. Li N., Han L., Peng M., Lv Y., Ouyang Y., Liu K. et al. Maternal and neonatal outcomes of pregnant women with coronavirus disease 2019 (COVID‑19) pneumonia: a case‑control study. Clin. Infect. Dis. 2020; 71(16): 2035‑41. https://dx.doi.org/10.1093/cid/ciaa352.
10. Cosma S., Carosso A.R., Cusato J., Borella F., Carosso M., Gervasoni F. et al. Preterm birth is not associated with asymptomatic/mild SARS‑CoV‑2 infection per se: Pre‑pregnancy state is what matters. PLoS One. 2021; 16(8): e0254875. https://dx.doi.org/10.1371/journal.pone.0254875.
11. von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J. Clin. Epidemiol. 2008; 61(4): 344‑9. https://dx.doi.org/10.1016/ j.jclinepi.2007.11.008.
12. Министерство здравоохранения Российской Федерации. Временные методические рекомендации. Организация оказания медицинской помощи беременным, роженицам, родильницам и новорожденным при новой коронавирусной инфекции COVID‑19. Версия 2 от 28.05.2020. Версия 3 от 25.01.2021. Версия 4 от 05.07.2021. [Ministry of Health of the Russian Federation. Organization of medical care for pregnant women, women in labor, women in labor and newborns with a new coronavirus infection COVID‑19. Methodological guidelines. Version 2, 28.05.2020. Version 3, 25.01.2021, Version 4, 05.07.2021 (in Russian)].
13. Процедуры рандомизации и отбора случайной выборки. ГОСТ Р ИСО 24153‑2012. Группа Т59. ОКС 03.120.30. [Statistical methods. Random sampling and randomization procedures. Standart ISO 24153‑2012. Group T59. OKS 03.120.30. (in Russian)].
14. Sealed Envelope Ltd. 2012. Power calculator for binary outcome superiority trial. Available at: https://www.sealedenvelope.com/power/binary‑superiority
15. Khong T.Y., Mooney E.E., Gordijn S.J., Morgan T.K., Nikkels P.G.J. et al. Pathology of the placenta: Practical guide. Springer: Nature; 2019. https://dx.doi.org/10.1007/978‑3‑319‑97214‑5.
16. Адамян Л.В., Вечорко В.И., Конышева О.В., Харченко Э.И. Беременность и COVID‑19: актуальные вопросы (обзор литературы). Проблемы репродукции. 2021; 27(3): 70‑7. [Adamyan L.V., Vechorko V.I., Konysheva O.V., Kharchenko E.I. Pregnancy and COVID‑19: current issues (literature review). Russian Journal of Human Reproduction. 2021; 27(3): 70‑7. (in Russian)]. https://dx.doi.org/10.17116/repro20212703170.
17. Eskenazi B., Rauch S., Iurlaro E., Gunier R.B., Rego A., Gravett M.G. et al. Diabetes mellitus, maternal adiposity, and insulin‑dependent gestational diabetes are associated with COVID‑19 in pregnancy: the INTERCOVID study. Am. J. Obstet. Gynecol. 2022; 227(1): 74.e1‑74. https://dx.doi.org/10.1016/ j.ajog.2021.12.032.
18. Мальгина Г.Б. Стресс и беременность: перинатальные аспекты. Екатеринбург; 2002. 188с. [Malgina G.B. Stress and pregnancy: perinatal aspects. Yekaterinburg; 2002. 188p. (in Russian)].
19. Гулькевич Ю.В., Маккавеева М.Ю., Никифоров Б.И. Патология последа человека и ее влияние на плод. Минск; 1968. 232с. [Gulkevich Yu.V., Makkaveeva M.Yu., Nikiforov B.I. Pathology of the human afterbirth and its effect on the fetus. Minsk; 1968. 232p. (in Russian)].
20. Юсенко С.Р., Нагорнева С.В., Коган И.Ю. Закономерности развития и становления интегративной функции центральной нервной системы плода в антенатальном периоде. Журнал акушерства и женских болезней. 2022; 71(5): 97‑110. [Yusenko S.R., Nagorneva S.V., Kogan I.Yu. Patterns of development and formation of the fetal central nervous system integrative function in the antenatal period. Journal of obstetrics and woman diseases. 2022; 71(5): 97‑110. (in Russian)]. https:/dx./doi.org/10.17816/ JOWD107183.
21. Hosier H., Farhadian S.F., Morotti R.A., Deshmukh U., Lu-Culligan A., Campbell K.H. et al. SARS‑CoV‑2 infection of the placenta. J. Clin. Invest. 2020; 130(9): 4947‑53. https://dx.doi.org/10.1172/JCI139569.
22. Wong S.F., Chow K.M., Leung T.N., Ng W.F., Ng T.K., Shek C.C. et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am. J. Obstet. Gynecol. 2004; 191(1): 292‑7. https://dx.doi.org/10.1016/ j.ajog.2003.11.019.
23. Jafari M., Pormohammad A., Neshin S.A.S., Ghorbani S., Bose D. et al. et al. Clinical characteristics and outcomes of pregnant women with COVID‑19 and comparison with control patients: Aasystematic review and meta‑ analysis. Rev. Med. Virol. 2021; 31(5): 1‑16. https://dx.doi.org/10.1002/ rmv.2208.
24. Martinez-Perez O., Prats Rodriguez P., Muner Hernandez M., Encinas Pardilla M.B., Perez Perez N., Vila Hernandez M.R. et al. The association between SARS‑CoV‑2 infection and preterm delivery: a prospective study with a multivariable analysis. BMC Pregnancy Childbirth. 2021; 21(1): 273. https://dx.doi.org/10.1186/s12884‑021‑03742‑4.
25. Филиппов О.С., Гусева Е.В. Основные показатели деятельности акушерско‑гинекологической службы в Российской Федерации в 2019 году. М.; 2020. 30с. [Filippov O.S., Guseva E.V. Key performance indicators of the obstetric and gynecological service in the Russian Federation in 2019. Moscow; 2020. 30p. (in Russian)].
26. Vivanti A.J., Vauloup-Fellous C., Prevot S., Zupan V., Suffee C., Do Cao J. et al. Transplacental transmission of SARS‑CoV‑2 infection. Nat. Commun. 2020; 11(1): 3572. https://dx.doi.org/10.1038/s41467‑020‑17436‑6.
27. Korebrits C., Ramirez M.M., Watson L., Brinkman E,. Bocking A.D., Challis J.R. Maternal corticotropin‑releasing hormone is increased with impending preterm birth. J. Clin. Endocrinol. Metab. 1998; 83(5): 1585‑91. https://dx.doi.org/10.1210/jcem.83.5.4804.
28. Щеголев А.И., Туманова У.Н., Серов В.Н. Поражения плаценты у беременных с SARS‑CoV‑2‑инфекцией. Акушерство и гинекология. 2020; (12): 44‑52. [Shchegolev A.I., Tumanova U.N., Serov V.N. Placental lesions in pregnant women with SARS‑¬CoV‑¬2 infection. Obstetrics and Gynecology. 2020; (12: 44‑52. (in Russian)]. https://dx.doi.org/10.18565/ aig.2020.12.44‑52.
Received 07.03.2023
Accepted 30.03.2023
About the Authors
Galina B. Malgina, Dr. Med. Sci., Director of the Ural Research Institute of Maternity and Child Care, Ministry of Health of Russia, galinamalgina@mail.ru, https://orcid.org/0000-0002-5500-6296, 620028, Russia, Yekaterinburg, Repin str., 1.Maria M. Dyakova, Junior Researcher, Ural Research Institute of Maternity and Child Care, Ministry of Health of Russia, +7(343)37-189-11, +7(950)550-06-52, mariadakova40@mail.ru, https://orcid.org/0000-0001-7911-6783, 620028, Russia, Yekaterinburg, Repin str., 1.
Svetlana V. Bychkova, PhD, Leading Researcher, Ural Research Institute of Maternity and Child Care, Ministry of Health of Russia, +7(343)37-189-11, simomm@mail.ru, https://orcid.org/0000-0002-8892-7785, 620028, Russia, Yekaterinburg, Repin str., 1.
Anastasia A. Grishkina, PhD, Pathologist, Department of Immunology, Clinical Microbiology, Pathomorphology and Cytodiagnosis, Ural Research Institute of Maternity and Child Care, Ministry of Health of Russia, xumukyc.ru@mail.ru, https://orcid.org/0000-0001-7433-2217, 620028, Russia, Yekaterinburg, Repin str., 1.
Oksana A. Melkozerova, Dr. Med. Sci., Deputy Director for Science, Ural Research Institute of Maternity and Child Care, Ministry of Health of Russia, abolmed@mail.ru, https://orcid.org/0000-0002-4090-0578, 620028, Russia, Yekaterinburg, Repin str., 1.
Nadezhda V. Bashmakova, Dr. Med. Sci., Professor, Chief Researcher, Ural Research Institute of Maternity and Child Care, Ministry of Health of Russia, bashmakovanv@niiomm.ru, https://orcid.org/0000-0001-5746-316X, 620028, Russia, Yekaterinburg, Repin str., 1.
Natalia A. Pepelyaeva, PhD, Chief Physician, Ural Research Institute of Maternity and Child Care, Ministry of Health of Russia, pepelyaevana@niiomm.ru, https://orcid.org/0000-0003-3278-2249, 620028, Russia, Yekaterinburg, Repin str., 1.
Sergey S. Olkov, PhD, Deputy Head of the Pediatrics Clinic, Ural Research Institute of Maternity and Child Care, Ministry of Health of Russia, olkovss@niiomm.ru, https://orcid.org/0000-0002-6142-370, 620028, Russia, Yekaterinburg, Repin str., 1.
Corresponding authors: Maria M. Dyakova, mariadakova40@gmail.com; Svetlana V. Bychkova, simomm@mail.ru



