Predicting response toneoadjuvant chemotherapy in cervical cancer: possibilities of radiomic analysis of MR images
Solopova А.Е., Bendzhenova B.B., Khokhlova S.V.
Objective: To develop and validate a radiomic model for predicting tumor response to neoadjuvant chemotherapy (NACT) in patients with locally advanced cervical cancer (LACC) based on MR imaging.
Materials and methods: A total of 182 patients aged 27 to 49 years with disease stages IB3-IIB and IIIC1 (two patients with metastatic pelvic lymph node involvement) according to FIGO 2018 were retrospectively enrolled in the study. The patients underwent treatment at the Academician Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow from 2018 to 2023. Radiomic parameters were extracted from T2-weighted (T2-WI) and diffusion-weighted (DWI) MR images. The most statistically significant characteristics were selected using LASSO (Least Absolute Shrinkage and Selection Operator) linear regression. The construction of the prediction model was based on a combination of radiomic signs and clinical data. In order to assess the prognostic effectiveness and clinical benefit of the developed model, ROC and decision curve analysis were used.
Results: Two models for predicting response to NACT were developed in the study. The first model included isolated radiomic signs and demonstrated a sensitivity of 79.6% in the main and 85.7% in the test group, specificity 80.6% and 72.1%, respectively. AUC was 0.90 in the main group and 0.83 in the validation group. In order to improve the quality of the model, clinical data (maximum linear size and degree of tumor differentiation, age of the patients, presence of metastatic lymphatic nodes) were included in the nomogram in addition to radiomic parameters. Radiomic nomogram showed sensitivity of 87.8% and 71.4%; specificity of 88.8% and 97.7% in the main and test groups, respectively. AUC was 0.96 and 0.94.
Conclusion: The constructed radiomic model is effective in predicting the therapeutic response of NACT in cervical cancer.
Authors’ contributions: Solopova A.E. – developing the concept of the publication; Bendzhenova B.B., Solopova A.E. – collecting and analyzing the sources, writing, editing the text of the article; Khokhlova S.V. – collecting and processing the material, checking the critical content, approving the final version of the article.
Conflicts of interest: The authors declare that there are no conflicts of interest.
Funding: The work was supported by the Russian Science Foundation grant “Development of technology for objective assessment of tumor progression potential in cervical cancer in order to create new approaches to the study of individual pathogenetic mechanisms of resistance to therapy using quantitative imaging methods, search for markers of treatment efficacy using mathematical modeling on real clinical data.” (No. 23-25-00445 dated of 24.01.2023).
Ethical Approval: The study was approved by the Ethical Review Board of Academician V.I. Kulakov National Medical Research Centre for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
Patient Consent for Publication: All patients participating in the study provided an informed consent for the publication of their data and associated images.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Solopova А.Е., Bendzhenova B.B., Khokhlova S.V.
Predicting response toneoadjuvant chemotherapy in cervical cancer: possibilities of radiomic analysis of MR images.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (12): 139-147 (in Russian)
https://dx.doi.org/10.18565/aig.2024.311
Keywords
Cervical cancer is the fourth most common cancer in the female reproductive system [1]. According to the International Federation of Gynecology and Obstetrics (FIGO 2018), the choice of treatment tactics usually depends primarily on the stage of the disease at the time of diagnosis. Treatment of early stages of cervical cancer (IA1, IA2, IB1, IB2, and IIA1) consists of surgery; the standard treatment of locally advanced cervical cancer (LACC) is chemoradiotherapy or neoadjuvant chemotherapy (NACT) followed by extended extirpation of the uterus [2]. Despite significant advances in chemotherapy for LACC, the recurrence rate remains high and reaches 35% at all stages of the disease. The side effects associated with radiation (hematologic and gastrointestinal toxicity, formation of urogenital vaginal and rectovaginal fistulas, etc.) also persist [3]. NACT for LACC usually leads to a reduction in tumor size and parametrial infiltration; therefore, radical surgery can be performed, which may improve long-term treatment outcomes. Radical surgical interventions can remove tumor clones in the second stage and reduce the incidence of adjuvant radiation therapy and associated potential post-radiation complications. The evaluation of the efficacy of NACT is based on the same methods that were used for the initial diagnosis. The FIGO (2018) staging system provides different diagnostic methods depending on their availability: ultrasonography, magnetic resonance imaging (MRI), computed tomography (CT) and positron emission tomography (PET-CT). MRI is the most preferable method at the stage of cervical cancer staging due to its high informative value in assessing the extent of the tumor process. The place and significance of MRI in the diagnosis and initial evaluation of cervical cancer and in the assessment of treatment efficacy are reviewed in the European Society of Urogenital Radiology (ESUR) guidelines from 2019 and by the experts on radiotherapy diagnosis of cervical cancer [4, 5].
Radiomics is a method that performs automated quantitative analysis of extracted data from medical images. The parameters potentially associated with tumor heterogeneity and aggressiveness can be extracted with the help of this method. Currently, there are no effective methods for predicting treatment response in patients with LACC after NACT, which remains a relevant clinical problem. Therefore, radiomic data obtained using MR imaging sequences as part of a standard protocol of the study performed at the initial staging phase can serve as a valuable additional non-invasive tool not only in the assessment of treatment efficacy but also in its prediction before the beginning of therapy.
The aim of the study was to develop and validate a model for predicting tumor response to NACT in patients with LACC.
Materials and methods
The clinical data (age, the FIGO stage of disease, degree of differentiation and maximum linear tumor size) and MRI findings of 236 female patients were included and analyzed in the study. All the patients had histologically verified LACC; they underwent NACT at the National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow, from 2018 to 2023.
The inclusion criteria were the presence of LACC in patients who underwent NACT at the Centre, disease stages IB3-IIB and IIIC1 (two patients had metastatic changes in pelvic lymph nodes), MRI of the pelvic organs performed before and after NACT at the Center, including T2-weighted (T2-WI) and diffusion-weighted (DWI) MR images.
The exclusion criteria were the reduced quality of the images T2-WI, DWI, which made it difficult to adequately segment the images to identify the region of interest (ROI); incomplete clinical data; conducting radiation therapy prior to NACT.
After applying the inclusion and exclusion criteria, 182 patients were enrolled in the study and divided into the main (n=132) and test (n=50) groups.
The response to NACT was assessed on the basis of the tumor size, and it was calculated using the Response Evaluation Criteria in Solid Tumors (RECIST 1.1). There are the following assessment criteria based on the RECIST 1.1 scale:
- complete response (CR) which refers to the disappearance of all foci, any of the enlarged lymph nodes should have a short axis <10mm;
- partial response (PR) which refers to the reduction of the diameter of all foci by more than 30%;
- progressive disease (PD) is an increased sum of the diameters of the main foci by 20% or more;
- stable disease (SD).
The patients were treated with NACT according to the following scheme: paclitaxel 175 mg/m2 + cisplatin 75 mg/m2 on day 1, given as 2–3 courses in a 21-day cycle with subsequent treatment evaluation.
The number of patients with progressive disease (PD) was 30, with partial (PR) and complete (CR) response was 152 according to the RECIST scale. Highly differentiated tumor (G1) was detected in 28 patients, moderately differentiated (G2) in 109 patients and poorly differentiated (G3) in 45 patients.
MRI protocol, imaging and tumor segmentation
The compulsory study protocol included: T2-WI (in three projections), T1-WI without and with MR signal suppression from the fat component (FatSat), DWI with b-factors 0, 500, 1000 m/mm2, with construction of parametric models of the measured diffusion coefficient (MDC), Dynamic 3D FatSat. The studies included in the retrospective analysis were performed on two magnetic resonance imaging scanners at the Center (GE SIGNA Architect 3.0T, Toshiba MS Vantage 1.5T). DICOM data of MR examinations were uploaded to an external solid state drive (SSD) for further processing with specialized free software. The open-source software LIFEx version 73.0 (www.lifexsoft.org) was used in the study. Manual segmentation of the entire primary tumor volume was performed for each image series (T2-WI, DWI) using a 3D region of interest (3D ROI). The example of segmentation of the region of interest is shown in Figures 1 and 2. After segmentation, the texture indices were automatically calculated for each sequence. The results were exported to Excel Files.
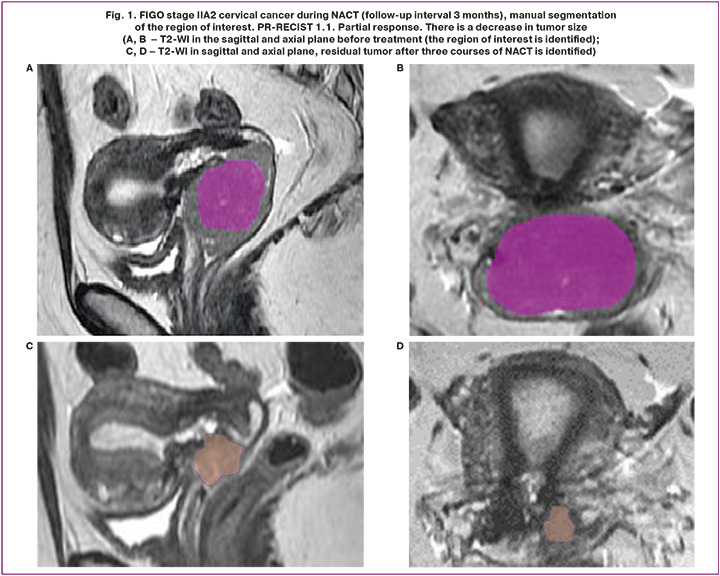
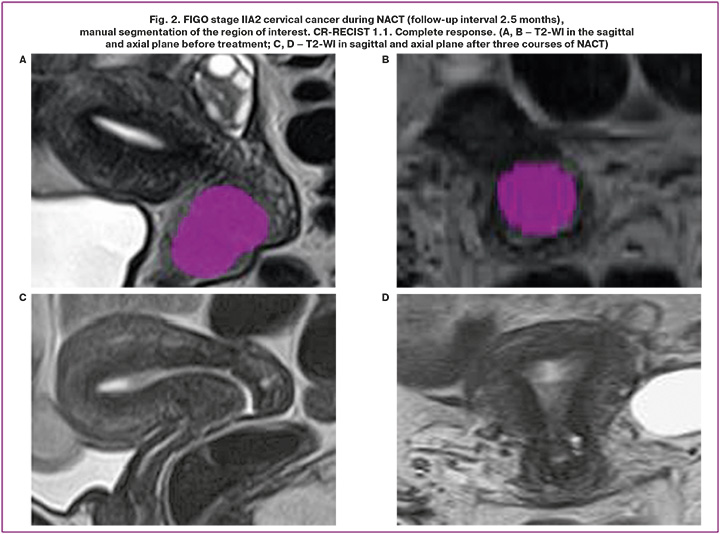
Choice of parameters, construction of rad-score and radiomic nomograms
LASSO linear regression was used for selecting the most statistically significant radiomic characteristics that had a correlation with response to NACT. Some parameters were excluded as their coefficients were reduced to zero; parameters with non-zero coefficients that were associated with treatment response were used. The rad-score (radiomics score, a scoring system for the assessment of the radiomic profile of a tumor) was calculated on the basis of the selected parameters. The combination of rad-score data and clinical risk factors was used to construct the nomogram. The prognostic performance of the radiomic nomogram was tested in the validation group based on ROC analysis, with calculation of sensitivity and specificity.
Statistical analysis
The data were collected, corrected, systematized, and the results were visualized in Microsoft Office Excel (2016) spreadsheets. The Python programming language (Python 3.11) was used for statistical processing of the results. The Shapiro–Wilk test was used to test the conformity of quantitative variables to a normal distribution. The test for normality of distribution showed that the data did not follow the normal distribution in the study, therefore subsequent calculations were made using non-parametric statistical methods.
The median was identified as the center of distribution, with quartiles (Me [Q1; Q3]) serving as metrics for variation. The Mann–Whitney U test was used to compare two samples that were not related to each other.
The qualitative results are expressed as absolute numbers with fractions (%). The nominal data were compared between groups using Pearson’s chi-squared test. Where the number of expected observations in any cell of the four-field table was less than 10, Fisher’s exact test was used to assess the level of significance of the differences.
In order to study the relationship between the phenomena represented by quantitative data, a non-parametric method was used, namely calculation of Spearman’s rank correlation coefficient (Rs) for dichotomous variables and the Matthews correlation coefficient (Rm). The obtained correlation values were interpreted according to the Chaddock scale: weak from 0.1 to 0.3; moderate from 0.3 to 0.5; strong from 0.5 to 0.7; high from 0.7 to 0.9; very high (strong) from 0.9 to 1.0. The differences were considered statistically significant at p≤0.05.
Results
The study included 182 patients aged 27 to 49 years with FIGO disease stages IB3-IIB and IIIC1 (two patients had metastatic changes in pelvic lymph nodes). The number of patients with progressive disease (PD) and stable disease (SD) was 30, with partial (PR) and complete (CR) response was 152 according to the RECIST scale. The patients were randomly divided into main (n=132) and test (n=50) groups. The table presents the clinical data of the patients who responded and did not respond to treatment. Age, tumor size and degree of tumor differentiation had no statistically significant correlation with response to therapy (p>0.05).
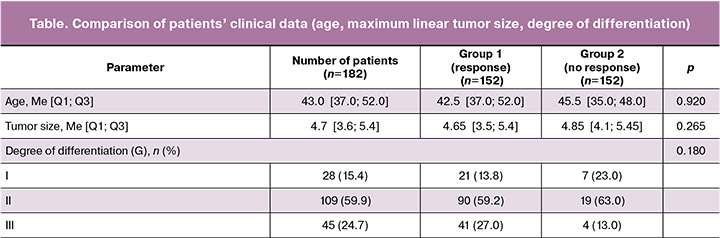
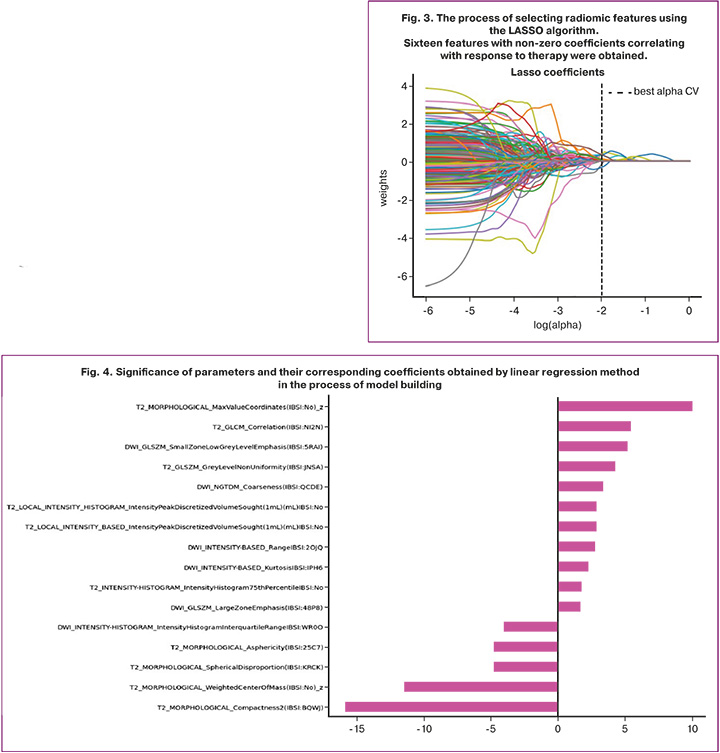
Radiomic analysis was performed using LIFEx software from MR images of T2-WI and DWI sequences. The statistical significance of the obtained model was determined using Pearson’s chi-squared test. The use of the LASSO method is based on taking some coefficients to zero and selecting features with non-zero coefficients indicating a strong association with response to therapy. As a result, 16 most prognostically significant features were obtained. The process of selecting the features and their coefficients are shown in Figures 3 and 4. Subsequently, the rad-score system was developed to evaluate the radiomic profile of each patient. This system was based on the coefficient values of the selected features, which were calculated by a linear combination of these features with their corresponding coefficients.
A model for predicting response to NACT was constructed using LASSO linear regression isolated on radiomic parameters. This model predicts response to therapy in the main sample with a sensitivity of 79.6% and specificity of 80.6%, ROC-AUC 0.90. In the validation group, the model showed a sensitivity of 85.7%, specificity of 72.1% and ROC-AUC of 0.83% (Fig. 5).
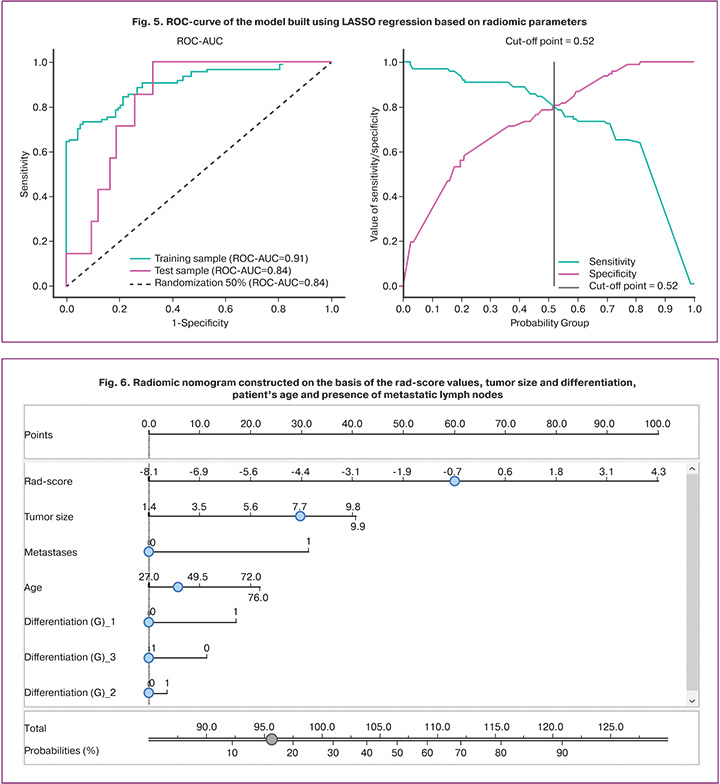
In order to improve the efficiency of the model, a radiomic nomogram including the rad-score and clinical data of the patients was constructed (Fig. 6). The application of the nomogram required the calculation of scores by drawing lines on the point axis according to the rad-score, patient’s age, tumor size and differentiation degree, the presence of metastatically changed pelvic lymph nodes, and then a vertical line was drawn to the lower axis of the nomogram.
The constructed model has a sensitivity of 87.8%, specificity of 88.8%, and ROC-AUC of 0.96. The test group has a sensitivity of 71.4%, specificity of 97.7%, and ROC-AUC of 0.94 (Fig. 7).
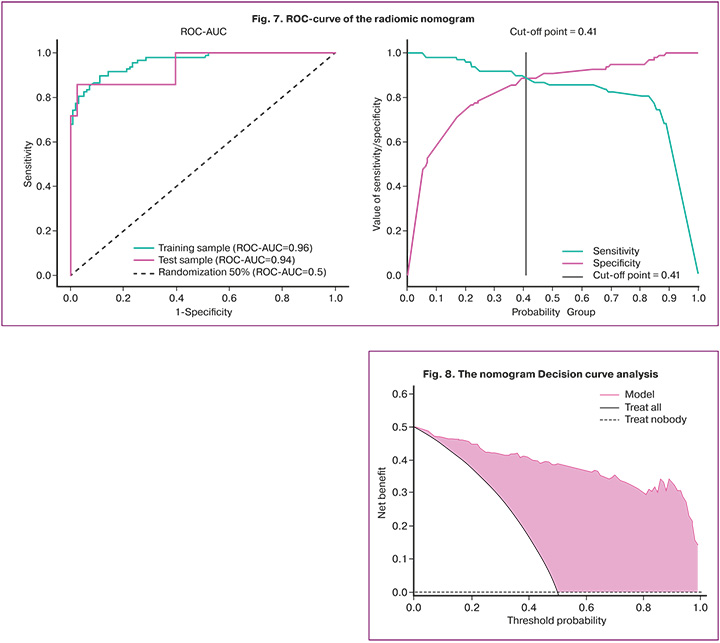
Decision Curve analysis (DCA) was used to assess the clinical utility of the nomogram. The analysis was performed by calculating the net benefits of the risk prediction model compared to the risk thresholds in the combined training and testing sets. The analysis of the decision curve showed that the range of threshold probabilities was between 0 and 1, where the model had a higher value than ‘treat all’ or ‘treat nobody’ (Fig. 8).
Discussion
Chemoradiotherapy is the standard in the treatment of cervical cancer, whereas NACT followed by radical hysterectomy has been proposed as an alternative approach. NACT has been effective in reducing tumor volume and parametrial infiltration in the majority of cases, and in some cases, it has been shown to lead to the complete elimination of parametrial infiltration. As a result, radical operations can be performed during the second stage. Five-year survival rates without recurrence for NACT followed by surgery range from 55.4% to 71%, and five-year overall survival rates range from 58.9% to 81% [6]. Currently, no effective clinical, biochemical, imaging, histological indicators have been developed, and there is a lack of information on biological markers that can predict response to NACT in cervical cancer. At the same time, the ability to predict response to NACT prior to therapy will dramatically improve efficacy; patients who at risk not to respond adequately to systemic treatment can avoid unnecessary chemotherapy-related toxicity as well as early disease progression due to ineffective treatment. Therefore, an effective approach to predict response to NACT prior to therapy is crucial in determining individual treatment strategy.
Currently, there is an increasing number of studies aimed at predicting NACT in cervical cancer. A study by Wang Y.C. et al. examined the possibility of predicting response to NACT using an improved intravoxel incoherent motion (IVIM) sequence. In this study, the values of the true diffusion coefficient (D), the pseudo diffusion coefficient associated with perfusion (D*) and the perfusion fraction (f) were determined. The values of D and MDC in patients who responded to treatment were significantly higher than those who did not respond to treatment. AUC was 0.771 and 0.806, respectively [7]. However, in a study by Dolciami M. et al., the authors also investigated intravoxel incoherent motion in the tumor structure as a predictor of response to NACT. The work found no significant differences in the groups that responded and did not respond to therapy between D* and ADC [8].
In comparison with non-radiomic studies, the model that was constructed in the study used a combination of multiple independent quantitative lesion structure features derived from MR images of the tumor. These features reflected different pathophysiological parameters, allowing for a more in-depth and objective analysis of differences.
The use of radiomic analysis in the diagnosis of cervical cancer is aimed at increasing the informative value of MRI in the lesions of the lymph nodes, parametrial and lymphovascular invasion, as well as in predicting the efficacy of antitumor treatment. For example, Li M. et al. in their study constructed a nomogram based on radiomic features and clinical data of patients with colorectal cancer for preoperative prediction of the lesions of the lymph nodes. The radiomic prediction model demonstrated AUC of 0.70 and 0.75 in the main and test groups, respectively [9]. A retrospective study by Ciolina M. et al. evaluated the role of texture analysis in predicting response to NACT. Twenty-eight female patients were included in the study, and texture analysis was applied on T2-WI sequences. AUC for the patients who responded and did not respond to treatment was 0.87 [10]. The obtained results may be related to the small sample size, isolated analysis of T2-WI sequence radiomic parameters and lack of clinical data of the patients. A multicenter study conducted by San C. built a prediction model for NACT in cervical cancer including 275 patients. The model included combined radiomic analysis of T1-WI and T2-WI sequences as well as clinical characteristics of the patients; AUC was 0.998 and 0.998 in the main and test groups, respectively, which correlates with our findings [11].
In our study, the construction of the nomogram was based on radiomic parameters extracted from T2-WI and DWI sequences and clinical data of the patients (maximum linear tumor size, degree of differentiation, age). It is worth noting that the region of interest (ROI) was defined in as homogeneous areas of the tumor as possible; moreover, the study did not use analysis of the ‘whole’ tumor volume, thus excluding volume-derived radiomic parameters from the comprehensive evaluation. Statistically significant radiomic features were chosen using LASSO logistic regression by selecting non-zero coefficients. The developed model demonstrated sensitivity 87.8% and 71.4%, specificity 88.8% and 97.7% and AUC 0.96 and 0.94 in the main and test groups, respectively.
The present study had several limitations:
- this was a single-center, retrospective study with a relatively small sample size (182 patients); the findings require further validation;
- due to insufficient information on the level of tumor markers (SCCA, CA125, CA153, CA19-9), they were not included in the analysis and formation of the prediction model.
Conclusion
The present study developed two models to predict response to NACT in cervical cancer. Radiomic nomogram combined MR data and clinical characteristics, which improved the prognostic performance of the model. It is possible to predict response to NACT before treatment using radiomic features obtained from MR images and clinical data. The timely identification of patients who do not respond to NACT will allow for the modification of their treatment plan. In addition, accurate prediction of response to therapy can optimize therapeutic approaches and improve overall survival and relapse-free survival. The developed prediction model, if subsequently validated, can be used in clinical practice as one of the factors determining the management tactics of patients in this group.
References
- Bray F., Laversanne M., Sung H., Ferlay J., Siegel R.L., Soerjomataram I. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024; 74(3):229-63. https://dx.doi.org/10.3322/caac.21834.
- Abu-Rustum N.R., Yashar C.M., Bean S., Bradley K., Campos S.M., Chon H.S. et al. NCCN Guidelines Insights: Cervical Cancer, Version 1.2020. J. Natl. Compr. Canc. Netw. 2020; 18(6): 660-6. https://dx.doi.org/10.6004/jnccn.2020.0027.
- Panici P.B., Di Donato V., Palaia I., Visentin V.S., Marchetti C., Perniola G. et al. Type B versus Type C radical hysterectomy after neoadjuvant chemotherapy in locally advanced cervical carcinoma: a propensity-matched analysis. Ann. Surg. Oncol. 2016; 23(7): 2176-82. https://dx.doi.org/10.1245/s10434-015-4996-z.
- Valentini A.L., Miccò M., Gui B., Giuliani M., Rodolfino E., Telesca A.M. et al. The PRICE study: The role of conventional and diffusion-weighted magnetic resonance imaging in assessment of locally advanced cervical cancer patients administered by chemoradiation followed by radical surgery. Eur. Radiol. 2018; 28(6): 2425-35. https://dx.doi.org/10.1007/s00330-017-5233-x.
- Рубцова Н.А., Березовская Т.П., Быченко В.Г., Павловская Е.А., Солопова А.Е., Агабабян Т.А., Ходжибекова М.М., Рыжкова Д.В., Чекалова М.А., Мешкова И.Е., Гажонова В.Е., Гус А.И., Багненко С.С., Медведева Б.М., Ашрафян Л.А., Новикова Е.Г., Берлев И.В., Демидова Л.В., Крикунова Л.И., Коломиец Л.А. Лучевая диагностика рака шейки матки. Консенсус экспертов. Медицинская визуализация. 2024; 28(1):141-56. [Rubtsova N.A., Berezovskaia T.P., Bychenko V.G., Pavlovskaya E.A., Solopova A.E., Agababyan T.A., Khodzhibekova M.M., Ryzhkova D.V., Chekalova M.A., Meshkova I.E., Gazhonova V.E., Gus A.I., Bagnenko S.S., Medvedeva B.M., Ashrafyan L.A., Novikova E.G., Berlev I.V., Demidova L.V., Krikunova L.I., Kolomiets L.A. Imaging of cervical cancer. Consensus of experts. Medical Visualization. 2024; 28(1): 141-56. (in Russian)]. https://dx.doi.org/10.24835/1607-0763-1341.
- Gadducci A., Cosio S. Neoadjuvant chemotherapy in locally advanced cervical cancer: review of the literature and perspectives of clinical research. Anticancer. Res. 2020; 40(9): 4819-28. https://dx.doi.org/10.21873/anticanres.14485.
- Wang Y.C., Hu D.Y., Hu X.M., Shen Y.Q., Meng X.Y., Tang H. et al. Assessing the early response of advanced cervical cancer to neoadjuvant chemotherapy using intravoxel incoherent motion diffusion-weighted magnetic resonance imaging: A pilot study. Chin. Med. J. (Engl.). 2016; 129(6): 665-71. https://dx.doi.org/10.4103/0366-6999.177995.
- Dolciami M., Capuani S., Celli V., Maiuro A., Pernazza A., Palaia I. et al. Intravoxel Incoherent Motion (IVIM) MR quantification in locally advanced cervical cancer (LACC): preliminary study on assessment of tumor aggressiveness and response to neoadjuvant chemotherapy. J. Pers. Med. 2022; 12(4): 638. https://dx.doi.org/10.3390/jpm12040638.
- Li M., Zhang J., Dan Y., Yao Y., Dai W., Cai G. et al. A clinical-radiomics nomogram for the preoperative prediction of lymph node metastasis in colorectal cancer. J. Transl. Med. 2020; 18(1): 46. https://dx.doi.org/10.1186/s12967-020-02215-0.
- Ciolina M., Vinci V., Villani L., Gigli S., Saldari M., Panici P.B. et al. Texture analysis versus conventional MRI prognostic factors in predicting tumor response to neoadjuvant chemotherapy in patients with locally advanced cancer of the uterine cervix. Radiol. Med. 2019; 124(10): 955-64. https://dx.doi.org/10.1007/s11547-019-01055-3.
- Sun C., Tian X., Liu Z., Li W., Li P., Chen J. et al. Radiomic analysis for pretreatment prediction of response to neoadjuvant chemotherapy in locally advanced cervical cancer: A multicentre study. EBioMedicine. 2019; 46: 160-9. https://dx.doi.org/10.1016/j.ebiom.2019.07.049.
Received 05.12.2024
Accepted 11.12.2024
About the Authors
Alina E. Solopova, Dr. Med. Sci., Leading Researcher at the Radiology Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4; Professor at the Department of Obstetrics, Gynecology and Perinatal Medicine, Sechenov University, Ministry of Health of Russia, dr.solopova@mail.ru, Scopus Author ID: 24460923200. Researcher ID: P-8659-2015, https://orcid.org/0000-0003-4768-115XBova B. Bendzhenova, PhD student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, https://orcid.org/0009-0004-4744-0422
Svetlana V. Khokholova, Dr. Med. Sci., Head of Department of Antitumor Drug Therapy, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, https://orcid.org/0000-0002-4121-7228
Corresponding author: Alina E. Solopova, dr.solopova@mail.ru



