Reference intervals for blood androgen levels in women of early and late reproductive age
Objective. To form reference intervals for blood androgen levels in women depending on their age. Materials and methods. The study included 1140 women who were divided into two groups depending on their age: late reproductive age (LRA) group consisting of women aged 35 years or older and early reproductive age (ERA) group which included patients under 35 years. To determine a number of hormones, patients of LRA group were divided into two subgroups: women of middle reproductive age (35–39 years) and women of late reproductive age (40–49 years). There was an assessment of the level of the following androgens: total testosterone (TT), free testosterone (FT), dehydroepiandrosterone sulfate (DHEAS), dihydrotestosterone (DHT), androstenedione (A) and sex hormone–binding globulin (SHBG). The calculation of 2.5 and 97.5 percentiles for these indicators was done according to the recommendations of the International Federation of Clinical Chemistry (IFCC) using statistical processing of reference values. Results. Reference intervals were obtained for TT, FT, DHT, DHEAS, A and SHBG in reproductive-aged women and significant differences were revealed in the indicators of LRA and ERA women (p<0.05). Conclusion. During the assessment of androgen levels in women, it is necessary to evaluate not only the upper reference value, as its excess indicates hyperandrogenism, but also the lower reference value, which can be indicative of androgen deficiency. It is also important to stratify patients correctly into groups depending on their age. The obtained data can be used in other clinical and diagnostic laboratories after the appropriate validation, according to the requirements of the Clinical and Laboratory Standards Institute (CLSI).Gavisova A.A., Ivanets T.Yu., Dolgushina N.V.
Keywords
Androgens play an important role in both men and women. They are not only the precursors of many steroid hormones including estrogens, but they also have a direct effect on many body systems [1–3].
Androgens (C19 steroids) are mainly produced in the ovaries and adrenal glands in women [1, 4, 5]. The major androgens are testosterone, dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulfate (DHEAS), androstenedione (A) and 5α-dihydrotestosterone (5α-DHT). The greatest biological activity is observed in testosterone and 5α-DHT, which directly bind to androgen receptors, however, A, DHEA and DHEAS are pro-androgens [1, 6, 7]. Testosterone and 5α-DHT in women are formed mainly by the peripheral transformation of their precursors in the liver, adipose tissue and skin [8].
Androgens, along with estrogens, play an important role in the processes of folliculogenesis [9]. Androgens have been proven to participate in the initiation of primordial follicles [9–11], as well as the development of a preantral follicle to antral one [12]. Thus, it is necessary to maintain optimal levels of androgens for the normal functioning of the ovaries [9]. The connection of androgens and sexual function in women is also of great importance. The level of DHEAS, testosterone and A in the blood serum below the tenth percentile is known to be associated with a decrease in libido [13, 14].
Due to insufficient clinical and laboratory data, the criteria for the diagnosis of androgen deficiency in women are ambiguous. Reference intervals for the level of androgens in women can be found in the literature, however, lower reference values have not been developed for all androgens, depending on the age of women; such values would be evident of the presence of androgen deficiency. According to the Princeton Consensus (June 28-29, 2001, Princeton, New Jersey), androgen deficiency is diagnosed if a woman meets three following conditions: (1) the presence of clinical symptoms of androgen deficiency (fatigue, decreased libido, decreased bone mass and muscle strength, memory changes, etc.); (2) absence of estrogen deficiency, as many of the above mentioned symptoms are often caused by it [15]; (3) a decrease in the level of free testosterone (FT) below the normal range [1, 16, 17].
The aim of this study was to form reference intervals for the level of androgens in the blood of women depending on their age.
Materials and Methods
The study included 1140 women whose age ranged from 18 to 49 years. The patients were divided into two groups depending on their age: late reproductive age (LRA) group consisting of women aged 35 years or older and early reproductive age (ERA) group which included patients under 35 years. To determine a number of hormones, patients of LRA group were divided into two subgroups: women of middle reproductive age (35-39 years) and women of late reproductive age (40-49 years). There were the following exclusion criteria: polycystic ovary syndrome and other causes of hyperandrogenism, premature ovarian insufficiency, surgical menopause, adrenal insufficiency, hormone-producing tumors, amenorrhea/oligomenorrhea, obesity (body mass index > 30 kg/m2), administration of hormonal therapy, pregnancy and lactation. Aliquots of serum samples were used for the study; the samples were obtained in accordance with the rules of sample preparation.
Blood samples were collected from the peripheral vein in the morning, on an empty stomach, in the early follicular phase (2nd -5th day of the menstrual cycle) using closed collection system “S-Monovette®” (Sarstedt, Germany) with a clot activator. The level of total testosterone (TT) in the blood serum was determined using electrochemiluminescent method on an automatic immunochemical analyzer “Cobas e411” (Roche Diagnostics GmbH, Germany). The level of DHEAS, A and sex hormone–binding globulin (SHBG) was determined using the immunochemiluminescent method on an automatic immunochemical analyzer “IMMULITE 2000” (Siemens, USA). The level of FT and dihydrotestosterone (DHT) in the blood serum was determined using a solid-phase enzyme immunoassay with the help of commercial kits Dihydrotestosterone (DHT) ELISA and Free Testosterone ELISA (DBC, Canada). The studies were carried out in the clinical and diagnostic laboratory of the National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow, Russia.
Statistical analysis
The statistical processing of the data was carried out using the statistical software package Statistica10. Variation series were verified for normality using the Shapiro-Wilk test. The calculation of 2.5 and 97.5 percentiles of reference intervals was done using statistical processing of reference values according to the recommendations of the International Federation of Clinical Chemistry (IFCC) and Clinical and Laboratory Standards Institute (CLSI). The series were compared using nonparametric methods (Mann-Whitney U test, Kruskal–Wallis test). Differences were considered statistically significant at p<0.05.
Results and Discussion
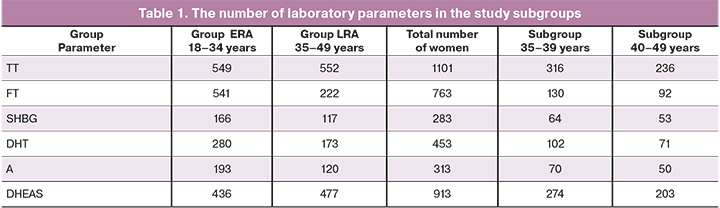
The number of women in each subgroup depending on the age, type of androgen and SHBG is presented in Table 1.
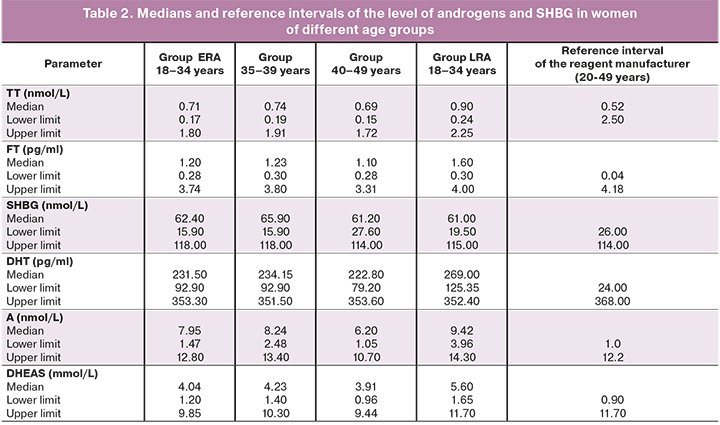
The medians and reference intervals of androgens and SHBG in women of different age groups are presented in Table 2. The calculation of medians and 2.5–97.5 percentile reference interval was performed for all androgens, both in groups LRA and ERA, and in the subgroups of 35-39 years and 40-49 years; however, calculation of parameters in the subgroups of 35-39 years and 40-49 years cannot be considered reliable due to insufficient sample size for FT, DHT, A and SHBG.
The statistical significance of the differences in androgen levels between the groups is presented in Table 3. The medians of all androgens were significantly lower in women of LRA group. The medians of SHBG did not differ in the groups of women.

According to the reagent manufacturer, reference intervals for TT are developed for the general group of women aged 20-49 years. The women of ERA group are known to have stable hormonal function of the ovaries [18], [19]. The impairment of ovarian function in women begins after 35 years (population data), therefore, the proposed stratification of patients based on age is more appropriate. The obtained data showed that the lower reference limit was 0.24 nmol/L for women aged 18-34 years, it was 0.19 nmol/L for women aged 35-39 years, and it was 0.15 nmol/L for women aged 40-49 years. Thus, hormonal function of the ovaries is most precisely reflected in the three-group stratification of patients in terms of TT: 18-34 years, 35-39 years and 40-49 years.
According to the data of the reagent manufacturer, the standard values for FT are 0.04-4.18 pg/ml. The obtained results of this study showed that the lower reference limit did not differ in women of groups ERA and LRA, and it was 0.30 pg/ml and 0.28 pg/ml, respectively. The level of FT is associated with the level of SHBG. There are three types of testosterone in the blood: FT, which is not bound to protein (1-3%), testosterone, which is bound with SHBG (60-70%), and testosterone, which is bound to albumin (poorly bound testosterone, 25-40%). The free and poorly bound forms refer to bioavailable testosterone, which is biologically active. When the level of SHBG is elevated or lowered, the evaluation of the FT level is more appropriate than determining TT.
The reagent manufacturer suggests that the standard values for SHBG are 26.0–114.0 nmol/L. There was no significant difference between women of groups LRA and ERA in our study, either. The lower reference value of SHBG for women of group ERA was 19.5 nmol/L, and it was 15.9 nmol/L for women of group LRA.
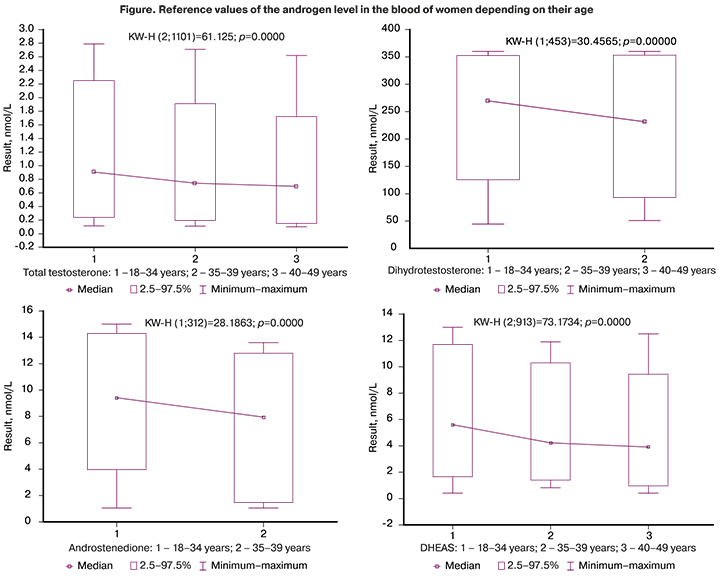
DHT is a biologically active form of testosterone, which derives from it in the cells of target organs under the influence of 5α-reductase. According to the reagent manufacturer, the standard values of DHT are 24.0-368.0 pg/ml. The results of our study showed a significant decrease in both the upper and lower reference values of DHT depending on the increase in the age of women, especially after 40 years. However, these data cannot be considered reliable due to the small sample size of patients aged 40-49 years. Therefore, women are proposed to be stratified by age and the lower standard value of DHT as follows: 18-34 years – 125.35 pg/ml, 35-49 years – 92.90 pg/ml.
Androstenedione is an intermediate product for the formation of both testosterone and estradiol. It has a relatively weak androgenic activity, but its content in the blood serum is often higher than content of testosterone, both in normal and pathological conditions. The concentration of serum androstenedione is a marker of androgen biosynthesis. According to the reagent manufacturer, the standard values for androstenedione level are 1.0–12.2 nmol/L. The results of our study demonstrated a significant decrease in both the upper and lower reference values of androstenedione depending on the increase in the age of women, especially after 40 years. Our data are consistent with the results of other researchers showing a decrease in the level of androstenedione in patients with diminished ovarian function [20]. However, these data cannot be considered reliable due to the small sample size of patients aged 40-49 years. Therefore, women are proposed to be stratified by age and the lower standard value of androstenedione as follows: 18-34 years - 3.96 nmol/L, 35-49 years - 1.47 nmol/L.
DHEAS is one of the androgens produced by the adrenal glands and paraluteal cells of the ovarian follicles. The concentration of DHEAS in the blood shows a strong dependence on age, namely, it increases rapidly after puberty with its peak at the age of 20 years, then gradually decreases, especially after 40 years [21]. According to the data of the reagent manufacturer, the standard values of DHEAS are 0.90–11.70 mmol/L. Taking into account the data of other studies and results of our research, the following age stratification and lower reference values are considered to be the most precise: 20-34 years – 1.65 mmol/L, 35-39 years - 1.40 mmol/L, 40-49 years – 0.96 mmol/L.
The reference values of the androgens are shown in Figure.
Conclusion
Thus, reference intervals for androgens were developed depending on the age of women according to the rules regulated by the guidelines of IFSS (http://www.ifcc.org/) and CLSI (https://clsi.org/). The obtained data can be used in other clinical and diagnostic laboratories after the appropriate validation based on the requirements of CLSI.
References
- Bachmann G., Bancroft J., Braunstein G., Burger H., Davis S., Dennerstein L. et al. Female androgen insufficiency: the Princeton consensus statement on definition, classification, and assessment. Fertil. Steril. 2002; 77(4): 660-5. https://dx.doi.org/10.1016/s0015-0282(02)02969-2.
- Burger H.G. Androgen production in women. Fertil. Steril. 2002; 77(Suppl. 4): S3-5. https://dx.doi.org/10.1016/s0015-0282(02)02985-0.
- Simpson E.R. Aromatization of androgens in women: current concepts and findings. Fertil. Steril. 2002; 77(Suppl. 4): S6-10. https://dx.doi.org/10.1016/s0015-0282(02)02984-9.
- Abraham G.E. Ovarian and adrenal contribution to peripheral androgens during the menstrual cycle. J. Clin. Endocrinol. Metab. 1974; 39(2): 340-6. https://dx.doi.org/10.1210/jcem-39-2-340.
- Longcope C. Adrenal and gonadal androgen secretion in normal females. Clin. Endocrinol. Metab. 1986; 15(2): 213-28. https://dx.doi.org/10.1016/s0300-595x(86)80021-4.
- Luu-The V., Dufort I., Pelletier G., Labrie F. Type 5 17beta-hydroxysteroid dehydrogenase: its role in the formation of androgens in women. Mol. Cell. Endocrinol. 2001; 171(1-2): 77-82. https://dx.doi.org/10.1016/s0303-7207(00)00425-1.
- Labrie F., Diamond P., Cusan L., Gomez J.L., Bélanger A., Candas B. Effect of 12-month dehydroepiandrosterone replacement therapy on bone, vagina, and endometrium in postmenopausal women. J. Clin. Endocrinol. Metab. 1997; 82(10): 3498-505. https://dx.doi.org/10.1210/jcem.82.10.4306.
- Walters K.A., Handelsman D.J. Role of androgens in the ovary. Mol. Cell. Endocrinol. 2018; 465: 36-47. https://dx.doi.org/10.1016/j.mce.2017.06.026.
- Montoya-Botero P., Rodriguez-Purata J., Polyzos N.P. Androgen supplementation in assisted reproduction: where are we in 2019? Curr. Opin. Obstet. Gynecol. 2019; 31(3): 188-94. https://dx.doi.org/10.1097/GCO.0000000000000532.
- Vendola K., Zhou J., Wang J., Famuyiwa O.A., Bievre M., Bondy C.A. Androgens promote oocyte insulin-like growth factor I expression and initiation of follicle development in the primate ovary. Biol. Reprod. 1999; 61(2): 353-7. https://dx.doi.org/10.1095/biolreprod61.2.353.
- Чернуха Г.Е., Найдукова А.А., Удовиченко М.А., Каприна Е.К., Иванец Т.Ю. Андрогенный профиль пациенток с синдромом поликистозных яичников и его взаимосвязь с метаболической дисфункцией. Акушерство и гинекология. 2019; 11: 122-8. [Chernukha G.E., Naidukova A.A., Udovichenko M.A., Kaprina E.K., Ivanets T.Yu. Androgen profile in patients with polycystic ovary syndrome and its association with metabolic dysfunction. Obstetrics and gynecology. 2019; 11: 122-8. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.11.122-128.
- Murray A.A., Gosden R.G., Allison V., Spears N. Effect of androgens on the development of mouse follicles growing in vitro. J. Reprod. Fertil. 1998; 113(1): 27-33. https://dx.doi.org/10.1530/jrf.0.1130027.
- Wåhlin-Jacobsen S., Pedersen A.T., Kristensen E., Laessøe N.C., Lundqvist M., Cohen A.S. et al. Is there a correlation between androgens and sexual desire in women? J. Sex. Med. 2015; 12(2): 358-73. https://dx.doi.org/10.1111/jsm.12774.
- Davis S.R., Davison S.L., Donath S., Bell R.J. Circulating androgen levels and self-reported sexual function in women. JAMA. 2005; 294(1): 91-6. https://dx.doi.org/10.1001/jama.294.1.91.
- Позднякова А.А., Шарашкина Н.В., Иванец Т.Ю., Бутарева Л.Б., Рунихина Н.К., Марченко Л.А. Особенности функционального состояния эндотелия и возможности его коррекции при преждевременной недостаточности яичников. Акушерство и гинекология. 2016; 11: 86-94. [Pozdnyakova A.A., Sharashkina N.V., Ivanets T.Yu., Butareva L.B., Runikhina N.K., Marchenko L.A. Features of endothelial functional state and feasibility of its correction in premature ovarian failure. Obstetrics and gynecology. 2016; 11: 86-94. (in Russian)]. https://dx.doi.org/10.18565/aig.2016.11.86-94.
- Sarrel P.M. Androgen deficiency: menopause and estrogen-related factors. Fertil. Steril. 2002; 77(Suppl. 4): S63-7. https://dx.doi.org/10.1016/s0015-0282(02)02967-9.
- Simon J.A. Estrogen replacement therapy: effects on the endogenous androgen milieu. Fertil. Steril. 2002; 77(Suppl. 4): S77-82. https://dx.doi.org/10.1016/s0015-0282(02)02986-2.
- Bulun S.E. Physiology and pathology of the female reproductive axis. In: Polonsky K.S., Larsen P.R., Kronenberg H.M., eds. Williams textbook of endocrinology. 12th ed. Philadelphia: Elsevier; 2011: ch.17. https://dx.doi.org/10.1016/B978-1-4377-0324-5.00017-1.
- Kotlyar A.M., Seifer D.B. Ethnicity/race and age-specific variations of serum AMH in women – a review. Front. Endocrinol. (Lausanne). 2021; 11: 593216. https://dx.doi.org/10.3389/fendo.2020.593216.
- Soman M., Huang L.C., Cai W.H., Xu J.B., Chen J.Y., He R.K. et al. Serum androgen profiles in women with premature ovarian insufficiency: a systematic review and meta-analysis. Menopause. 2019; 26(1): 78-93. https://dx.doi.org/10.1097/GME.0000000000001161.
- Nunes-Souza E., Silveira M.E., Mendes M.C., Nagashima S., de Paula C.B.V., Silva G.G.V.C. et al. From adrenarche to aging of adrenal zona reticularis: precocious female adrenopause onset. Endocr. Connect. 2020; 9(12): 1212-20. https://dx.doi.org/10.1530/EC-20-0416.
Received 30.04.2021
Accepted 10.06.2021
About the Authors
Alla A. Gavisova, Ph.D., Senior Researcher of the 1st Gynecology Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecologyand Perinatology, Ministry of Healthcare of Russian Federation. E-mail: gavialla@yandex.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Tatyana Yu. Ivanets, Dr. Med. Sci., Head of the Clinical Diagnostic Laboratory, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology
and Perinatology, Ministry of Healthcare of Russian Federation. E-mail: Т_ivanets@oparina4.ru.
Nataliya V. Dolgushina, Dr. Med. Sci., Professor, Deputy Director – Head of the Department of Research Administration, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of Russian Federation. E-mail: n_dolgushina@oparina4.ru.
117997, Russia, Moscow, Ac. Oparina str., 4.
For citation: Gavisova A.A., Ivanets T.Yu., Dolgushina N.V. Reference intervals for blood androgen levels in women of early and late reproductive age.
Akusherstvo i Ginekologiya / Obstetrics and gynecology. 2021; 8: 127-132 (in Russian)
https://dx.doi.org/10.18565/aig.2021.8.127-132



