Regional experience with virological cervical screening in the Sverdlovsk Region
Objective. To evaluate the clinical sensitivity and specificity of a care system for human papillomavirus (HPV) for the identification of its DNA in an organized screening program versus conventional cytology in the remote areas of the Sverdlovsk Region.Kononova I.N., Bashmakova N.V., Gaeva O.E., Krestyaninova T.V.
Subjects and methods. A total of 857 women underwent cervical screening by the hybrid capture HPV DNA test using the careHPV testing system, with a parallel cytological examination by the conventional method, followed by a colposcopic, pathomorphological study in patients with a positive HPV test and/or altered cytological patterns.
Results. A high-titer high-risk HPV (hr-HPV) positive test using the careHPV diagnostic system was seen in 84 patients: 35 (41.67%) hrHPV-positive women were found to have cervical precancer and cancer, while 16 (2.07%) hrHPV-negative patients with the visually altered cervix had cervical intraepithelial neoplasia 1 (CIN 1). Virological screening was ascertained to have a significantly higher sensitivity in high-grade CIN and cervical cancer (CC), which demonstrates the feasibility of using this method during CC screening in the remote areas.
Conclusion. This investigation and calculations of screening methods demonstrated that the hybrid capture test using the careHPV diagnostic system versus conventional cytology had a significantly higher sensitivity in identifying high-grade CIN and CC, but cytology had a higher specificity.
Keywords
Cervical cancer (CC) is one of the most preventable and curable forms of cancer if detected at the stage of cervical intraepithelial neoplasia (CIN). Cervical cancer is the second most common genital cancer among women worldwide, with an estimated annual 400,000 new cases and more than 200,000 deaths. According to the Ministry of Health of the Russian Federation, there were 15,342 new cases of cervical cancer in Russia in 2016. The incidence of cervical cancer has increased significantly, especially in women of reproductive age [3]. Of the total number of patients with pre-invasive malignancy in Russia (7,187), the majority (4,318) were women with cervical cancer [4].
It has been known since 1995 that the human papillomavirus (HPV) DNA is present in all cases of cervical cancer [5], often combined with the anal, vaginal and vulvar forms both in patients and their partners [6, 7]. Worldwide, HPV is found in almost all cervical carcinomas, and 65.0 - 87.0% of precancerous diseases [8]. Persistent HPV is a major risk factor for the progression of neoplastic transformation due to virogeny, the integration of HPV DNA into the host genome and the synthesis of E7 and E6 oncoproteins with resulting in local immune dysfunctions. At the same time, the presence of high-risk types of HPV in cervical smears with normal cytomorphology increases the risk of developing CIN III by a factor of 116 [9, 10, 11].
The currently existing screening strategy with cytology does not lead to the expected early detection of cervical cancer [12, 13]. Numerous studies published over the past 15 years have demonstrated the value of HPV DNA testing during primary cervical cancer screening because HPV is diagnosed longer than cytological changes [14]. It has been systematically concluded that HPV DNA testing is more sensitive than cytology for identifying women with cervical cancer. In some countries, HPV testing is used as the main screening test, or a combination of HPV and cytology tests [15, 16]. The active introduction of cervical cancer screening in European countries and debatable questions of cervical cancer screening in Russia and across the globe create the prerequisites for creating a unified diagnostic and prognostic algorithm to optimize the diagnosis and treatment of this malignancy.
The digene HC2 HPV DNA test (QIAGEN) is widely used in screening for cervical cancer in cities with a high level of medical services that are not available in rural, remote areas or in low- and middle-income settings [17]. To address the unmet need for HPV testing in such low resource areas, Qiagen developed a new rapid HPV test (careHPV) based on hybrid capture technology (Hybrid Capture 2 = HC2). The test is designed for the qualitative detection of 14 high-risk types of HPV DNA (HR-HPV) in cervical and vaginal specimens; the test takes only 2.5 hours to perform. This fast, portable, and easy-to-use test does not require large separate facilities. Clinical studies conducted in the European Union showed that its sensitivity in detecting precancerous cervical lesions was identical to that of the digene HC2 HPV DNA Test (QIAGEN) [18]. We conducted a cross-sectional study to evaluate the diagnostic accuracy of the careHPV system as a rapid screening test in the provincial city of the Sverdlovsk region.
The study aimed to investigate the sensitivity and specificity of careHPV test for detection of HPV DNA in an organized screening program, compared to conventional cytology in remote areas of the Sverdlovsk region.
Materials and methods
Women aged 30 to 60 years and living the town of Verkhnyaya Pyshma of the Sverdlovsk region with a population of 50,132 people were invited to participate in the study. The inclusion criteria for the study patient selection were as follows: women aged 30–60, informed consent to undergo virologic and cytologic cervical cancer screening. Exclusion criteria were: a pregnancy, a history of hysterectomy, a history of previously diagnosed or treated CIN, HIV-positive status. Potential participants received a study information booklet; written informed consent was obtained from all patients enrolled in the study. Cervical specimens for cytologic examination were collected using both the conventional cytobrush on a glass slide and a careBrush in a test tube containing the careHPV Collection Medium. Before the examination, the test tubes were placed in a refrigerator. After collecting 90 tubes, a single-step careHPV test was performed at the maternity clinic in Verkhnyaya Pyshma. Cytological samples were examined independently of DNA testing in the cytological laboratory in accordance with the Papanicolaou classification with adaptation for adequate analysis to the Bethesda system for reporting cervical cytology: normal cytology, atypical squamous cells of unspecified significance (ASCUS), characterizing the boundary state or equivalent, LSIL - low-grade squamous intraepithelial lesion; the HPV effect corresponds to mild dysplasia (CIN I), HSIL to moderate dysplasia and severe dysplasia (CIS) or CIN II and CIN III; cervical cancer. All women with abnormal HPV-test and cervical cytology underwent colposcopy at the maternity clinic of the Verkhnyaya Pyshma CCH. The findings were interpreted according to the colposcopic terminology of the IFCPC (International Federation for Cervical Pathology and Colposcopy) 2011 Classification. Patients with abnormal colposcopic findings underwent cervical biopsy with histologic examination of the obtained biopsy specimens. After examination, the findings were assessed according to the histological classification as normal, CIN I (LSIL), CIN II, CIN III (HSIL), and cervical cancer.
Statistical analysis was performed using SPSS Statistics version 20.0 software. Categorical variables (reported as detection rates) were compared using the two-sided Fisher’s exact test; p values of <0.05 were considered to be statistically significant. Sensitivity, specificity, – PV (negative predictive value), + PV (positive predictive value), and diagnostic accuracy of diagnostic tests.
Sensitivity is the proportion of people with the disease who have a positive test for the disease. Specificity is the proportion of people without the disease who have a negative test. The calculations are as follows:
Sensitivity = a / (a+c);
Specificity = d/ (d+b),
Note: a – true positive results, b - false-positive results, d - true negative results; с - false-negative results.
Positive predictive value is the probability of disease in a patient with a positive (abnormal) test result + PV = a / (a + b). Negative predictive value is the probability of not having the disease when the test result is negative (normal) test result -PV = d / (c + d). The diagnostic accuracy is the proportion of true results among all the results: [19].
Diagnostic accuracy =(a+d)/ (a+b+c+d) [19].
The sensitivity and specificity were evaluated using a ROC analysis with the calculation of the area under the ROC curve.
Results and discussion
A total of 857 women were included in the screening program. The careHPV test for HR-HPV was positive in high titers in 84 (9.8%) women. Further colposcopic and histological examinations showed that these HR-HPV-positive women were significantly more likely to have CIN and cervical cancer than HPV-negative women. It should be noted that the majority of HPV-positive women had high grade precancerous or cancerous cervical lesions. It is noteworthy that CIN was also detected in HPV-negative women with abnormal findings on visual inspection of the cervix. However, they mainly had CIN I (Table 1). Our findings did not differ significantly from the results obtained in the European countries and demonstrated the key role of human papillomavirus infection in the development of high-grade cervical neoplasia [20].
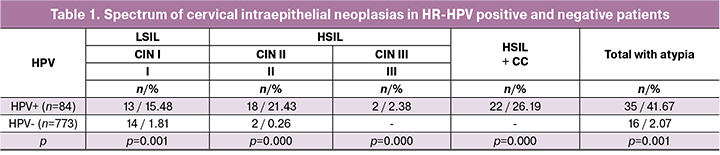
The specificity and sensitivity of the careHPV test were significantly higher for the detection of high-grade cervical neoplasia and cervical cancer (p = 0.002 for CINII, p = 0.001 for CIN III, and p = 0,000 for cervical cancer), which is in line with studies conducted in other Eurasian countries [21]. Of note is high both positive and negative predictive values of the careHPV test. The area under the ROC curve for CIN III was 0.900 ± 0.016 with 95% CI: [0.623 ÷ 0.969] with p = 0.001, which corresponds to high diagnostic test accuracy (table 2).
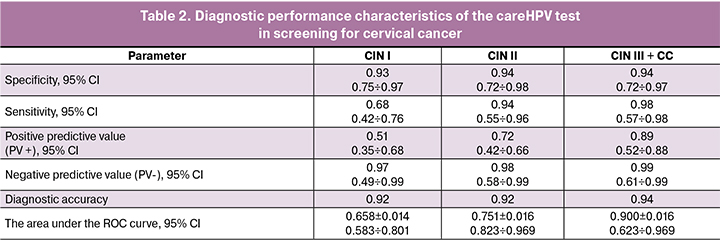
There was a significant difference in detecting cervical precancerous lesions between conventional cervical cytology and virological examination confirmed by cervical morphology (p = 0.002) (Table 3).
The cervical cytology identifies such a lesion as ASCUS, which requires further monitoring of the state of the exo-and endocervix. Analysis of HSIL + cervical cancer showed a significant discrepancy between the results of conventional cervical cytology and virological examination (p = 0.004), which also in favor of using virological screening for HSIL and cervical cancer as primary screening in remote areas. The literature has reported that 50-60% of CIN I cases are highly likely to show spontaneous regression. These observations indicate the advisability of detecting only high-grade CINs in remote areas, since CIN I is not considered as a precancerous condition by foreign researchers, and the issue of treatment of CIN I is debatable in the Russian Federation because it requires substantial health care spending. Since the concept of cervical cancer prevention in the Russian Federation includes the treatment of LSIL for preventing cervical cancer, it is feasible to use co-screening in megapolis territories, which allows higher sensitivity and specificity for detecting CIN I-III and administer adequate personalized therapy in combination with physio medical treatments.
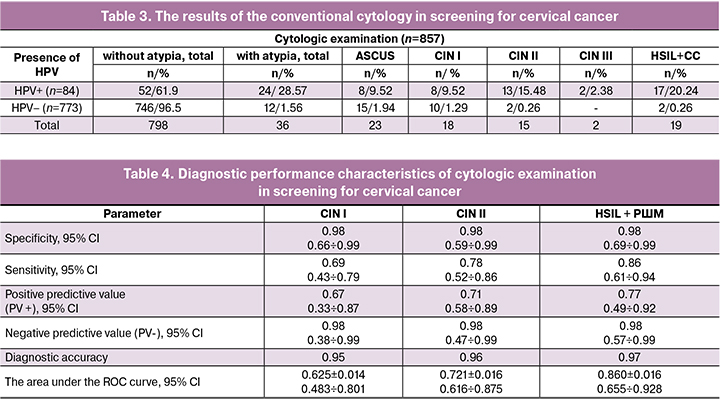
The calculation of the specificity and sensitivity of conventional cervical cytology demonstrated a significantly higher specificity of the method compared to virological screening; this observation did not differ significantly from the results of studies conducted in the European countries [22]. Sensitivity for the detection of high-grade CIN was higher; however, it was significantly lower than that of virological screening (Table 4).
The algorithm of the management strategy of patients with identified precancerous cervical lesions involved complex therapy including the correction of vaginal microbiocenosis based on vaginal pH monitoring using vaginal PH balance test strips and antiviral and immunomodulating therapy with ultrasonically cavitated solutions of peptide immune regulators [23]. After the control examination, CIN I lesions were subjected to argon plasma ablation by the Fotek device. All patients with high-grade neoplasia underwent microbiocenosis correction followed by electrosurgical excision or conization and received rehabilitation to accelerate healing.
Conclusion
Our findings on diagnostic accuracy of cervical cancer screening tests showed that the sensitivity of the careHPV test in detecting CIN II, CIN III and cervical cancer was significantly higher than that of conventional cervical cytology, thus implying the feasibility of using this method in cervical cancer screening programs, especially in remote areas of the Russian Federation.
References
- Tang Y., Zheng L., Yang S., Li B., Su H., Zhang L.P. Epidemiology and genotype distribution of human papillomavirus (HPV) in Southwest China: a cross-sectional five years study in non-vaccinated women. Virol. J. 2017; 14(1): 84.
- Обоскалова Т.А., Кононова И.Н., Севостьянова О.Ю., Берзин С.А. Эпидемиологические особенности рака шейки матки у жительниц крупного промышленного города. Уральский медицинский журнал. 2014; 4: 69-72. [Oboskalova T.A., Kononova I.N., Sevostyanova O.Yu., Berzin S.A. Epidemiological features of cervical cancer in women of a large industrial city. The Urals Medical Journal. 2014; 4(118):69–72 (In Russ.)].
- WHO/ICO Information Centre on HPV and Cancer. Russian Federation: Human Papillomavirus and Related Cancers, Fact Sheet 2016. Available at: http//www.hpvcentre.net
- Аксель Е.М., Виноградова Н.Н.. Статистика злокачественных новообразований женских репродуктивных органов. Онкогинекология. 2018; 3: 64-9. [Aksel Е.M., Vinogradova N.N. Statistics of malignant neoplasms of female reproductive organs. Oncogynecology. 2018; 3: 64-69. (In Russ.)]
- Zur Hausen H. Are human papillomavirus infections not necessary or sufficient causal factors for invasive cancer of the cervix? Int. J. Cancer. 1995; 63(2): 315-6.
- Heard I., Etienney I., Potard V. et al. Factors associated with prevalent anal cancer precursors in asymptomatic HIV-infected women. In: Abstracts 15th World congress for cervical pathology and colposcopy. 26 May-30 May London 2014: 40-1.
- Macbalek D.A., Poynten M., Jin F., Fairley C.K., Farnsworth A., Garland S.M. et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol. 2012; 13(5): 487-500.
- Castle P.E., Qiao Y.L., Zhao F.H., Chen W., Valdez M, Zhang X. et al. Clinical determinants of a positive visual inspection after treatment with acetic acid for cervical cancer screening. BJOG. 2014; 12(6): 739-46.
- Кононова И.Н., Ворошилина Е.С. Особенности местного иммунитета при цервикальных интраэпителиальных неоплазиях, ассоциированных с папилломавирусной инфекцией. Российский иммунологический журнал. 2014; 8(3): 809-11. [Kononova I.N., Voroshilina E.S. Features of local immunity in cervical intraepithelial neoplasia associated with papillomavirus infection. Russian Immunological Journal. 2014; 8.3(17): 809-811.(In Russian)].
- Fontecha N., Arrese E., Andia D., Cisterna R., Basaras M. RNA extraction method is crucial for human papillomavirus E6/E7 oncogenes detection. Virol. J. 2017; 14(1): 50.
- Кононова И.Н. Эпидемиология папилломавирусной инфекции в крупном промышленном городе. Охрана материнства и детства (Витебск). 2015; 1: 9-13. [Kononova I.N. Epidemiology of papillomavirus infection in a large industrial city. Protection of motherhood and childhood. Belarus (Vitebsk) . 2015; 1(25): 9-13.(In Russ.)].
- Рекомендации ВОЗ по выявлению и лечению предраковых поражений для профилактики рака шейки матки. М.; 2013. [WHO recommendations on the detection and treatment of precancerous lesions for the prevention of cervical cancer/2013; 27.(In Russ.)]
- Blatt A.J., Kennedy R., Luff R., Austin R.M., Rabin D.S. Comparison of cervical cancer screening results among 256,648 women in multiple clinical practices. Cancer Cytopathol. 2015; 123(5): 282-8.
- Keegan H., Mc Inerney J., Pilkington L., Grønn P., Silva I., Karlsen F. et al. Comparison of HPV detection technologies: Hybrid Capture 2, PreTect HPV-Proofer and analysis of HPV DNA viral load in HPV16, HPV18 and HPV33 E6/E7 mRNA positive specimens. J. Virol Methods. 2009; 155(1): 61-6.
- Monteiro C., Rebelo T., Figueiredo Dias M. New molecular markers in HPV infection risk stratification: systematic review. In: Abstracts 18th International multidisciplinary HPV congress EUROGIN. Lisbon, Portugal, December 2018.
- Varl J., Ivanus U., Jerman T., Nolde N., Prevodnik V.K. Clinical significance of HC2 Test results in grej zone range used in Slovenian cancer screening program zora. In: Abstracts 18th International multidisciplinary HPV congress EUROGIN. Lisbon, Portugal, December 2018.
- Meijer C.J., Berkhof J., Castle P.E., Hesselink A.T., Franco E.L., Ronco G. et al. Guidelines for human papillomavirus DNA test requirements for primary cervical cancer screening in women 30 years and older. Int. J. Cancer. 2009; 124(3): 516-20.
- Carozzi F.M., Del Mistro.A., Confortini.M., Sani.C., Puliti.D., Trevisan.R. еt al. Reproducibility of HPV DNA testing by hybrid capture 2 in a screening setting. Am. J. Clin. Pathol. 2005; 124(5): 716-21.
- Флетчер Р., Флетчер С., Вагнер Э. Клиническая эпидемиология. Основы доказательной медицины. М.: Медиа Сфера; 1998. 352 с. [Fletcher R.H., Fletcher S.W., Wagner E.H. Clinical epidemiology. The essentials. 3rd ed. Williams &Wilkins; 1996.]
- Wright T., Zhang G., Behrens C.M. Screening with prejudice; how knowledge of patients HPV status impacts the performance of cervical cytology in the ATHENA trial. In: Abstracts 15th World congress for cervical pathology and colposcopy. 26 May-30 May London2014: 56-7.
- Camara Hawa, Nosi Somu, Guy Rebecca, Lafferty Lise, Vallely Andrew, Kelly-Hanku Angela. Apceptability of self-collected POC HPV-based testing: a qualitative meta-synthesis. In: Abstracts 18th International multidisciplinary HPV congress EUROGIN. Lisbon, Portugal, December 2018.
- Coutinho F., Coutinho L., Oliveira J., Ramos V., Carvalho MJ., Caramero O. et al. Atypycal glandular cells on pap testing and its correlations to HPV. Abstracts 18th International multidisciplinary HPV congress EUROGIN. Lisbon, Portugal, December 2018.
- Обоскалова Т.А., Кононова И.Н., Ворошилина Е.С. Иммунокоррекция кавитированными ультразвуком растворами в комплексном лечении цервикальных интраэпителиальных неоплазий, ассоциированных с папилломавирусной инфекцией. Уральский медицинский журнал. 2013; 4: 46-51. [Oboskalova TA, Kononova IN, Voroshilina E.S. Immunocorrection by ultrasonically cavitated solutions in the complex treatment of cervical intraepithelial neoplasias associated with papillomavirus infection. The Urals Medical Journal. . 2013; 4(109): 46-51. (In Russian)].
Received 13.08.2018
Accepted 21.09.2018
About the Authors
Kononova Irina N., MD, associate professor, acting Leading Researcher, Head of the Training Center of the ««Ural Research Institute for Maternal and Child Care»» of the Ministry of Health of Russia. Phone: + 7 (343) 371-8911; e-mail: irkonmed@mail.ru620028 Russia, Yekaterinburg, ul. Repin, d.1.
Bashmakova Nadezhda V., MD, professor, chief obstetrician-gynecologist of the Ural Federal District, chief researcher of the «Ural Research Institute for Maternal and Child Care» of the Russian Ministry of Health, Honored Doctor of the Russian Federation, Chief Obstetrician-Gynecologist of the Ural Federal District. Tel .: +7(343)371-8768; e-mail: dr@niiomm.ru. 620028 Russia, Yekaterinburg, ul. Repin, d.1.
Gaeva Oksana E., graduate student obstetrician-gynecologist of the Federal State Budgetary Institution «Ural Research Institute for Maternal and Child Care» of the Russian Ministry of Health. E-mail: eletty@mail.ru. 620028 Russia, Yekaterinburg, ul. Repin, d.1.
Krest'yaninova Tamara V., doctor obstetrician-gynecologist, head. Women's consultation GBUZ SB «Vyshnepyshminsky central city hospital named after P.D. Borodin»
Phone: + 7 (922) 149-2119. 624090 Russia, Sverdlovsk region, Verkhnyaya Pyshma, ul. Tchaikovsky, d. 32.
For citation: Kononova I.N., Bashmakova N.V., Gaeva O.E., Krestyaninova T.V. Regional experience with virological cervical screening in the Sverdlovsk Region.
Akusherstvo i ginekologiya/Obstetrics and gynecology. 2019; 8: 84-89(in Russian).
https://dx.doi.org/10.18565/aig.2019.8.84-89



