Гормональная регуляция молочной железы
Молочная железа является частью репродуктивной системы женщины. На фоне заболеваний гинекологического профиля доброкачественные заболевания молочных желез встречаются у 76–97,8% женщин [1]. В том числе поэтому, согласно Приказу МЗ РФ от 20 октября 2020 г. N 1130н «Об утверждении порядка оказания медицинской помощи по профилю "акушерство и гинекология"», при исключении злокачественных новообразований женщины с доброкачественными заболеваниями молочных желез находятся под диспансерным наблюдением врача-акушера-гинеколога, который оказывает медицинскую помощь по диагностике доброкачественной патологии молочных желез и лечению доброкачественных диффузных изменений с учетом сопутствующей гинекологической патологии [2].
На сегодняшний день общепринятая терминология доброкачественных заболеваний молочных желез отсутствует. В нашей стране наиболее часто пользуются термином «мастопатия», за рубежом – «фиброзно-кистозная болезнь» [1].
Для понимания патогенеза дисгормональных заболеваний молочных желез важно учитывать, что регуляция роста и развития молочной железы происходит под сложным гормональным контролем. Основная роль в данных процессах принадлежит половым гормонам. В первой фазе пубертатного периода на развитие молочных желез влияют эстрогены, во второй – эстрогены и прогестерон. Эстрогены ответственны за рост и развитие протоков и соединительной ткани, прогестерон – за рост и развитие железистой ткани, увеличение числа альвеол, рост долек. Основная роль пролактина – стимуляция секреции молока лактоцитами; под влиянием пролактина увеличивается число эстрогеновых рецепторов в молочной железе. В регуляции развития молочной железы участвует и гормон роста, сходный по физиологическому действию с пролактином [3].
Вне беременности молочная железа подвержена непосредственному влиянию эстрогенов, прогестерона, пролактина и гормона роста, тогда как во время беременности – хорионического гонадотропина, ингибина, плацентарных эстрогенов, прогестерона и пролактина.
Помимо этого на ткани молочной железы влияют тиреоидные гормоны, глюкокортикоиды, минералокортикоиды, инсулин и витамин D [4].
Клинические и экспериментальные данные о роли гормонов в развитии фиброзно-кистозной болезни (ФКБ) молочных желез зачастую противоречивы. Тем не менее, мастопатия является гормонозависимым заболеванием, обусловленным дисбалансом в гипоталамо-гипофизарно-яичниковой системе [5].
На сегодняшний день полагают, что ФКБ не является предраком или стадией канцерогенеза в молочной железе. Но при этом риск развития рака молочной железы (РМЖ) на фоне ФКБ во многом зависит от выраженности пролиферативных процессов в ткани молочной железы. Ключевым в оценке риска РМЖ у больных с мастопатией является морфологическое исследование ткани молочной железы, полученной при биопсии [6].
В таблице 1 указана степень риска РМЖ при мастопатии, согласно Santen R.J. и Mansel R. [7].

Также независимым фактором риска развития РМЖ является высокая маммографическая плотность. Продемонстрировано, что ее повышение ассоциируется с увеличением риска развития РМЖ в 3–6 раз, что существенно выше, чем при многих других факторах риска. У женщин от 35 до 74 лет с пролиферативными формами ФКБ высокая маммографическая плотность дополнительно повышала риск РМЖ на 9,3–27,8% [6].
Как указано выше, молочная железа является органом-мишенью для большого количества гормонов и факторов роста, где одну из ведущих ролей играют половые гормоны и экспрессия соответствующих рецепторов [5].
Современные представления о функции половых гормонов при раке молочной железы невероятно обширны. Постоянно появляются новые исследования в этой области, в том числе исследования, посвященные потенциальному терапевтическому влиянию на рецепторы различных стероидных гормонов [4].
Семейство стероидных рецепторов, которое включает рецепторы также рецепторы половых гормонов – эстрогенов (ЭР), прогестерона (ПР), андрогенов (АР), играет важную роль в патогенезе заболеваний молочной железы. Они функционируют преимущественно как ядерные рецепторы, регулирующие экспрессию большого числа генов, однако их полный спектр действия выходит далеко за рамки этого основного механизма. Они участвуют во множестве взаимодействий с другими белками, включая обширные перекрестные взаимодействия друг с другом. Их влияние на биологию клеток молочной железы зависит от таких факторов, как посттрансляционные модификации, экспрессия корегуляторов и корепрессоров, а также какая изоформа стероидных рецепторов преимущественно синтезируется в данном клеточном контексте [4]. Рецепторы половых гормонов экспрессируются в здоровой молочной железе человека, а также при различных типах РМЖ.
В случае половых гормонов стероидогенез происходит главным образом в яичниках и надпочечниках, но определенные этапы, особенно превращение тестостерона в дигидротестостерон или эстрадиол (ароматизация), могут иметь место во многих тканях, которые часто одновременно являются мишенями для стероидных гормонов, в том числе, в молочной железе. К ферментам, участвующим в локальном синтезе и метаболизме эстрогенов, относятся ароматаза (переход андрогенов в эстрогены), сульфатаза (переход неактивных сульфатных форм эстрогенов в активные), 17β-дигидрогеназа I и II (из эстрона в эстрадиол и наоборот), сульфотрансфераза (образование сульфатных форм) [5].
В лиганд-зависимых механизмах передачи сигналов ЭР связывание эстрогена с ЭР вызывает конформационное изменение, которое позволяет различным корегуляторам стимулировать транскрипцию генов-мишеней ЭР. Как и для других рецепторов стероидных гормонов, эстроген-зависимые механизмы далее подразделяются на прямой геномный, или классический; непрямой геномный, или неклассический; а также негеномный механизмы действия [8, 9].
Помимо своей способности напрямую регулировать экспрессию генов, эстрогены также влияют на передачу сигналов и клеточную функцию посредством быстрых мембранных механизмов [10].
Значимость гонадного стероидогенеза в нормальном развитии молочных желез и в канцерогенезе, в том числе отражается в том факте, что раннее менархе и поздняя менопауза связаны с более высоким риском рака молочной железы. Точно так же позднее менархе и преждевременная недостаточность яичников ассоциированы со снижением риска РМЖ [11]. При этом большинство случаев РМЖ возникает в постменопаузе, когда уровень циркулирующих эстрогенов низкий. Возможно, высокая распространенность гормонозависимого рака в постменопаузе обусловлена отчасти ролью данных ферментных систем. Активность эстронсульфатазы в опухолевой ткани молочной железы в 10-500 раз выше активности ароматазы. Также важную роль играют гидроксиметаболиты эстрогенов и ряд метаболитов прогестерона (которые оказывают пролиферативные либо антипролиферативные эффекты) [5].
Другой рецептор, который играет важнейшую роль как в норме, так и при РМЖ, – это рецептор прогестерона (ПР). ПР способен оказывать как активирующее, так и тормозящее действие на транскрипцию большого числа генов. Геномный результат действия ПР зависит также от локальной экспрессии корегуляторов, которая, как было доказано, различается, в зависимости от ткани. На эти процессы также влияет фаза менструального цикла или сам процесс канцерогенеза [12].
В здоровой молочной железе основным эффектом от стимуляции прогестероном является пролиферация и дифференцировка клеток, ведущие к развитию и росту молочных желез, – процессы, которые происходят в основном в период полового созревания и лактации [12].
Однако на большинство клеток прогестерон не влияет напрямую, поскольку исследования на мышах показывают, что ПР экспрессируют только около 20–40% люминальных эпителиальных клеток молочной железы [12, 13]. Следовательно, пролиферация, индуцированная прогестероном, происходит в две фазы. Во-первых, в течение первых 24 часов после воздействия прогестерона ПР-положительные клетки пролиферируют и синтезируют паракринные митогенные факторы, наиболее важным из которых является RANKL (receptor activator of nuclear factor κβ ligand, лиганд рецептора-активатора ядерного фактора κβ), которые затем запускают пролиферацию рядом расположенных клеток, которые не экспрессируют ПР [13].
Исследования на мышиных моделях показали решающую роль действия ПР в инициации канцерогенеза. Более того, наблюдается увеличение относительной численности ПР-положительных клеток с вышеупомянутых 20–40% в здоровой молочной железе до примерно 50% при инвазивном раке, что предполагает переход от паракринного к аутокринному режиму передачи сигналов в качестве основного фактора прогрессирования опухоли. [13]. С другой стороны, на более поздних стадиях канцерогенеза потеря экспрессии ПР связана с менее дифференцированным и более агрессивным фенотипом, что приводит к худшему прогнозу [12].
В нормальной ткани изоформы ПР присутствуют почти в равных количествах, но в опухолевых клетках соотношение ПРA: ПРB часто нарушено [13, 14].
В здоровой ткани молочной железы примерно 20% эпителиальных клеток экспрессируют андрогеновые рецепторы, где они отвечают за рост и дифференцировку клеток [15].
Есть данные, что рецепторы андрогенов присутствуют примерно в 80% клеток инвазивного рака молочной железы, с наибольшей частотой (95%) в случае ЭР-позитивного и наименьшей (10–35%) в случае трижды негативного РМЖ [16, 17]. Фактически, АР является наиболее часто экспрессируемым ядерным рецептором при раке молочной железы. В некоторых случаях (25%) АР является единственным рецептором половых гормонов, экспрессируемым отдаленными метастазами [16].
Сигнальные пути АР могут повышать резистентность к эндокринной терапии (тамоксифен, анастрозол, фулвестрант) при ЭР-позитивном раке за счет перекрестного взаимодействия с сигнальными путями ЭР.
Исследование влияния АР при трижды негативном РМЖ дало смешанные результаты, начиная от повышенной смертности и метастазов до отсутствия связи, а также лучшим прогнозом и меньшим вовлечением лимфатических узлов [17].
Потенциальные возможности терапии РМЖ препаратами, влияющими на рецепторы андрогенов, включает две основные стратегии: применение агонистов АР при раке ЭР-позитивном РМЖ и применение антагонистов в случае трижды негативной опухоли, экспрессирующей АР [17].
Некоторые синтетические гестагены с андрогенным эффектом, входящие в состав МГТ, а также глюкокортикоиды и минералокортикоиды могут стимулировать рост опухоли, возможно, также за счет общности в структуре их рецепторов.
Влияние комбинированных оральных контрацептивов и менопаузальной гормонотерапии на риск рака молочной железы
Таким образом, общепринятым является положение о том, что изменения синтеза и метаболизма эстрогенов и прогестерона имеет фундаментальное значение для риска РМЖ [18].
В связи с этим, при назначении комбинированных оральных контрацептивов (КОК) или менопаузальной гормонотерапии (МГТ) врачи-гинекологи зачастую сталкиваются с обеспокоенностью пациенток относительно их влияния данных препаратов на риск РМЖ.
Согласно данным обзора современной литературы от 2018 г., относительный риск РМЖ на фоне текущего приема КОК составляет 1,2 (95% ДИ 1,14–1,26) – то есть, 1 дополнительный случай на 7690 женщин, использующих КОК. Указанный риск, по-видимому, нивелируется при прекращении приема в течение 5 лет [19].
Рассматривая влияние МГТ на риск РМЖ необходимо учитывать тот факт, что в постменопаузе в опухолевой ткани молочной железы концентрация локального эстрадиола в 20–50 раз выше, чем в плазме (результат локальной конверсии из андрогенов, эстрона и эстрона сульфата). При этом не наблюдаются значимые различия в концентрациях эстрадиола: в крови при РМЖ и в норме; в самой молочной железе при раке в репродуктивном возрасте и в постменопаузе; локально при раке в постменопаузе на фоне приема МГТ и без МГТ. Таким образом, опухолевая концентрация эстрадиола не зависит от уровня в плазме [5].
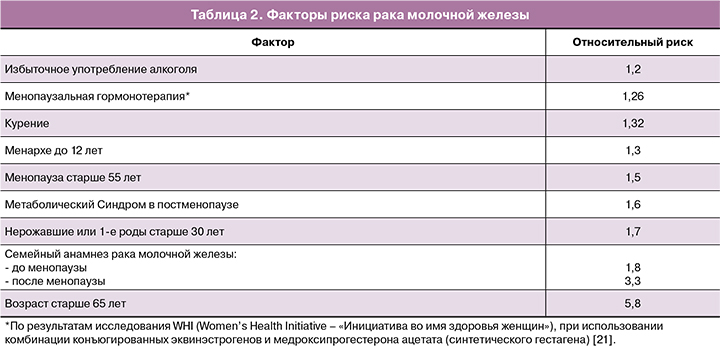
При этом, согласно данным Singletary et al., факторами риска РМЖ, являются факторы, приведенные в таблице 2 [20].
Согласно позиции Североамериканского общества по менопаузе от 2017 г. [22], отягощенный семейный анамнез по РМЖ не является противопоказанием к МГТ.
Согласно рекомендациям Международной ассоциации по менопаузе от 2016 г., возможное повышение риска рака молочной железы, связанное с МГТ, невелико и оценивается в менее чем 0,1% в год, или менее 1,0 случая на 1000 женщин на год использования гормонотерапии [23]. Этот уровень схож или ниже, чем повышение риска, связанное с такими частыми факторами образа жизни, как сниженная физическая активность, ожирение и употребление алкоголя. Данные, полученные в исследовании WHI, не показали повышения риска у женщин, впервые применяющих МГТ в течение 5–7 лет после начала терапии [21]. Исследование WHI также показало, что монотерапия конъюгированными эквинэстрогенами в течение 7,1 лет снижала риск диагностирования рака молочной железы и смертности у женщин с удаленной маткой [24].
В трех исследованиях было высказано предположение, что микронизированный прогестерон или дидрогестерон могут быть связаны с более низким риском, чем синтетический прогестаген [25–27].
Тиболон снижает маммографическую плотность, тогда как высокая маммографическая плотность является одним из значимых факторов риска РМЖ [28].
Доступные в настоящее время данные не позволяют предположить различия в риске между пероральным и трансдермальным путями применения эстрадиола [29]. Тем не менее, в настоящее время недостаточно данных клинических исследований адекватной мощности для полной оценки возможной разницы в частоте развития РМЖ при использовании разных типов, доз и путей введения эстрогенов, типа прогестагена и применения андрогенов.
В августе 2019 г. в журнале «The Lancet» был опубликован нашумевший метаанализ эпидемиологических данных «Тип и длительность менопаузальной гормонотерапии и риск рака молочной железы» от группы авторов из Оксфорда [30]. В анализ вошли исследования с 1992 по 2018 гг. Указывается, что в развитых странах при приеме МГТ в течение 5 лет, начиная с 50 лет, частота РМЖ в возрасте 50–69 лет возрастет на 1 случай на каждые 50 женщин (c 6,3 до 8,3%), использующих монофазную эстроген-гестагенную терапию; на 1 случай на 70 женщин (с 6,3 до 7,7%), использующих циклическую эстроген-гестагенную терапию; на 1 случай на каждые 200 женщин (с 6,3 до 6,8%), использующих монотерапию эстрогенами, при этом вагинальные эстрогены не повышают риски. Соответствующие риски при использовании МГТ в течение 10 лет, по мнению авторов, увеличатся еще вдвое [30].
После данной публикации последовала совместная позиция ведущих международных сообществ, главное заключение которой состояло в том, что нельзя накладывать произвольные ограничения на дозу или длительность МГТ, необходимо руководствоваться также результатами предшествующих масштабных плацебо-контролируемых и обсервационных исследований. Также был сделан вывод о том, что решение о назначении МГТ должно основываться на индивидуальном соотношение пользы и риска, с учетом позитивного влияния МГТ на климактерический синдром, качество жизни, сердечно-сосудистую и костную системы [31].
В октябре 2020 г. было опубликовано исследование Британской национальной базы, которое дает более обобщенные оценки различных рисков РМЖ, связанных с конкретными прогестагенными компонентами МГТ, при этом подтверждая отсутствие повышенных рисков от краткосрочного использования монотерапии эстрогенами, комбинации эстрадиола с дидрогестероном и тиболона. Увеличение продолжительности приема (более 5 лет) было связано с некоторым повышением рисков, при этом тиболон и эстрадиол с дидрогестероном демонстрировали наименьшие риски [32].
Таким образом, можно сделать вывод, что с точки зрения любого увеличения риска РМЖ более значим именно прогестагенный компонент МГТ и КОК, а не эстроген. Согласно рекомендациям Международной ассоциации по менопаузе от 2016 г., риск РМЖ, связанный с применением МГТ, невелик, зависит от ее длительности и неуклонно снижается после ее прекращения. До назначения МГТ следует оценить соответствующий риск, включая обязательное проведение маммографии. Возможное увеличение риска РМЖ, связанное с МГТ, может быть частично снижено путем отбора женщин с исходно более низким индивидуальным риском, включая низкую плотность молочных желез, а также путем образования по вопросам превентивных мер, направленных на коррекцию образа жизни (снижение массы тела, приема алкоголя и увеличение физической активности). У женщин, принимающих МГТ, следует проводить ежегодную маммографию [23].
Применение комбинированных оральных контрацептивов и менопаузальной гормонотерапии у групп высокого риска рака молочной железы (носительниц мутаций BRCA1/2)
У женщин-носительниц мутаций одного из аллелей гена BRCA1 риск развития в течение жизни РМЖ составляет около 75% в возрасте до 50 лет, а к 70 годам достигает 85–97%. При мутациях гена BRCA2 риск развития РМЖ ниже, чем при мутациях BRCA1, и колеблется от 65 до 95% [33–36].
Применение КОК ассоциировано со снижением риска рака яичников (РЯ) до 45–50% у носительниц мутаций BRCA 1 и до 60% у носительниц мутаций BRCA 2. Показано, что более длительное применение КОК коррелирует с более значимым снижением риска РЯ [36]. Возможное, однако до сих пор не подтвержденное, небольшое повышение риска РМЖ у носительниц мутаций BRCA1/2 при приеме КОК значимо компенсируется доказанным профилактическим эффектом в отношении риска РЯ [35, 37].
С учетом негативно влияния мутаций BRCA1/2 на овариальный резерв, а также высокого риска рака яичников и других видов онкологических заболеваний, пациенткам следует рекомендовать превентивное сохранение генетического материала (ооциты, эмбрионы). С учетом высокого риска (50%) наследования мутации генов BRCA 1/2, женщине может быть предложена преимплантационная генетическая диагностика для селекции BRCA1/2-негативных эмбрионов. Согласно данным ряда исследований, применение вспомогательных репродуктивных технологий у носительниц мутаций BRCA1/2 не связано с повышением риска РМЖ и/или РЯ [35, 38, 39].
Риск-редуцирующая сальпингоофорэктомия, произведенная до наступления менопаузы, может приводить к развитию большого спектра климактерических расстройств, более выраженных, чем у женщин с естественной менопаузой [40].
Согласно данным систематического обзора современной литературы и позиции Североамериканского общества по менопаузе [22], носительницы мутаций после проведения риск-редуцирующей тубовариоэктомии могут получить пользу от использования МГТ (улучшение качества жизни, профилактика сердечно-сосудистых заболеваний, остеопороза и когнитивных нарушений) без значимого повышения риска РМЖ [41, 42].
Препаратами выбора для МГТ в данной ситуации являются эстрогены в случае проведения гистерэктомии или комбинация эстрогенов с метаболически нейтральными гестагенами в случае сохраненной матки.
Применение препаратов половых гормонов у больных раком молочной железы
РМЖ (независимо от типа, стадии и прошедшего времени после радикального или адъювантного лечения) является противопоказанием к комбинированным гормональным контрацептивам, МГТ и тиболону [43]. Наблюдательные исследования и РКИ указывают как на нейтральный эффект, так и на повышение рисков рецидива [22].
Согласно позиции Североамериканского общества по менопаузе, рекомендовано принимать решение о назначении системной (а также локальной при генитоуринарном менопаузальном синдроме) МГТ женщинам, перенесшим РМЖ, в исключительных случаях – при тяжелой степени симптомов, после неудачных попыток терапии негормональными методами, после подробного консультирования, с принятием решения совместно с онкологом [22]. Однако, в инструкциях к препаратам КОК, МГТ и вагинальных эстрогенов в нашей стране РМЖ является противопоказанием к их применению, что указано в клинических рекомендациях.
Таким образом, в случае наличия показаний к применению КОК или МГТ у пациенток (в том числе с ФКБ) нужно учитывать тот факт, что при отсутствии узловых образований молочных желез, а также отсутствии высокой маммографической плотности (ACR: D), при наличии категории 1 или 2 по шкале BI-RADS, а также отсутствии РМЖ в анамнезе, как и других значимых факторов риска РМЖ (выявленные ранее мутации в генах BRCA1/2, за исключением перенесенной риск-редуцирующей двусторонней сальпингоофорэктомии и др.), возможно полагать, что у пациентки нет противопоказаний к КОК или МГТ со стороны молочных желез. Тогда как РМЖ (независимо от типа, стадии и прошедшего времени после радикального или адъювантного лечения) является противопоказанием к данным видам терапии.
На сегодняшний день в мире активно изучается альтернатива классической МГТ – тканеселективный эстрогеновый комплекс – комбинация конъюгированных эквинэстрогенов с базедоксифеном (селективным модулятором эстрогеновых рецепторов) [44]. Базедоксифен способен блокировать эстрогеновые рецепторы в молочной железе и эндометрии, благодаря чему эстрогены обладают указанными выше положительными эффектами без выраженного влияния на молочную железу и эндометрий [45]. Данный препарат способствует уменьшению плотности молочных желез [46], но требуются дополнительные данные для подтверждения ее влияния на заболеваемость РМЖ [47]. Отсутствие при применении данной комбинации выраженных «гормональных» (эстрогенных, гестагенных и других) эффектов на молочную железу может быть прогностически более благоприятным для носительниц мутаций BRCA 1/2 [35].
Также одним из перспективных направлений является изучение эффектов эстетрола в составе КОК и МГТ, а также при РМЖ. Эстетрол – эстроген Е4 – вырабатывается только в печени плода человека с 9-й недели гестации. Он является агонистом эстрогеновых рецепторов во влагалище, эндометрии, костной ткани, головном мозге, однако антагонистом эстрогеновых рецепторов в молочной железе. В связи с этим, предполагается, что эстетрол обладает противоопухолевым эффектом в молочной железе [47–50].
Заключение
Таким образом, с учетом того, что молочная железа является органом-мишенью для половых гормонов, при назначении КОК или МГТ ключевым является проведение тщательного обследования молочных желез перед и во время терапии (согласно действующим нормативным документам), отбора пациенток из группы низкого риска РМЖ, а также выбора препаратов с доказанным отсутствием значимого влияния на риски РМЖ.



