Application of molecular genetic methods for the diagnosis and prediction of severe dysplasia and cervical cancer
Objective: Development of a test for detection of "severe" HPV-associated cervical lesions and the risk progression of "minor" forms of lesions using molecular genetic methods.Burmenskaya O.V., Nazarova N.M., Cysheva E.G., Prilepskaya V.N., Trofimov D.Yu., Sukhikh G.T.
Materials and methods: The study included 466 patients of Outpatient Department of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology of the Ministry of Health of Russia. Cytological examination, HPV testing, colposcopy, cervical biopsy (when indicated), histological examination, and gene- expression analysis in real-time RT-PCR for mRNA expression profile of 11 genes were performed. 90 patients with "minor" forms of cervical intraepithelial neoplasia underwent dynamic observation every 6 months within 24 months.
Results: A method for detection of severe dysplasia, cervical cancer and the risk of progression of "minor" forms of HPV-associated diseases was developed. Using regression analysis of MKI67, CDKN2A, CCNB1, MYBL2, EXO, ANLN, UBE2T, PGR, ESR1, BAG1, BCL2 mRNA expression and considering high oncogenic risk of HPV, an algorithm for calculation of the risk index (RI) of cervical lesions was proposed. 88.3% of HSIL and 100% of CC cases were detected using the RI. For "minor" cervical lesions, unfavorable and favorable prognosis were confirmed in 81.5% and in 84.1% of cases, respectively.
Conclusion: The developed model can be prospectively applied in clinical practice for screening and prevention of cervical cancer as an additional test for sorting the samples with "severe" lesions and assessment of the risk of the disease progression, as well as selecting the patients for colposcopy and biopsy according to indications.
Keywords
Cervical cancer (CC) is one of the most common tumors and is listed the fourth among all oncological diseases. In 2020 the incidence of the disease was 604,127 cases, and the mortality rate was 341,831 cases [1]. For prevention of the disease screening of CC is strongly recommended.
In Russia, CC screening is performed by cytological examination and is recommended for women aged 21–29 years every 3 years, and for women of 30–65 – every 5 years a co-testing (cytological examination and analysis for high-risk HPV) is recommended [2]. For a long time, screening included only cytological examination (Pap smear or liquid-based cytology). Despite high specificity, this method has some disadvantages: insufficient sensitivity, which is 53.0% (30–87%), and a large number of discordant results when performed by different clinicians, and when comparing the results with histological examination [3–5]. Cytology histology correlation (CHC) is an important criterion in assessing the quality of screening for cervical cancer [6]. Thus, when comparing the results of cytological and histological examination in 415 patients with cervical diseases, the concordance of the diagnosis was observed in 194 (46.7%) patients. The total number of insignificant inconsistency was 192 (46.4%) cases, and the total number of significant inconsistencies between cytological and histological examination was 29 (6.9%) cases [7]. Bergeron C. and von Knebel Doeberitz M. found that HPV testing can detect earlier stages of high grade squamous intraepithelial lesions (HSIL) than cytological examination [8].
Due to a higher sensitivity (96.1%), HPV testing was included in cervical cancer screening programs, and in some countries it is used as a primary test. However, this method has low specificity because of the high prevalence of HPV infections, which is estimated from 16 to 24% [9–10]. Typically, papillomavirus infection (PVI) is transient, and eliminates within 12–18 months. Persistence of the high carcinogenic risk virus for more than 24 months can lead to the development of cervical intraepithelial neoplasia (CIN). If HPV infection persists longer, the risk of disease progression to CIN 3 within 5 years is: with normal cytological results (NILM) – 5–7.4%, with ASCUS – 8–10%, with LSIL – 15–17% [11]. However, most women with mild dysplasia have a high risk of invasive procedures on the cervix, that could have been avoided if low risk of disease progression was confirmed.
Therefore, the task of revealing patients with “severe” cervical lesions (HSIL, CC) and rapid progression of the disease with “minor” lesions in HPV-infected women is highly important. Molecular genetic methods are useful in solving this problem. The study of Melnikova N.V. et al. demonstrated the possibility of distinguishing the patients with cervical intraepithelial neoplasia CIN 2 and ≤CIN 1 by using the mRNA expression of 21-gene panel using quantitative real-time polymerase chain reaction [12].
The aim of the study was to develop a method for determining “severe” HPV-associated cervical lesions and the risk of progression of “minor” lesions using molecular genetic methods.
Materials and methods
The study included 466 patients aged 20 to 49 years who had applied to the Outpatient Department of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology, Perinatology of the Ministry of Health of the Russian Federation from December 2015 to March 2019. Inclusion criteria: reproductive age from 20 to 49 years; regular menstrual cycle; for the study group: cytological and/or histological confirmation of NILM, ASCUS, LSIL, HSIL or CC, and the presence of carcinogenic HPV risk; for the control group: absence of HPV and cytological confirmation of NILM; ability to follow the protocol instructions; signed informed consent. Exclusion criteria: pregnancy; postpartum period and lactation; hormonal therapy; acute inflammatory disease (of specific or nonspecific etiology); no possibility to follow the protocol instructions.
Additionally, 90 women with “minor” cervical epithelial lesions were included in a prospective observational study, where they were examined every 6 months for 24 months.
Cytological examination, molecular genetic testing, colposcopy, and biopsy (when indicated, with subsequent histological examination of the biopsy sample) were performed within the study. Cytological examination was performed using automatic BD Sure Path technology with cell preparations on a liquid basis and interpretation of cell composition both automatically and manually according to Bethesda classification system (revised in 2014).
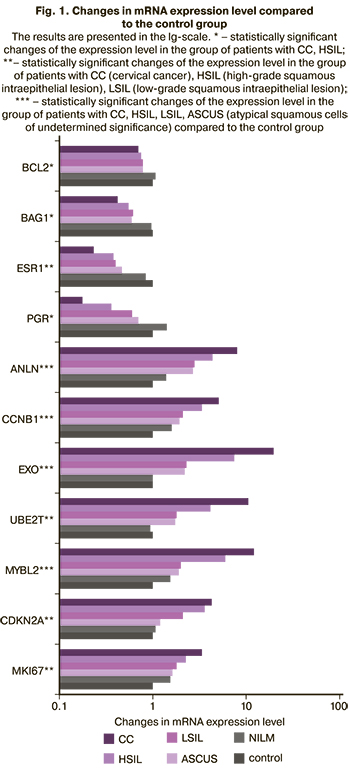 Materials for laboratory examinations were cervical canal scrapings of cells taken in special transport media for liquid cytology (BD Sure Path), HPV typing (Stor-F), and gene expression (Stor-ex). HPV DNA was isolated using a PREP-GC kit, RNA was isolated using PREP-NK. Then, reverse transcription polymerase chain reaction was performed to determine the level of expression of 11 target genes: ESR1, PGR (estrogen and progesterone receptors), MKI67, MYBL2, CCNB1, CDKN2A (genes regulating cell proliferation and cell cycle), ANLN (gene regulating invasion), BCL2, BAG1 (apoptotic genes), EXO1 (gene regulating DNA replication and repair), UBE2T (protein utilization), and two reference genes B2M and GUSB. The transcriptional profile of genes was determined by multiplex real-time PCR (two genes in one tube), in accordance with the instructions of the manufacturer, with primers and fluorescently labeled probes (Fam, Cy5) for encoding gene fragments. Reagents and detecting instrument “DTprime” were used in accordance with the instructions of the manufacturer (“DNA-Technology LLC”, Russia). After amplification, the level of transcripts was calculated by comparing the indicator cycles (∆Cq) with norms relatively reference genes B2M and GUSB.
Materials for laboratory examinations were cervical canal scrapings of cells taken in special transport media for liquid cytology (BD Sure Path), HPV typing (Stor-F), and gene expression (Stor-ex). HPV DNA was isolated using a PREP-GC kit, RNA was isolated using PREP-NK. Then, reverse transcription polymerase chain reaction was performed to determine the level of expression of 11 target genes: ESR1, PGR (estrogen and progesterone receptors), MKI67, MYBL2, CCNB1, CDKN2A (genes regulating cell proliferation and cell cycle), ANLN (gene regulating invasion), BCL2, BAG1 (apoptotic genes), EXO1 (gene regulating DNA replication and repair), UBE2T (protein utilization), and two reference genes B2M and GUSB. The transcriptional profile of genes was determined by multiplex real-time PCR (two genes in one tube), in accordance with the instructions of the manufacturer, with primers and fluorescently labeled probes (Fam, Cy5) for encoding gene fragments. Reagents and detecting instrument “DTprime” were used in accordance with the instructions of the manufacturer (“DNA-Technology LLC”, Russia). After amplification, the level of transcripts was calculated by comparing the indicator cycles (∆Cq) with norms relatively reference genes B2M and GUSB.
HPV typing (high oncogenic risk types: 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82; low oncogenic risk types: 6, 11, and 44(55)) was performed by real-time PCR using “HPV QUANT-21” kit (“DNA-Technology LLC”, Russia).
The study was approved by the Ethics Committee of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology of the Ministry of Health of the Russian Federation (Protocol No. 4, dated October 12, 2015). Patients included in the study signed informed consent.
Statistical analysis
The central tendency of the expression level was presented as median (Me). The significance of differences between the groups was determined using the Mann–Whitney U-test. The difference was considered statistically significant at p<0.05. The development of algorithms for diagnosis of “severe” HPV-associated cervical lesions and the risk of progression of “minor” lesions (Risk Index) was performed using multiple logistic regression analysis. The ln values of gene expression levels and the presence of high-risk HPV were included as independent (predictor) variables. The response variables were the presence and absence of progressive neoplastic transformation of the epithelium. ROC analysis was used to assess the quality of the binary classifier. The results were processed using SPSS Statistics version 17.0.
Results
According to the results of the cytological and histological examination, the following groups were formed: HPV-infected women with normal cytology results (NILM, group 1, n=97), ASCUS (group 2, n=28), LSIL (group 3, n=61), HSIL (group 4, n=60), and CC (group 5, n=21); the control group included 199 women without HPV with NILM confirmed by cytological examination.
It was found that the mRNA expression level of genes MKI67, MYBL2, CCNB1, CDKN2A, ANLN, EXO1, UBE2T increased with the severity of HPV-associated lesion of the epithelium, while the mRNA expression level of ESR1, PGR, BCL2, BAG1 decreased (Figure 1).
The results of regression analysis are presented in Table 1, the results of sample separation in the studied groups depending on RI value are presented in Table 2.
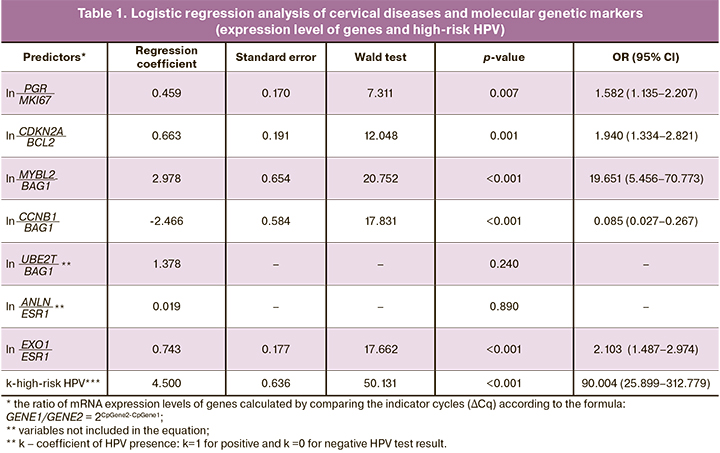
High risk was noted in 53 of 60 samples with cytologically confirmed HSIL and in all samples with CC. The diagnostic sensitivity was 88.3% (80.2–96.5%) for HSIL and 100% (86.5–100%) for CC (Table 2). The area under the ROC was 0.968 ± 0.010 (p=2.9×10-45). The discrimination threshold for cut-off was at 50 points.
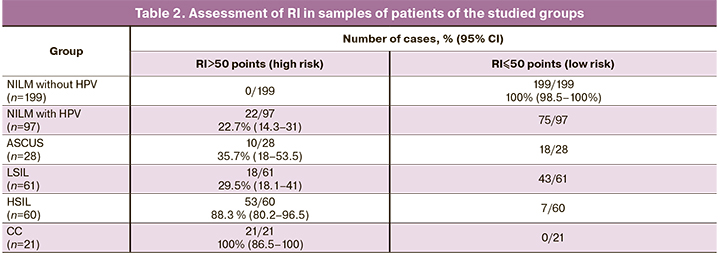
RI less than 50 points was observed in all 199 samples without HPV with a cytologically confirmed NILM. The diagnostic specificity was 100% (98.5–100%).
The following clinical observation is presented as an example.
Clinical observation
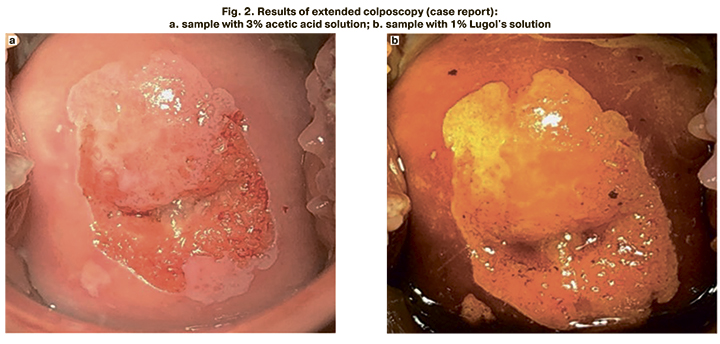
Patient V., 27 years old. The cytological examination confirmed NILM; HPV type 16 was determined. The Risk Index value was calculated using the formula RI= 1/(1+e^(-z) )×100%, where e=2.71828 – is the value of the natural logarithm; z – is the value of the boundary function calculated from experimental data, z=0.459×ln 226.1-30.8 + 0.663×ln 231.8-29.5 + 2.978×ln 228.4-29.4 - 2.466×ln 228.4-28.6 + 0.743×ln 224.7-32.7 + 4.500×1. The RI value was 79 points, and therefore the patient underwent colposcopy. Colposcopy revealed mild changes in the epithelium (Figure 2). After target biopsy, the histological examination confirmed CIN 2.
The high-risk group included 22.7 to 35.7% of samples with “minor” cervical lesions (≤CIN 1). 90 women were followed-up for 24 months with an examination every 6 months. The results of the follow-up in the studied groups are presented in Table 3.
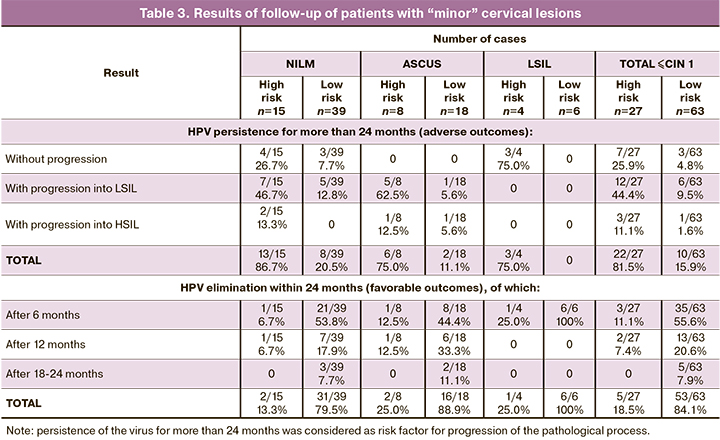
In the NILM group (at risk), the virus persisted for more than 24 months in 13 of 15 cases with progression into LSIL and HSIL in 7 and 2 cases, respectively. In the ASCUS group (at-risk), the virus persisted for more than 24 months in 6 of 8 cases with progression into LSIL and HSIL in 5 and 1 case, respectively. In the LSIL group (at risk), the virus persisted for more than 24 months without a progression in 3 of 4 cases. At risk, the virus eliminated in the NILM group – in 2 of 15 cases, in the ASCUS group – in 2 of 8 cases, and in the LSIL group – in 1 of 4 cases.
At no risk, the virus eliminated in the NILM group – in 31 of 39 cases, in the ASCUS group – in 16 of 18 cases, and in the LSIL group – in all 6 cases. In the NILM group (with no risk), the virus persisted for more than 24 months in 8 of 39 cases with progression into LSIL in 5 cases; in the ASCUS group, the virus persisted for more than 24 months with progression into LSIL and HSIL in 1 case in each.
In patients at risk with “minor” cervical lesions (≤CIN 1), the virus persisted for more than 24 months in 22 of 27 cases (81.5%) with progression into LSIL and HSIL in 12 and 3 cases, respectively (true positive prognosis). Elimination of the virus was observed in 5 of 26 cases (18.5% false positive prognosis).
At no risk, the virus eliminated in 53 of 63 cases (84.1% true negative prognosis). The persistence of the virus for more than 24 months was observed in 10 of 63 cases (15.9% false negative prognosis) with progression into LSIL and HSIL in 6 and 1 case, respectively.
Thus, at 24-month follow-up of patients with “minor” cervical lesions, poor prognosis was confirmed in 81.5% of cases and a good prognosis – in 84.1% of cases.
Discussion
HPV testing is widely used in the diagnosis of cervical lesions. HPVs of high oncogenic risk (types 16, 18, 26, 30, 31, 33, 34, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 67, 68, 69, 70, 73, 82, 85, 97) are common – from 75 to 80% of sexually active women become infected with HPV [13]. In most cases the virus eliminated in 375-542 days after infection [14]. In our study, a 24-month follow-up showed that high-risk HPV eliminated in 38 (42.2%) of 90 patients after 6 months, in 15 (16.7%) – after 12 months, and in 5 (5.6%) – after 18-24 months, i.e. in more than half of cases (64.4%). Similar results were obtained in previous studies [15]. The results of the current study are comparable with the studies of Rodriguez A.C. et al., Weaver B. et al., where in 85% of HPV-infected women the virus eliminated within 8-24 months after infection [16, 17]. The study of Turanova O.V. et al. showed that the virus eliminated after 6 months in 57.1% of patients [18].
A meta-analysis of A.F. Rositch et al., revealed that the elimination of the virus depends on the type and occurs in 10.9-11.5 months after infection. In 58.7% of patients, HPV eliminated in the first year of follow-up, and only in 9.5% and in 3.2% HPV eliminated after 18 and 24 months, respectively [19].
Due to the high prevalence of papillomavirus infection and the high elimination rate of the virus, the task is both to detect “severe” forms of cervical lesions and to identify patients with “minor” cervical lesions in HPV-infected women who are at risk of progression to CIN 3. This study was aimed at solving these problems. We used markers (human mRNA and DNA of high-risk HPV) whose expression levels reversed (increased or decreased) as the severity of cervical lesions increased.
We developed a model for the integral assessment of gene expression levels that helped to identify “severe” of the cervix, including 3 additional cases of high grade squamous intraepithelial lesion (HSIL) missed with cytological examination, but further confirmed by histological examination. One such example is presented in the results of the study (clinical observation). Due to the significant discrepancy between the cytological (NILM) and histological (HSIL) findings, assessment of RI in this patient lead to an early diagnosis of precancerous cervical lesions. The developed model can be potentially used in clinical practice as an additional method for selecting patients for colposcopy and biopsy.
However, we think that the developed method of integral assessment of gene and HPV expression may be of greatest interest for the prognosis of PVI. In patients in the risk group with “minor” cervical lesions (RI>50 points), HPV was more likely to persist for 24 months (81.5% vs. 15.9%) and to progress to LSIL (44.4% vs. 10.5%) and HSIL (12.5% vs. 1.6%).
A similar approach is implemented in the QIAsure Methylation Test (Qiagen, Germany), which can detect hypermethylation of DNA of FAM19A4 genes and microRNA of hsa-mir124-2. DNA hypermethylation leads to decreased expression of these markers and is associated with CC and severe CIN 2/3 lesions on the background of high-risk HPV that persists for more than 5 years, and shows an increased level of methylation and chromosomal aberrations (“cancerous” (epi) genetic profile). The clinical sensitivity of QIAsure test is 66–67% for CIN 3 and 100% for CC when used on samples taken by a clinician or on self-sampled biomaterial. Positive results are believed to provide effective detection of CC and severe CIN 2/3 lesions with an expected high short-term risk of progression to CC [20]. A negative QIAsure test indicates a low risk of CC over the next 14 years [21].
The QIAsure test (approved by FDA) was designed to detect “severe” cervical lesions and to select HPV-positive samples or samples with atypical squamous cells of undetermined significance (ASCUS) in cytological examination for colposcopy or subsequent biopsy.
The test has approximately the same rate (24.9%) of positive results as in our study at ≤CIN 1 [22]. However, the manufactures of the QIAsure test do not give any explanation of this phenomenon. As we think, this may be due to rapidly progressing forms of HPV-associated lesions.
This is a pilot study, and it needs further validation on a larger cohort in accordance with the requirements of the TRIPOD statement, which will be used in planning, conducting, analyzing, and reporting of future studies using this model [23].
Conclusion
The developed model is subject to further testing and can be used in clinical practice for screening and prevention of cervical cancer as a supplementary method for selecting samples with “severe” lesions and the risk of progression, as well as for selecting patients for colposcopy and biopsy if medically required.
References
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021; 71(3):209-49. https://dx.doi.org/10.3322/caac.21660.
- Официальный интернет-портал правовой информации. Доступно по: http://publication.pravo.gov.ru/Document/View/0001202011130037 [Official Internet portal of legal information] (in Russian). Available at: http://publication.pravo.gov.ru/Document/View/0001202011130037
- Bhatla N., Singhal S. Primary HPV screening for cervical cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2020; 65: 98-108. https://dx.doi.org/10.1016/j.bpobgyn.2020.02.008.
- Cuzick J., Clavel C., Petry K-U., Meijer C.J.L.M., Hoyer H., Ratnam S. et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int. J. Cancer. 2006; 119(5): 1095-101. https://dx.doi.org/10.1002/ijc.21955.
- Segura S.E., Ramos-Rivera G., Hakima L., Suhrland M., Khader S. Low-grade squamous intraepithelial lesion, cannot rule out high-grade lesion: diagnosis, histological outcomes and human papillomavirus results. Cytopathology. 2019; 30(1): 99-104. https://dx.doi.org/10.1111/cyt.12629.
- Crothers B.A., Jones B.A., Cahill L.A., Moriarty A.T., Mody D.R., Tench W.D. et al. Quality improvement opportunities in gynecologic cytologic-histologic correlations: findings from the College of American Pathologists Gynecologic Cytopathology Quality Consensus Conference working group 4. Arch. Pathol. Lab. Med. 2013; 137(2): 199-213. https://dx.doi.org/10.5858/arpa.2012-0250-OA.
- Аттоева Д.И., Асатурова А.В., Назарова Н.М., Прилепская В.Н., Стародубцева Н.Л., Шешко П.Л., Уруймагова А.Т. Сопоставление результатов клинических и морфологических методов исследований при ВПЧ-ассоциированных заболеваниях шейки матки (ретроспективное исследование). Гинекология. 2021; 23(1): 78-82. [Attoeva D.I., Asaturova A.V., Nazarova N.M., Prilepskaya V.N., Starodubtseva N.L., Sheshko P.L., Uruimagova A.T. Comparison of the results of clinical and morphological research methods in HPV-associated diseases of the cervix (retrospective study). Gynecology. 2021; 23(1): 78-82. (in Russian)]. https://dx.doi.org/10.26442/20795696.2021.1.200647.
- Bergeron C., von Knebel Doeberitz M. The role of cytology in the 21st century: the integration of cells and molecules. Acta Cytol. 2016; 60(6): 540-2. https://dx.doi.org/10.1159/000449402.
- Guan P., Howell-Jones R., Li N., Bruni L., de Sanjosé S., Franceschi S. et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int. J. Cancer. 2012; 131(10): 2349-59. https://dx.doi.org/10.1002/ijc.27485.
- Bruni L., Diaz M., Castellsagué X., Ferrer E., Bosch F.X., de Sanjosé S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J. Infect. Dis. 2010; 202(12): 1789-99. https://dx.doi.org/10.1086/657321.
- Gage J.C., Hunt W.C., Schiffman M., Katki H.A., Cheung L.S., Cuzick J. et al. Risk stratification using human papillomavirus testing among women with equivocally abnormal cytology: results from a State-Wide Surveillance Program. Cancer Epidemiol. Biomarkers Prev. 2016; 25(1): 36-42. https://dx.doi.org/10.1158/1055-9965.EPI-15-0669.
- Мельникова Н.В., Боженко В.К., Антонова И.Б., Бабаева Н.А., Яровая Н.Ю., Болотина Н.А., Захаренко М.В., Сенчукова А.П., Акопова Н.Б., Александрова Н.В., Бурменская О.В., Ашрафян Л.А. Цервикальные интраэпителиальные неоплазии: анализ профиля мРНК в практике жидкостной цитологии. Акушерство и гинекология. 2017; 4: 95-100. [Melnikova N.V., Bozhenko V.K., Antonova I.B., Babaeva N.A., Yarovaya N.Yu., Bolotina N.A. et al. Cervical intraepithelial neoplasia: analysis of the mRNA profile in the practice of liquid cytology. Obstetrics and gynecology. 2017; 4: 95-100. (in Russian)]. https://dx.doi.org/10.18565/aig.2017.4.95-100.
- Burd E.M. Human papillomavirus laboratory testing: the changing paradigm. Clin. Microbiol. Rev. 2016; 29(2): 291-319. https://dx.doi.org/10.1128/CMR.00013-15.
- Li W., Meng Y., Wang Y., Cheng X., Wang C., Xiao S. et al. Association of age and viral factors with high-risk HPV persistence: a retrospective follow-up study. Gynecol. Oncol. 2019; 154(2): 345-53. https://dx.doi.org/10.1016/j.ygyno.2019.05.026.
- Сычева Е.Г., Назарова Н.М., Бурменская О.В., Прилепская В.Н., Трофимов Д.Ю., Сухих Г.Т. Персистенция ВПЧ высокого онкогенного риска и другие молекулярно-генетические предикторы развития цервикальных интраэпителиальных неоплазий. Акушерство и гинекология. 2018; 12: 104-10. [Sycheva E.G., Nazarova N.M., Burmenskaya O.V., Prilepskaya V.N., Trofimov D.Yu., Sukhikh G.T. Persistence HPV high oncogenic risk and other molecular genetic predictors of cervical intraepithelial neoplasia. Obstetrics and gynecology. 2018; 12: 104-10. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.12.104-110.
- Rodriguez A.C., Schiffman M., Herrero R., Wacholder S., Hildesheim A., Castle P.E. et al. Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. J. Natl. Cancer Inst. 2008; 100(7): 513-7. https://dx.doi.org/10.1093/jnci/djn044.
- Weaver B., Shew M., Qadadri B., Tu W., Tong Y., Denski C. et al. Low-level persistence of human papillomavirus 16 DNA in a cohort of closely followed adolescent women. J. Med. Virol. 2011; 83(8): 1362-9. https://dx.doi.org/10.1002/jmv.22116.
- Туранова О.В., Белокриницкая Т.Е., Белозерцева Е.П., Авраченкова А.В. ВПЧ-инфекция: проспективное наблюдение элиминации и оценка факторов риска персистенции. Доктор.Ру. 2019; 4: 31-5. [Turanova O.V., Belokrinitskaya T.E., Belozertseva E.P., Avrachenkova A.V. HPV infection: prospective observation of elimination and assessment of risk factors for persistence. Doctor.Ru. 2019; 4: 31-5. (in Russian)]. https://dx.doi.org/10.31550/1727-2378-2019-159-4-31-35.
- Rositch A.F., Koshiol J., Hudgens M.G., Razzaghi H., Backes D.M., Pimenta J.M. et al. Patterns of persistent genital human papillomavirus infection among women worldwide: a literature review and meta-analysis. Int. J. Cancer. 2013; 133(6): 1271-85. https://dx.doi.org/10.1002/ijc.27828.
- Floore A., Hesselink A., Oštrbenk A., Alcaniz E., Rothe B., Pedersen H. et al. Intra‐ and inter-laboratory agreement of the FAM19A4/mir124‐2 methylation test: results from an international study. J. Clin. Lab. Anal. 2019; 33(4): e22854. https://dx.doi.org/10.1002/jcla.22854.
- De Strooper L.M., Berkhof J., Steenbergen R.D., Lissenberg-Witte B.I., Snijders P.J., Meijer C.J. et al. Cervical cancer risk in HPV‐positive women after a negative FAM19A4/mir124‐2 methylation test: a post hoc analysis in the POBASCAM trial with 14 year follow-up. Int. J. Cancer. 2018; 143(6): 1541-8. https://dx.doi.org/10.1002/ijc.31539.
- Luttmer R., De Strooper L.M., Dijkstra M.G., Berkhof J., Snijders P.J., Steenbergen R.D. et al. FAM19A4 methylation analysis in self-samples compared with cervical scrapes for detecting cervical (pre)cancer in HPV-positive women. Br. J. Cancer. 2016; 115(5): 579-87. https://dx.doi.org/10.1038/bjc.2016.200.
- Moons K.G., Altman D.G., Reitsma J.B., Ioannidis J.P.A., Macaskill P., Steyerberg E.W. et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): explanation and elaboration. Ann. Intern. Med. 2015; 162(1): W1-73. https://dx.doi.org/10.7326/M14-0698.
Received 08.06.2021
Accepted 01.07.2021
About the Authors
Olga V. Burmenskaya, Dr. Sci. (Bio), Head of the Laboratory of Oncological Genetics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, +7(495)438-22-92, o_bourmenskaya@oparina4.ru, 117997, Russia, Moscow, Ac. Oparin str., 4.Niso M. Nazarova, Dr. Med. Sci., Senior Researcher, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology,
Ministry of Health of the Russian Federation, +7(495)438-14-03, grab2@yandex.ru, 117997, Russia, Moscow, Ac. Oparin str., 4.
Elena G. Sycheva, PhD, doctor, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology Ministry of Health of the Russian Federation, +7(910)492-54-55, el.bona@mail.ru, 117997, Russia, Moscow, Ac. Oparin str., 4.
Vera N. Prilepskaya, Dr. Med. Sci., Professor, Academiciam V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology Ministry of Health
of the Russian Federation, +7(495)438-69-34, vprilepskaya@mail.ru, 117997, Russia, Moscow, Ac. Oparin str., 4.
Dmitry Yu. Trofimov, Dr. Sci. (Bio), Professor, Director of the Institute of Reproductive Genetics, Academiciam V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology Ministry of Health of the Russian Federation, +7(495)438-49-51, d_trofimov@oparina4.ru, 117997, Russia, Moscow, Ac. Oparin str., 4.
Gennady T. Sukhikh, Academician RAS, Professor, Director, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, +7(495)438-18-00, g_sukhikh@oparina4.ru, 117997, Russia, Moscow, Ac. Oparin str., 4.
Authors' contributions: Suklhikh G.T. – the concept and design of th study; Nazarova N.M., Sycheva E.G. – material collection and processing; Trofimov D.Yu., Burmenskaya O.V. – PCR analysis performance; Burmenskaya O.V., Sycheva E.G. – statistical data processing; Burmenskaya O.V., Nazarova N.M. – writing the text of the article; Prilepskaya V.N. – editing the text of the article.
Conflicts of interest: The authors have no conflicts of interest.
Funding: The study was carried out with a partial financial support for scientific research in the framework of the State Assignment No. 19.18-2021 “Elaboration and introduction of the protocols taking into account new technologies in early and differential diagnostics, prediction of the risk of prediction of HPV-associated precancerous lesions and oncological diseases in women of reproductive age”.
Patient Consent for Publication: All patients provided informed consent for the publication of their data (and associated images)
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Burmenskaya O.V., Nazarova N.M., Cysheva E.G., Prilepskaya V.N., Trofimov D.Yu., Sukhikh G.T. Application of molecular genetic methods
for the diagnosis and prediction of severe dysplasia and cervical cancer.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 10: 85-92 (in Russian)
https://dx.doi.org/10.18565/aig.2021.10.85-92



