Retrospective analysis of human papillomavirus prevalence in women with cervical pathology
Andreev A.O., Bayramova G.R., Ilyasova N.A., Asaturova A.V., Trofimov D.Yu.
Objective: This study aimed to compare the distribution of different human papillomavirus (HPV) genotypes in various cervical lesions and estimate the prevalence of low-grade squamous intraepithelial lesions (LSIL) and high-grade squamous intraepithelial lesions (HSIL) when one or more HPV genotypes are detected.
Materials and methods: We conducted an analysis of HPV testing using the Quantum-21 test system in 19236 women aged 18–81 years who visited the V.I. Kulakov NMRC for OG&P between November 2011 and April 2022. Among these women, 5700 tested positive for HPV and were included in this analysis. We retrospectively examined the laboratory findings used to confirm this diagnosis. The patients were divided into three groups based on the histological verification of the diagnosis. Group 1 included 186 patients with chronic cervicitis, Group 2 included 341 patients with LSIL, and Group 3 included 292 patients with HSIL.
Results: Analysis of HPV genotype distribution revealed that HPV type 16 was the most prevalent (23.6%), followed by genotypes 44 (12.3%), and 31 (12.3%). The evaluation of HPV genotype prevalence showed that in 66.6% of cases, only one HPV type was detected, whereas in 20.8% of cases, two types were detected. Three types were found in 7.4% of cases and four or more types were detected in 5.2% of cases. Examination of the ratio between single and multiple HPV types revealed that the detection of multiple genotypes was significantly more frequently associated with LSIL than with HSIL (24.9% vs. 5.5%, respectively).
Conclusion: The results of this study demonstrated that the distribution and representation of HPV genotypes in cervical pathology in our sample differed from global trends. Furthermore, the study showed that the detection rate of multiple HPV types decreased with increasing grades of cervical lesions.
Authors' contributions: Andreev A.O., Bayramova G.R. – conception and design of the study; Bayramova G.R., Asaturova A.V. – data collection and analysis; Ilyasova N.A. – statistical analysis; Andreev A.O., Bayramova G.R. – manuscript drafting; Bayramova G.R., Trofimov D.Yu. – manuscript editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Andreev A.O., Bayramova G.R., Ilyasova N.A., Asaturova A.V., Trofimov D.Yu. Retrospective analysis of human papillomavirus prevalence in women with cervical pathology. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (11): 140-149 (in Russian)
https://dx.doi.org/10.18565/aig.2023.225
Keywords
Human papillomavirus (HPV) is one of the most common sexually transmitted diseases worldwide. It is responsible for 4.5% of all malignant neoplasms in both genders [1]. The list of malignant neoplasms associated with HPV continues to expand annually. Recent studies have shown that HPV-positive status is associated with an increased risk of bladder cancer [2]. However, the most prevalent HPV-associated malignancy is cervical cancer [3]. The percentage of HPV-positive cervical cancer cases has been steadily increasing, from 5.9% in 1990 to 92.9% in 2010, reaching 99% in 2020 [4, 5]. This upward trend can be attributed to improvements in the HPV detection techniques. To date, 449 papillomavirus genome variants have been identified. Among these, 229 were HPV genotypes and 220 were papillomaviruses found in animals.
Notably, the papillomavirus genome library has been regularly updated since 2013. In May 2023, new HPV genotypes, 228 and 229, were discovered and added. This suggests the possibility of identifying new genotype variants in the future [6]. The choice of a diagnostic panel for genotyping plays a crucial role in HPV DNA testing. In Russia, current recommendations suggest the determination of at least 12 HPV genotypes [7]. However, some studies on HPV prevalence and distribution have argued for using a panel that identifies an even larger number of HPV genotypes [8–10]. In recent years, the scientific community has also focused on the detection of multiple HPV genotypes in individual patients. The frequency of such cases ranges from 25% to 40%, as reported by various authors [11–13]. It is important to note that there is a fundamental divergence of opinion regarding the interpretation of these cases.
This study aimed to investigate the specific characteristics of HPV distribution and the influence of one or more HPV genotypes on the risk of developing cervical lesions.
Materials and methods
We conducted an analysis of HPV testing using the Quantum-21 test system in 19236 women aged 18–81 years who visited V.I. Kulakov NMRC for OG&P of the Ministry of Health of the Russian Federation between November 2011 and April 2022.
HPV DNA was tested by determining the number of genomic equivalents of the virus by real-time polymerase chain reaction using the Quant-21 HPV reagent kit, designed for detection, typing, and quantification of HPV DNA of low (HPV 6, 11, 44 types) and high (HPV 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82 types) carcinogenic risk. A total of 5700 HPV-positive women were included in the analysis.
We retrospectively examined the laboratory findings used to confirm this diagnosis. Laboratory methods included histological examination of cervical tissue samples obtained by targeted biopsy, excision, or conization of the cervix. The analysis included 819 histological report protocols, and the patients were divided into three groups based on the histological verification of the diagnosis. Group 1 included 186 patients with chronic cervicitis, Group 2 included 341 patients with low-grade squamous intraepithelial lesions (LSIL), and Group 3 included 292 patients with high-grade squamous intraepithelial lesions (HSIL).
Statistical analysis
Statistical analysis was performed using the IBM SPSS Statistics Version 20 statistical software. The distribution of continuous variables was tested for normality using the Kolmogorov-Smirnov test. Regardless of whether the variables met the normality assumption, quantitative data were presented as medians with quartiles (Me (Q1; Q3)). Categorical data are presented as percentages with an indication of 95% confidence interval (95% CI), calculated using Wilson's method. Non-parametric tests (Mann–Whitney test, Chi-square) were used to compare variables according to their properties (quantitative or categorical). Species diversity was measured by calculating the Shannon biodiversity index, and evenness of species distribution was analyzed by determining the Shannon fairness index. A value of p<0.05 was taken as the critical level of significance.
Results
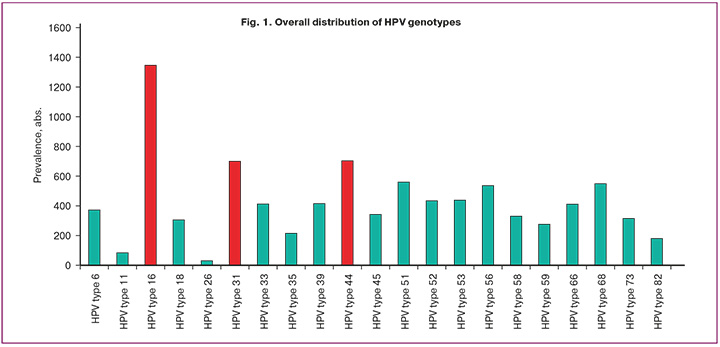
HPV-positive status was detected in 5700 female patients. The mean age of the patients was 31 years (range, 26–37 years). Analysis of the HPV genotype distribution showed that HPV genotype 16 was the most common (1346/5700, 23.6%), followed by genotypes 44 (703/5700, 12.3%) and 31 (700/5700, 12.3%). Importantly, the prevalence of HPV genotype 18 was inferior to that of most highly oncogenic types and was 305/5700 (5.4%), followed by genotype 35 at 215/5700 (3.8%), genotype 82 at 180/5700 (3.2%), and genotype 26 at 30/5700 (0.5%). Meanwhile, from the identified diagnostic panel of 21 HPV types, 2749/5700 (48.2%) of all detected HPV cases were distributed among the three leading genotypes: 16, 44, and 31. The prevalence of low-oncogenic HPV types (6, 11, 44) was 1159/5700 (20.3%) of high-oncogenic HPV types (16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 52, 53, 56, 58, 59, 66, 68, 73, 82) was 4541/5700 (79.7%) (Fig. 1).
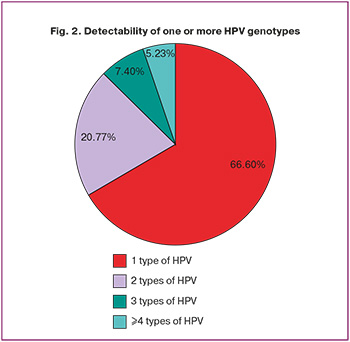
The evaluation of HPV genotype prevalence showed that only one HPV type was detected in 3796/5700 (66.6 %) cases, whereas two types were identified in 1184/5700 (20.8 %) cases. Three types were found in 422/5700 (7.4%) cases and four or more types were detected in 298/5700 (5.2%) cases (Fig. 2).
In 819 cases, histological examination of the cervical biopsy specimen was performed to definitively verify the diagnosis. Of the 819 cases, 186, 341, and 292 specimens corresponded to histologic diagnoses of chronic cervicitis, LSIL, and HSIL, respectively. The age of the patients in Group 3 was statistically significantly (p<0.001) higher than that in groups 1 and 2 and was 34 (29;40), 31 (19;53), and 31 (26;35) years, respectively.
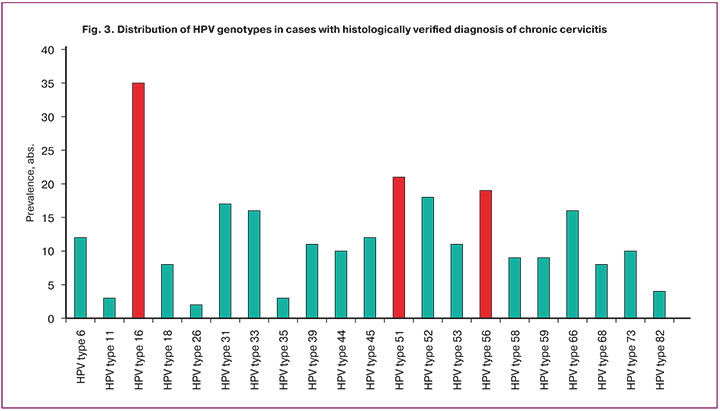
In Group 1, only one HPV type was detected in 141/186 (75.8%) cases, and two or more HPV genotypes were detected in 45/186 (24.2%) cases. It should be emphasized that every fourth patient diagnosed with chronic cervicitis had more than one HPV type. Thus, the data obtained indicated that HPV detection was registered 260 times out of 186 cases of chronic cervicitis. Thus, the evaluation of genotype distribution in this group revealed that the dominant HPV genotypes were types 16 (35/260, 13.5%), 51 (21/260, 8.1%), and 56 (19/260, 7.3%) (Fig. 3). Moreover, HPV genotype 16 was significantly more frequent than HPV types 51 and 56 (p<0.05).
In Group 2, only one HPV type was detected in 209/341 (61.3%) patients, and 132/341 (38.7%) had two or more HPV genotypes.
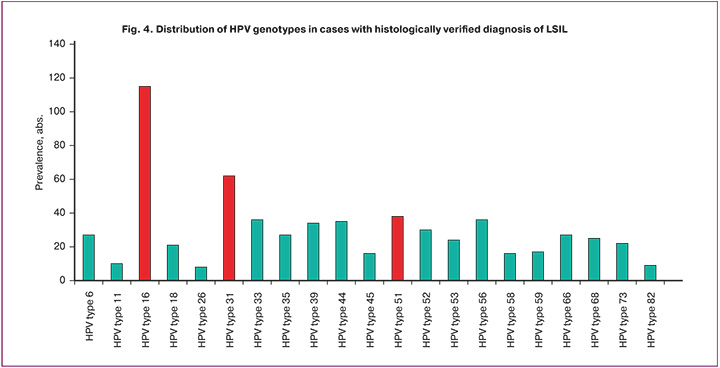
A total of 635 HPV cases were detected in the samples with LSIL. Based on the total number of HPV genotype detections, evaluation of their distribution showed that 16 (115/635, 18.1%), 31 (62/635, 9.8%), and 51 (38/635, 5.9%) HPV types were the dominant types (Figure 4). Moreover, HPV genotype 16 was significantly more frequent than HPV types 31 and 51 (p<0.0001).
In Group 3, of the 292 patients, only one HPV type was detected in 229/292 (78.4%) cases, and two or more HPV types were detected in 63/292 (21.6%) samples. HPV detection occurred 380 times in samples with HSIL. Considering the total number of HPV genotype detections, evaluation of their distribution showed that 16 (150/380, 39.5%), 31 (51/380, 13.4%), 33 (30/380, 7.9%), and 44 (30/380, 7.9%) HPV types were dominant (Figure 5). Moreover, HPV genotype 16 was significantly more frequent than HPV types 31, 33, and 44 (p<0.0001).
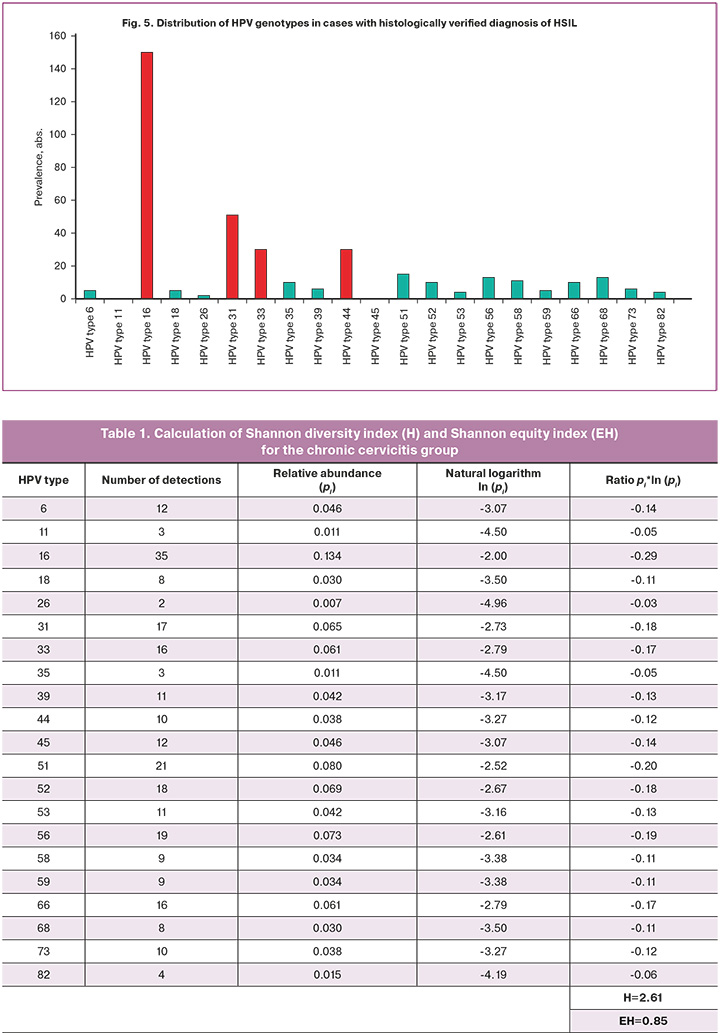
As can be seen from the data presented, the leading position in terms of prevalence in all groups is occupied by HPV type 16, amounting to 13.5%, 18.1% and 39.5%, respectively.
It should be noted that the prevalence of HPV type 16 in Group 3 was significantly higher than that in groups 1 and 2 (p<0.01), whereas no differences were found between groups 1 and 2.
The detection of multiple HPV genotypes (three or more types) in the same sample was significantly (p<0.0001) more frequently associated with LSIL than with HSIL (24.91% vs. 5.47%) (OR=2.36, 95% CI: 1.66–3.35) (Fig. 6). At the same time, no significant difference in the detectability of LSIL and HSIL was found when only one or two HPV types were detected (p>0.05).
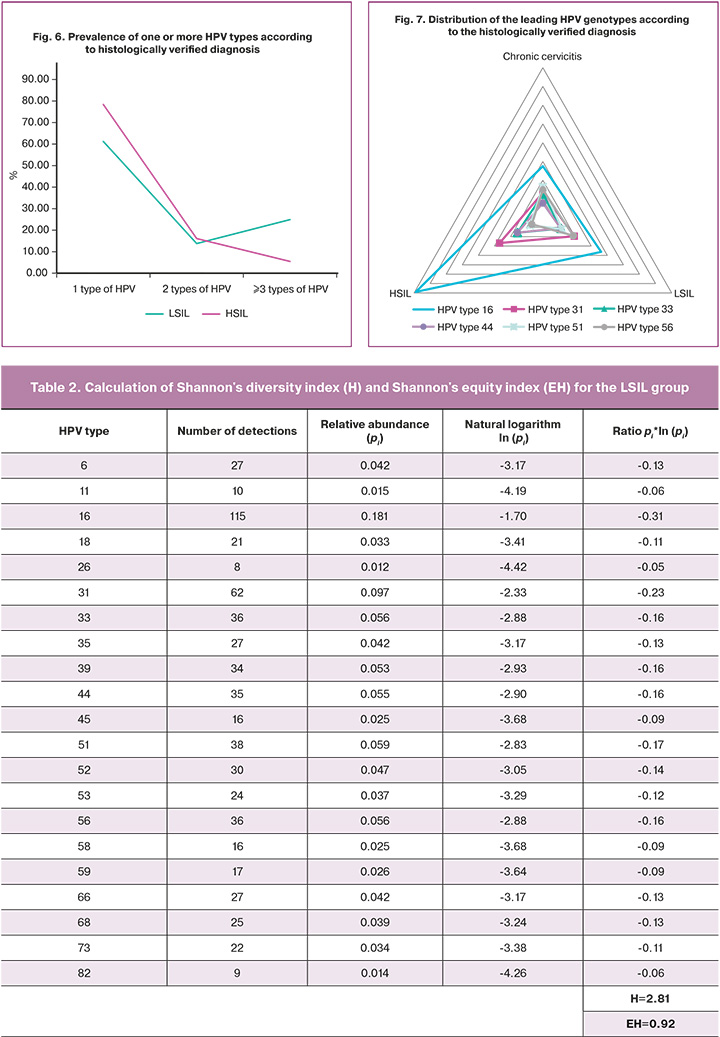
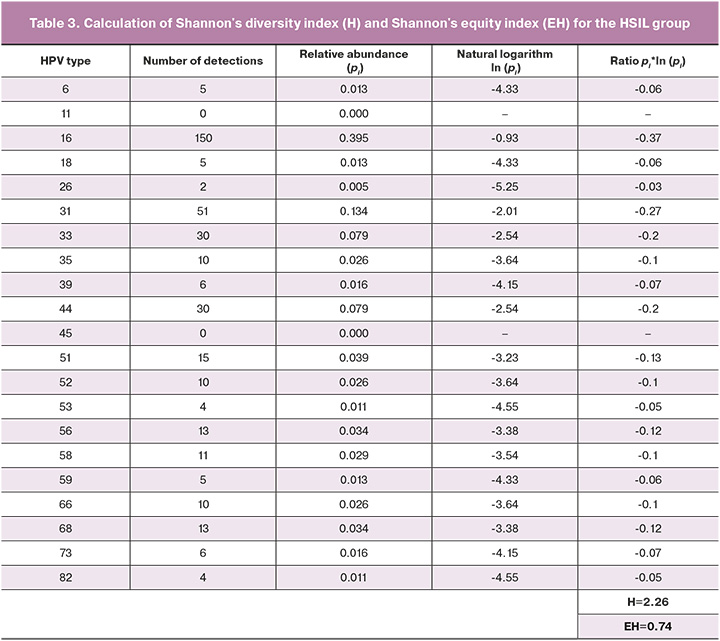
Analysis of the distribution pattern of the leading HPV genotypes in the hierarchy of association with histologically verified diagnosis showed that 51 HPV types tended to decrease in their representation with increasing severity of cervical lesions (21/260 (8.3%) cases with chronic cervicitis versus 38/635 (5.9%) cases with LSIL versus 15/380 (3.9%) cases with HSIL). However, in cases where HPV types 16, 31, and 44 were detected, a well-defined inverse relationship was observed; the highest prevalence of these types was found in cases with HSIL and the lowest in cases with chronic cervicitis. The distribution of HPV type 16 in the histological findings was as follows: chronic cervicitis (13.5%), LSIL (18.1%), and HSIL (39.5%). At the same time, HPV genotype 16 was statistically significantly more frequently detected in Group 3 than in groups 1 and 2 (p<0.01). The distribution of HPV type 31 followed the same trend in chronic cervicitis (6.7%), LSIL (9.8%), and HSIL (13.4%). Moreover, 31 HPV genotypes were significantly less common in Group 1 than in groups 2 and 3 (p<0.01 and p<0.05, respectively). It should be noted that the distribution of HPV type 44 also showed an increased prevalence depending on the cervical lesion grade: 3.9% for chronic cervicitis, 5.5% for LSIL, and 7.9% for HSIL. However, no statistically significant differences in the prevalence of the 44 HPV genotypes were found between groups (p>0.05) (Fig. 7).
A more in-depth study of the data showed that the structure of HPV diversity changes depending on a histologically verified diagnosis. Thus, the three leading HPV genotypes had different cumulative distributions: 75/260 (28.8%) for chronic cervicitis, 215/635 (33.9%) for LSIL, and 231/380 (60.8%) for HSIL. As part of the study, we analyzed the diversity of HPV types for each group by calculating the Shannon diversity index (H) and Shannon equity index (EH). The diversity index score was 2.61 for the chronic cervicitis group, 2.81 for the LSIL group, and 2.26 for the HSIL group. The higher the "H" index, the higher the type diversity in the sample. The Shannon fairness index reflects the uniformity of the prevalence of all the types in the studied sample. The values of the "EH" index can range from 0 to 1. A value of 1 indicates absolute uniformity of the distribution. Thus, for the chronic cervicitis group, the fairness index value was 0.85, for the LSIL group – 0.92, and for the HSIL group – 0.74. Thus, the least pronounced diversity of detectable HPV genotypes was observed in the HSIL group, combined with minimal evenness of distribution among all studied groups (Tables 1–3).
Discussion
Our results indicate marked heterogeneity in the representation of HPV genotypes, both in the general population and in different histological groups. Additionally, we obtained unique data regarding the distribution of HPV genotypes, which are not commonly observed by researchers in other countries. For instance, while HPV type 16 remains the most prevalent, types 44 and 31, which follow, significantly stand out among the other dominant genotypes described in similar studies [8–10, 12, 14]. This variation may be attributed to geographical differences, resulting in variations in the distribution of HPV genotypes even within regions of the same country [15].
Detection of multiple HPV genotypes is a matter of significant concern because of the ambiguous interpretation of the clinical picture described above. Two opposing theories exist regarding the prognosis associated with the detection of 2 or more HPV types in relation to the development of precancerous lesions of the cervix. For instance, our data indicated that multiple genotypes detected in a sample were more likely to be associated with LSIL (24.9%) than HSIL (5.5%). These findings are consistent with those of Bruno et al., in which cervical intraepithelial neoplasia (CIN) I accounted for 60.4% of cases with multiple HPV genotypes, CIN II accounted for 43.7%, and CIN III accounted for 22% [13]. However, contradictory results have been reported in literature. For example, a recently published Korean study found that HSIL was significantly more associated with the detection of multiple HPV types compared to the detection of a single genotype (33.2% vs. 18.3%) [12]. Notably, the distribution of HPV genotypes and their potential relevance to the development of more severe grades of cervical lesions should also be highlighted [16–19]. In our sample, HPV types 16, 31, and 44 were not only the most common types found in the general population but also the most strongly associated with progression to squamous intraepithelial lesions of the cervix. The three genotypes exclusively exhibited this pattern in our sample.
It is important to note that data on biodiversity and distribution among detectable HPV genotypes are scarce in the current literature. However, these parameters are crucial indicators of the internal hierarchical HPV processes. Our findings indicate that the diversity index and uniformity of HPV type distribution decrease with increasing lesion severity (0.92 for LSIL vs. 0.74 for HSIL). Such values may indirectly support the hypothesis that the simultaneous detection of several HPV types is more favorable in terms of prognosis than the detection of a single genotype.
Conclusion
Our data demonstrated significant variability in the distribution, prevalence, biodiversity indices, and evenness of different HPV genotypes across different grades of cervical lesions, indicating the association of certain HPV types with specific stages of SIL. The results of our study strongly indicate that the detection rate of multiple HPV types decreases as the grade of the cervical lesions increases. Nevertheless, further research is needed to consider additional factors related to HPV distribution and potential pathogenicity in order to definitively address this issue.
References
- Nelson C.W., Mirabello L. Human papillomavirus genomics: Understanding carcinogenicity. Tumour Virus Res. 2023;15:200258. https://dx.doi.org/10.1016/10.1016/j.tvr.2023.200258.
- Sun J.X., Xu J.Z., Liu C.Q., An Y., Xu M.Y., Zhong X.Y. et al. The association between human papillomavirus and bladder cancer: Evidence from meta-analysis and two-sample mendelian randomization. J. Med. Virol. 2023; 95(1): e28208. https://dx.doi.org/10.1002/jmv.28208.
- Добровольская Д.А., Байрамова Г.Р., Асатурова А.В., Теврюкова Н.С. Прогностическая значимость биомаркеров вируса папилломы человека в дифференциальной диагностике плоскоклеточных интраэпителиальных поражений шейки матки. Акушерство и гинекология. 2022;6:20-5. [Dobrovolskaya D.A., Bairamova G.R., Asaturova A.V., Tevryukova N.S. Prognostic value of human papillomavirus biomarkers in the differential diagnosis of squamous intraepithelial lesions of the cervix. Obstetrics and Gynecology. 2022;(6):20-5. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.6.20-25.
- Bedell S.L., Goldstein L.S., Goldstein A.R., Goldstein A.T. Cervical cancer screening: past, present, and future. Sex Med. Rev. 2020;8(1):28-37. https://dx.doi.org/10.1016/j.sxmr.2019.09.005.
- Lee J.E., Chung Y., Rhee S., Kim T.H. Untold story of human cervical cancers: HPV-negative cervical cancer. BMB Rep. 2022;55(9):429-38. https://dx.doi.org/10.5483/BMBRep.2022.55.9.042.
- National Institute of Allergy and Infectious Diseases. PaVE: the papillomavirus episteme. Release Note, 19 May 2023. https://pave.niaid.nih.gov/release_notes
- Министерство здравоохранения Российской Федерации. Клинические рекомендации «Цервикальная интраэпителиальная неоплазия, эрозия и эктропион шейки матки». 2020. [Ministry of Health of the Russian Federation. Clinical guidelines "Cervical intraepithelial neoplasia, erosion and ectropion of the cervix". 2020. (in Russian)].
- Farahmand M., Moghoofei M., Dorost A., Abbasi S., Monavari S.H., KianiS.J., Tavakoli A. Prevalence and genotype distribution of genital human papillomavirus infection in female sex workers in the world: a systematic review and meta-analysis. BMC Public Health. 2020;20(1):1455. https://dx.doi.org/10.1186/s12889-020-09570-z.
- Yang X., Li Y., Tang Y., Li Z., Wang S., Luo X. et al. Cervical HPV infection in Guangzhou, China: an epidemiological study of 198,111 women from 2015 to 2021. Emerg. Microbes Infect. 2023; 12(1):e2176009. https://dx.doi.org/10.1080/22221751.2023.2176009.
- Derbie A., Mekonnen D., Yismaw G., Biadglegne F., Van Ostade X., Abebe T. Human papillomavirus in Ethiopia. Virusdisease. 2019;30(2):171-9. https://dx.doi.org/10.1007/s13337-019-00527-4.
- Yang-Chun F., Yuan Z., Cheng-Ming L., Yan-Chun H., Xiu-Min M. Increased HPV L1 gene methylation and multiple infection status lead to the difference of cervical epithelial cell lesion in different ethnic women of Xinjiang, China. Medicine (Baltimore). 2017;96(12):e6409. https://dx.doi.org/10.1097/MD.0000000000006409.
- Kim M., Park N.J., Jeong J.Y., Park J.Y. Multiple human papilloma virus (HPV) infections are associated with HSIL and persistent HPV infection status in Korean patients. Viruses. 2021;13(7):1342. https://dx.doi.org/10.3390/v13071342.
- Bruno M.T., Scalia G., Cassaro N., Boemi S. Multiple HPV 16 infection with two strains: a possible marker of neoplastic progression. BMC Cancer. 2020; 20(1):444. https://dx.doi.org/10.1186/s12885-020-06946-7.
- Wang X., Zeng Y., Huang X., Zhang Y. Prevalence and genotype distribution of human papillomavirus in invasive cervical cancer, cervical intraepithelial neoplasia, and asymptomatic women in Southeast China. Biomed. Res. Int. 2018;2018:2897937. https://dx.doi.org/10.1155/2018/2897937.
- Xia C., Li S., Long T., Chen Z., Chan P.K.S., Boon S.S. Current updates on cancer-causing types of human papillomaviruses (HPVs) in East, Southeast, and South Asia. Cancers (Basel). 2021;13(11):2691. https://dx.doi.org/10.3390/cancers13112691.
- Zhang J., Cheng K., Wang Z. Prevalence and distribution of human papillomavirus genotypes in cervical intraepithelial neoplasia in China: a meta-analysis. Arch Gynecol Obstet. 2020;302(6):1329-37. https://dx.doi.org/10.1007/s00404-020-05787-w.
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021:71(3):209-49. https://dx.doi.org/doi:10.3322/caac.21660.
- Inturrisi F., Bogaards J.A., Heideman D.A.M., Meijer C.J.L.M., Berkhof J. Risk of cervical intraepithelial neoplasia grade 3 or worse in HPV-positive women with normal cytology and five-year type concordance: a randomized comparison. Cancer Epidemiol. Biomarkers Prev. 2021; 30(3):485-91. https://dx.doi.org/10.1158/1055-9965.
- Костава М.Н. ВПЧ-ассоциированные заболевания в вопросах и ответах. Акушерство и гинекология. 2021; 11: 267-76. [Kostava M.N. HPV-associated diseases: questions and answers. Obstetrics and Gynecology. 2021;(11):267-76. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.11.267-276.
Received 27.09.2023
Accepted 09.10.2023
About the Authors
Alexander O. Andreev, Ph.D. Student, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(903)613-00-26, sasha.grash2010@yandex.ru,https://orcid.org/0000-0002-9835-440X, 117997, Russia, Moscow, Ac. Oparin str., 4.
Guldana R. Bayramova, Dr. Med. Sci., Merited Doctor of the Russian Federation, Professor at the Department of Obstetrics and Gynecology of the Department of Professional Education, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, Clinical Care Supervisor at the Research and Outpatient Department, V.I. Kulakov
NMRC for OG&P, Ministry of Health of Russia, +7(926)660-48-77, bayramova@mail.ru, https://orcid.org/0000-0003-4826-661X, 117997, Russia, Moscow, Ac. Oparin str., 4.
Natalya A. Ilyasova, Researcher at the Department of International Cooperation, Obstetrician-Gynecologist at the Research and Outpatient Department, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, natalia_ilyasova@mail.ru, https://orcid.org/0000-0003-0665-3515, 117997, Russia, Moscow, Ac. Oparin str., 4.
Alexandra V. Asaturova, Dr. Med. Sci., Head of the 1st Anatomic Pathology Department, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
a_asaturova@oparina4.ru, https://orcid.org/0000-0001-8739-5209, 117997, Russia, Moscow, Ac. Oparin str., 4.
Dmitry Yu. Trofimov, Corresponding Member of the RAS, Professor, Dr. Bio. Sci., Director of the Institute of Reproductive Genetics, V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia, +7(495)438-49-51, d_trofimov@oparina4.ru, 117997, Russia, Moscow, Ac. Oparin str., 4.



